Based on the literature review, it is concluded that esterification is the most preferred upgrading technique due to the properties of bio-oil resulting from the process and its low production cost. For this project, heterogeneous catalyst is used, while the scope is within the application of solid acid and solid base. The research methodology in this project consists of artificial bio-oil preparation, catalyst preparation, esterification experiment and characterization of bio-oil.
Finally, both solid acid and solid catalysts converted the bio-oil into esters through esterification experiment. It is also concluded that this project provides a fast reaction rate to achieve the equilibrium conversions and that the properties of upgraded bio-oil in this project are improved to standard biodiesel properties. The chemical composition of bio-oil is complicated and mainly consists of water, carboxylic acids, carbohydrates and lignin-derived substances.
The aim of this project is to upgrade bio-oil by catalytic esterification over different solid catalysts. Bio-oil has advantages in transportation, storage, combustion, retrofitting and flexibility in production and marketing. Virtually any form of biomass can be considered for fast pyrolysis to produce bio-oil.
Since bio-oil is obtained by a dry distillation process, the liquid is practically ash-free.
RECENT UPGRADING TECHNIQUES
Although some technical problems related to the application remain, bio-oil emulsification can be a useful tool to help pyrolysis enter the market. Hydrogen is a clean energy source and very important in the chemical industry, and the increasing focus on converting the aqueous fraction of bio-oil is promising. Hydrogen production from bio-oil for reforming has been extensively investigated, including fixed or fluidized bed reactions and studies of reforming mechanisms or model compounds.
The fixed bed used in conventional natural gas reforming is not suitable because of its tendency to thermally decompose and form carbon deposits on the upper layer of the catalyst and in the reactor freeboard. Ultimately, the physical properties of the bio-oil are changed in such a way that they are similar to conventional diesel fuels (Zhang et al, 2006). Emulsification of bio-oil with diesel provides a short-term approach to the use of bio-oil in diesel engines, but the fuel stability and properties were unsatisfactory.
Upgrading bio-oil through steam reforming still requires the development of a catalyst that has both high activity and mechanical strength during the steam reforming process. Esterification is the most preferred technique to upgrade bio-oil because the properties of the bio-oils upgraded through this process are comparable to those of conventional diesel fuels. The basic strength of the Group II oxides and hydroxides increased in the order Mg > Ca > Sr > Ba.
In recommending the selection of suitable solid acid catalysts, the activity should be related to the basic properties of the catalysts and their validity. A didactic ionic liquid HS04 was synthesized and used as a catalyst for bio-oil upgrading through ethanol esterification reaction at room temperature. The pH value of the improved bio-waft increased compared to that of the raw bio-waft, while the moisture content decreased.
The results showed that the supercritical upgrading process worked efficiently and the components of the upgraded bio-oil were highly optimized. Aldehydes, such as furfural and vanillin, which are typically present in crude bio-oil, were removed during the supercritical upgrading process and were not found in the upgraded bio-oil. The residue of distilled upgraded bio-oil obviously decreased compared to the residue of distilled crude bio-oil.
The density and kinematic viscosity of upgraded bio-oil decreased compared to that of crude bio-oil. On the other hand, the pH value and heating value increased compared to that of crude bio-oil.

MATERIALS AND EQUIPMENTS
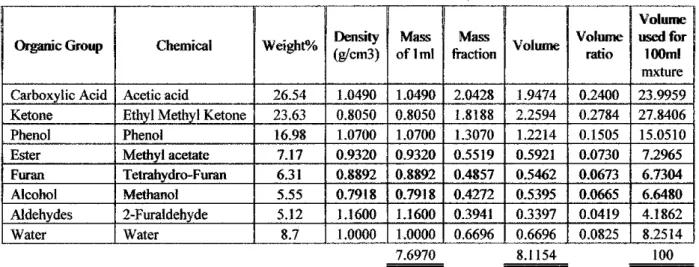
Knowing the percentage of this composition and the density of each component, 100 ml of artificial bio-oil will be produced accordingly. The materials and procedure for this preparation are described as in the following section (Zhang et al, 2006). 1 The esterification reaction with ethanol and bio-oil in a molar ratio of 2.5:1 is carried out in a three-necked flask equipped with a reflux condenser and magnetic stirrer.
