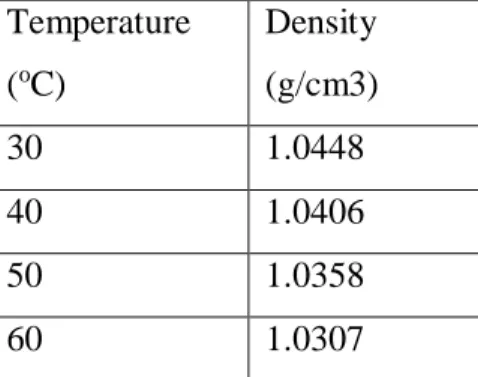
The amount of CO2 present in the atmosphere has increased significantly and will show a tremendous increase if appropriate actions are not taken to deal with this issue [1]. The higher presence of CO2 in natural gas lowers the octane number or heating value and also due to its acidic nature creates corrosion problems in pipelines during transmission and during its processing in equipment [3]. The removal of CO2 gas by adsorption contains two steps: (1) adsorption of the gas on the adsorbent to reach equilibrium, (2) regeneration of the adsorbents for reuse.
The biggest disadvantage of this process is that most of the energy is wasted in cooling the process, mainly in the dilute gas streams. So far there are no industrial applications of the membrane for the capture of CO2 gas from. To achieve effective separation, multiple membrane stages or recycle streams are required to be used in the system, this results in further complications and more energy consumption and cost.
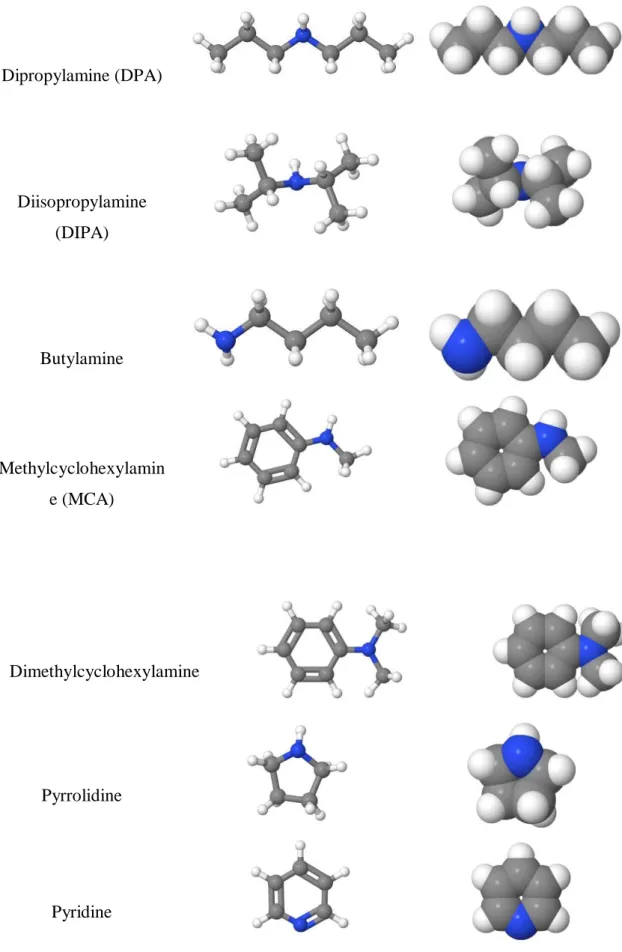
The dissolved gas develops a weak interaction with solvent, which helps in solvent recovery through desorption. Chemical absorption is used instead of physical absorption for many technological processes, such as absorption of CO2 using alkanolamines such as monoethanolamine (MEA), diethanolamine (DEA), methyldiethanolamine (MDEA), 2-amino-2-methyl-1-propanol (AMP ) , mixtures of amines piperazine (PZ), lipophilic amine solvents such as dipropylamine (DPA) & n,n-dimethylcyclohexylamine (DMCA) and their mixtures [8].
Problem Statement
Therefore, new solvents with improved characteristics of lower heat of regeneration, high CO2 loading capacity and fast reaction kinetics should be explored [9]. To evaluate solvent performance with respect to CO2 loading for a wide variety of temperature and pressure conditions for industrial applications and to obtain optimized mixture concentration for effective CO2 series.
Scope of the study
The rate of hydrolysis of these carbamates to bicarbonates depends on the stability of the carbamate. A new proposed solvent, which is the amino acid salt solution, is supposed to have more advantages compared to the conventional alkanolamine [13]. After the CO2 loading exceeds the set value, the solid precipitate is formed from the amino acid salt solution [14].