After the molar ratio, the mass balance of ethanol and bio-oil per 100 g of total solution mass is 71.43 g and 28.57 g, respectively.
CHARACTERIZATION OF BIO-OIL
The density of the improved bio-oil was measured by considering the mass for 5. The result showed that the improved bio-oil with solid base catalyst has the highest density compared to those with solid acid and no catalyst, although the differences are not significant . The same case occurred in terms of viscosity, while bio-oil improved with solid base catalyst has the highest dynamic viscosity compared to the other two.
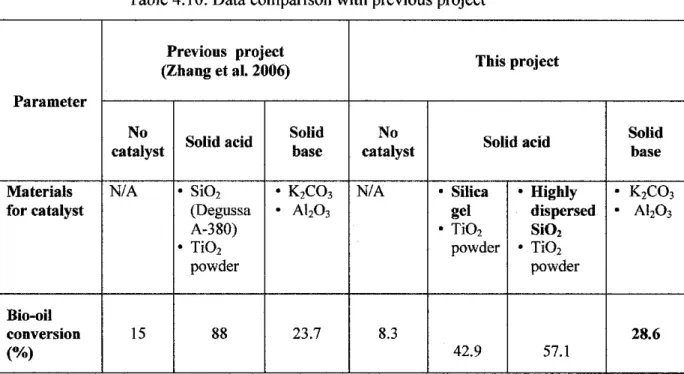
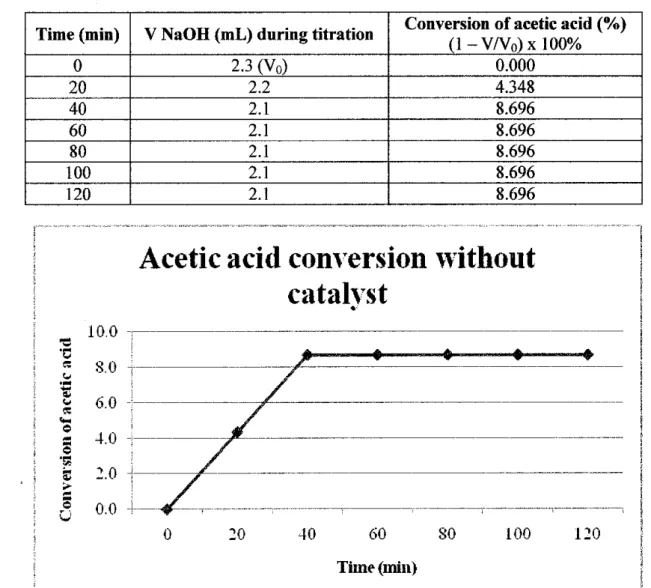
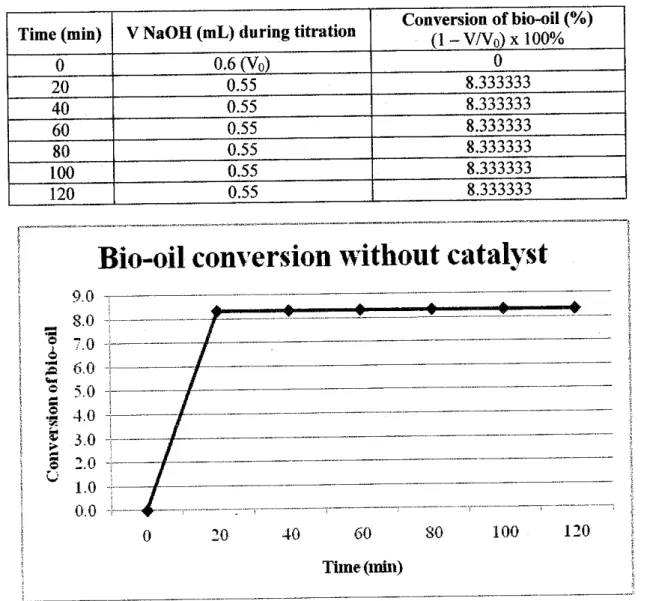
The acidity of the upgraded bio-oil varies differently depending on the type of catalyst used. As shown in Table 4.7, the pH value of upgraded bio-oil without catalyst is 3.013, which is considered quite acidic. This is due to the large amount of acid in the artificial bio-oil itself.