Here, amino acid salts are used for CO2 absorption [16]. So increasing the alkyl group will further increase the basic strength of the amine molecule. Consequently, in the gas phase, the total basic strength of the amine molecule is mainly influenced by the electronegativity of the central nitrogen atom, which is in the order: R3N > R2NH > RNH2 > NH3.
But in the case of aqueous solution, other factors such as solubilization and steric effect also play a role in influencing the basic strength of the amine molecule. We now know that increasing the electron density of the nitrogen atom will further increase the basic strength of the amine molecule. Most amine molecules are randomly distributed, so their interaction with water forms an intermolecular hydrogen bond interaction, which is weak (13-42.
An alkyl group with a linear chain has the lowest solubility compared to an alkyl substituent with a branched chain and a cyclic structure, because as the sphericity of the molecule increases, its hydrophobic character decreases. The steric effect will play its role in addition to the solubilizing effect to affect the basic strength of the amine molecules. But in the case of tertiary amines, the combination of solubility and steric effect is dominant in determining the basic strength of amines [19].
In this proposed work, we will use several solvents, gases and equipment to analyze the performance and characterization of the solvents [21].
Materials
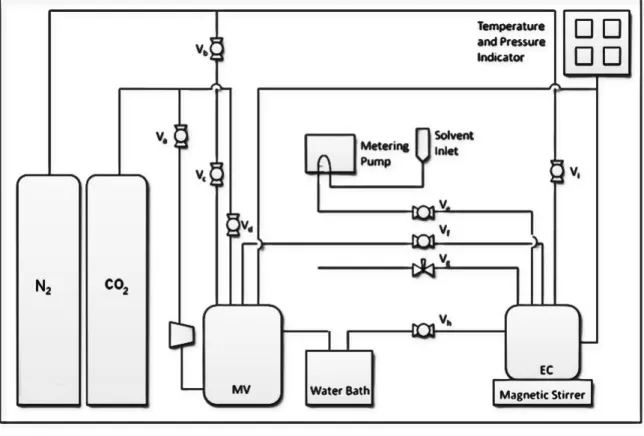
The devices used follow several principles and operation which are discussed in the next section. The solubility of a gas is a thermodynamic property in which the solvent and gas are brought into contact with each other under specific operating conditions of pressure and temperature resulting in the absorption of the gas into the solvent [21]. This high pressure solubility cell is capable of measuring pressure up to 100 bar and temperature up to 353.15 K.
The setup involves two containers, first mixing container (3L) in which gas pressure is increased to 6000kPa and another one is equilibrium cell (50mL) where solubility measurements are to be done. Initially, both containers are purged with nitrogen and then the mixing container is pressurized from 50kPa to 6000kPa to avoid any contamination in the containers. The pressure of the system is measured using digital pressure indicator (Druck DPI 150) with a precision of ± 1.0kPa for a range of 0kPa to 10,000kPa.
The temperature of the system is maintained by the thermostatic water bath Julabo with an uncertainty of ± 0.1ºC and the internal temperature of the mixing vessel and the equilibrium cell is measured by the Yokogawa (7653) digital thermometer with an accuracy of ± 0.01ºC. Initially, a vacuum is created in the equilibrium cell and 5 ml of aqueous solution is supplied using a dosing pump. The temperature of the cell is then brought to the desired value and the pressure is noted. The CO2 is then transferred from the mixing vessel to the equilibrium cell with the stirrer on.
The transferred moles of CO2 can be calculated using the pressure drop, container volume and temperature using the following equation: After almost 2-3 hours, when there is no change in the pressure drop in the solubility cell, we can assume that thermodynamic equilibrium has been achieved. The remaining moles of CO2 in the gas phase ng can be calculated at equilibrium pressure (PCO2), temperature and overhead gas volume using the following equation:. 8) The solubility is then calculated as the volume of CO2, assuming the solution is equimolar, using the gas equation:.

Thermophysical Properties of Amino Acid Salt Solvents
Later, for measurement setting, the procedure of accurate density measurement is completely dependent on the measurement settings which include equilibrium mode, viscosity correction and temperature scanning steps. The settings for density measurement of all samples are adjusted to operate at medium equilibrium mode with automatic viscosity correction method of <700 mPa.s and 5K step increase of temperature within the initial and final temperature range. The predetermined amount of the sample (about 2 ml) is filled into a syringe and slowly injected into U-tube measuring cell (0.7 ml) to ensure that there is no bubble in the cell by looking through the optical glass window.
The default method is activated to start the density measurement and simultaneously record the data in the device's memory. The refractive index of the corresponding amino acid salt aqueous solutions is measured using a digital refractometer (Atago, RX-5000 alfa). The refractometer is calibrated before each sample measurement using distilled water and checked with pure liquids of known refractive index.
The pH value of each aqueous solution of the amino acid salt solution is measured using a pH meter. A comparison of data for selected solutions of amino acid salts with conventional amines currently used for CO2 absorption has been studied [14]. Made correction to the extended proposal report and submitted it to the supervisor for evaluation.
Thermophysical Properties of Amino Acid Salt Solution For this part the results obtained are as followed
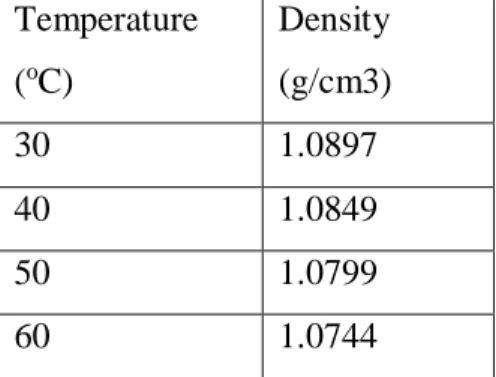
The density of the solution is found to be higher as the composition increases but decreases as the temperature increases. This is due to the presence of both amino acid and amine in the solution which will cause the greater density compared to unique solution. As observed, the pH value of the proposed solution is found to be lower as the composition of the solution increases.
This is due to the fact that the presence of piperazine, which is amine, has slightly acidic properties that affect the pH value of the solution. The refractive index of the proposed solution is greater with the composition of 30% sodium glycinate. The presence of the mixture of both amino acid and amine will influence the observed RI value.
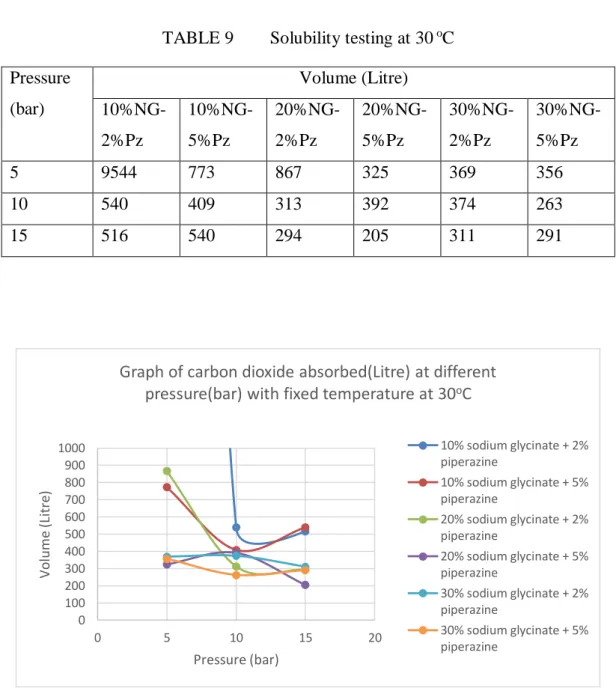
Solubility Testing
From the solubility test performed, it is observed that the variation of different temperature and pressure gives different variation of the absorption or loading capacity. At 30oC, it is observed that the solution does not induce any positive load capacity as desorption of CO2 occurs at the moment. At 40oC and 50oC, a certain positive load capacity is observed for a certain composition of the solution used.
In general, it can be induced that the variation of different composition of the mixture gives different loading capacity as the parameters: temperature and pressure are increased. As shown in the figure above, it is observed that for the available conventional amino salt solution, the loading capacity measured in terms of CO2 solubility decreases as the temperature increases. Consequently, it is concluded that the mixture of the proposed solution has significant impact at certain conditions to increase the loading capacity of CO2.
It can be concluded that different composition of amino acid salt solution will give different loading capacity of CO2. This is due to the sensitivity of the solution at different composition to tolerate the different variation of the temperature and pressure. In the experimental work done, the best condition, when composed of 10% sodium glycinate, 5% piperazine, gives the best loading capacity of the CO2.
In comparison with the pure amino acid salt solution, the loading capacity will decrease as the temperature increases. The proposed solution has an optimal loading capacity under specific operating conditions, as this result shows that it is significant over the available amino acid salt solution. The use of the base consists of group 2 elements such as calcium hydroxide and magnesium hydroxide to see the difference in terms of solubility and thermophysical properties of the substance.
Wessling, “Highly selective solutions of amino acid salts as absorption fluids for capturing CO2 in gas–.