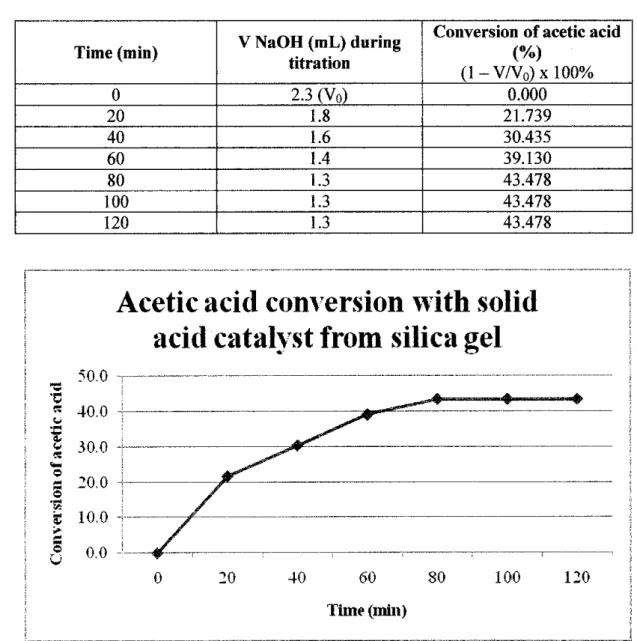
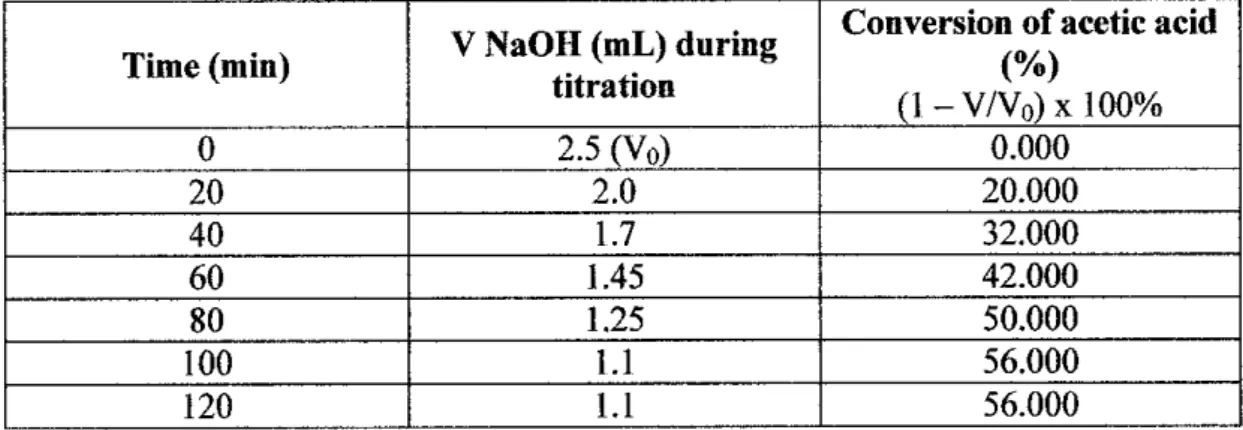
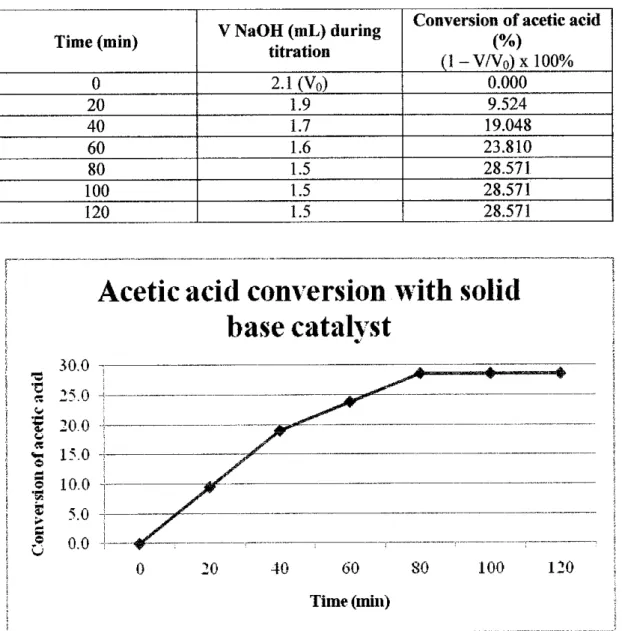
Since the solid acid catalyst was used, the pH value of the upgraded bio-oil becomes more acidic, which is 1.045. Regarding catalyst material, as shown in the table above, this project used silica gel and highly dispersed SiO2 as new approaches to generate solid acid catalyst for bio-oil upgrading through esterification reaction. Regarding bio-oil conversion, Table 4.8 showed that the conversion achieved by using the solid base catalyst in this project is 28.6%.
Another case study analyzed in this project concerns the properties of the upgraded bio-oil. Based on the above table, it is concluded that the properties of upgraded bio-oil correspond to standard biodiesel. 0.8508 gr/cm3 for solid acid, solid base and no catalyst, respectively. Both solid acid and solid base catalysts successfully converted the bio-oil into esters through esterification experiment.
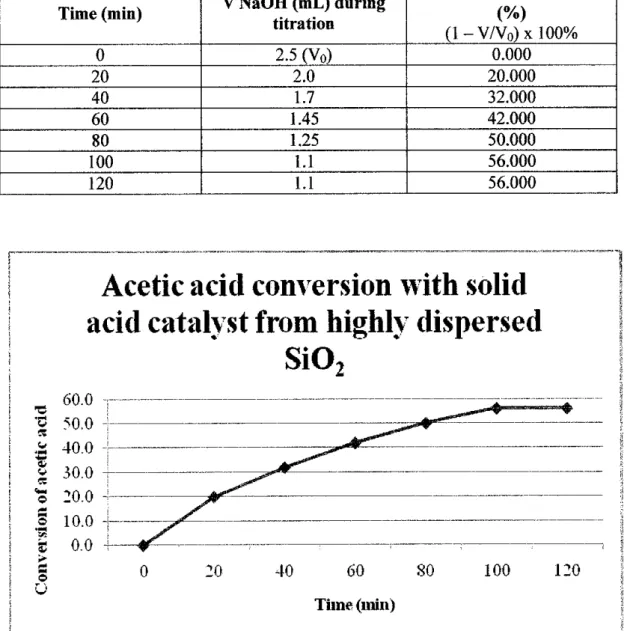
The solid acid catalyst generated by the highly dispersed SiO2 converted the bio-oil the most, while the conversion increased to 57.14%. Compared to the previous project using the same catalyst, this project successfully increased the conversion of upgraded bio-oil using solid base catalysts by 4.9%. The properties of upgraded bio-oil are improved in this project to standard biodiesel properties.
Based on the characterization results, the bio-oil improved with solid base catalyst has the highest dynamic density and viscosity, while the bio-oil improved with solid acid catalyst has the highest acidity. For recommendation, it is encouraged to study other parameters of improved bio-waft such as ester content, water content and flash point.