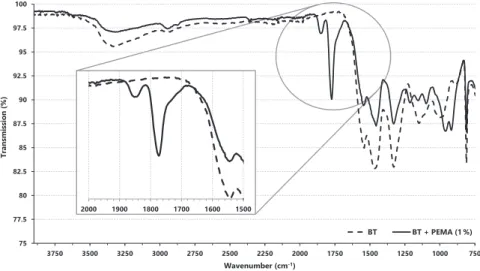
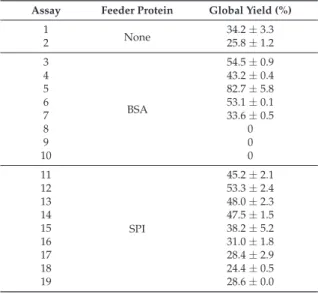
The FTIR results support that the immobilization reaction occurred, as the peaks of the whole cells immobilized on agar show changes relative to the position and intensity of the characteristic peaks for native agar. The FTIR results support that the immobilization reaction occurred, as the peaks for the whole cells immobilized on calcium alginate show changes relative to the position and intensity of the characteristic peaks for native calcium alginate].
Materials and Methods
For workup, the cells were discarded by filtration and 1 ml of the supernatant was saturated with NaCl, followed by extraction with 1 x 1 ml HPLC eluent (n-hexane/i-PrOH = 95/5, v/v) with shaking for 5 min. Then the cells were discarded by filtration and 1 ml of the supernatant was saturated with NaCl followed by extraction with 1 x 1 ml of HPLC eluent (n-hexane/i-PrOH = 95/5, v/v) with shaking for 5 min.
Conclusions
Immobilization of marine fungi on silica gel, silica xerogel and chitosan for biocatalytic reduction of ketones.J. A comparative study of asymmetric reduction of ketones using the growing and resting cells of marine-derived fungi.Mar.
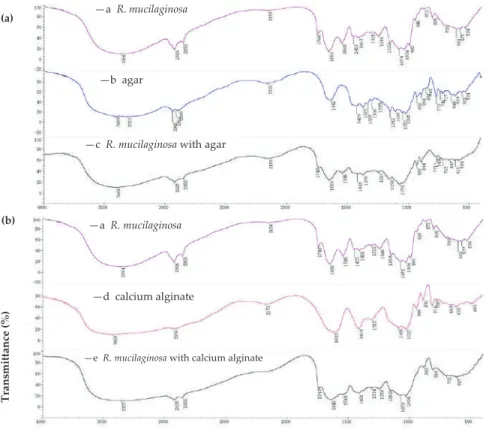
Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its
- Introduction
- Results
- Discussion
- Materials and Methods
These data suggest that AtaA-mediated immobilization is persistent enough to enable continuous hydrogen production in the flow reactor. The hydrogen production in each batch of the triple batch reactions was 0.4–0.6 mol/mol glucose, and the hydrogen production rate in the flow reactor was 42 mL·h−1·L−1.
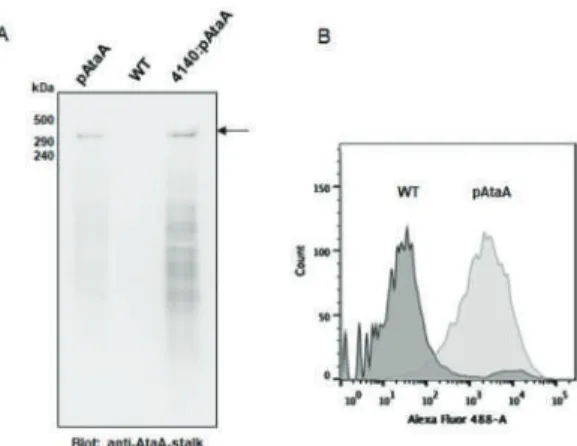
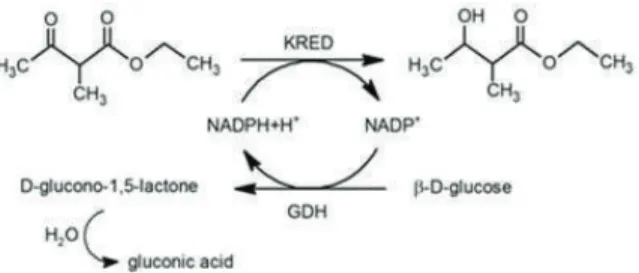
Co-Immobilization of Ketoreductase and Glucose Dehydrogenase
Results and Discussion
Five different ratios and concentrations of KRED and GDH were used for co-immobilizations (Table 2). Furthermore, the concentrations of KRED used (Table 2) were very low compared to the 100 g·dm-3 KRED used in the immobilization on EC-HFA resin [1].
Approaching Immobilization of Enzymes onto Open Porous Basotect ®
Conclusions
As a result, interactions between BT and laccase molecules lead to almost complete coverage of the BT surface with the formation of an enzyme monolayer. Evaluation of different lipase biocatalysts in biodiesel production from used cooking oil: critical role of immobilization support. Fuel.
One-Pot, One-Step Production of Dietary Nucleotides by Magnetic Biocatalysts
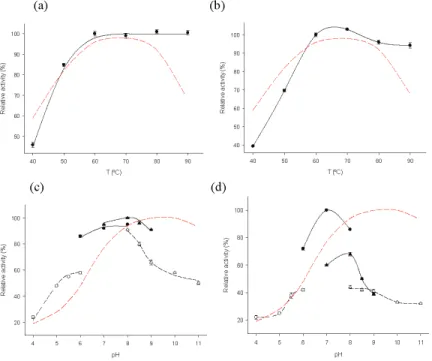
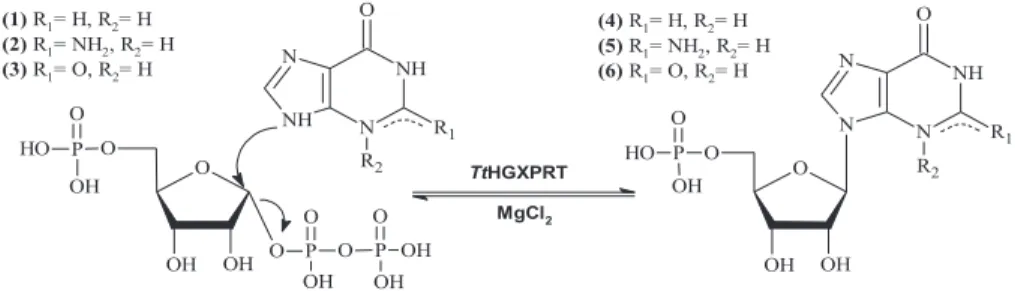
Considering the experimental results, MTtHGXPRT3 and MTtHGXPRT5 were chosen as the best derivatives for further biochemical studies. MTtHGXPRT3 showed high activity (relative activity >80%) in the pH range of 7-9 and there were no substantial differences depending on the nature of the buffer solution (similar activity at pH 7 in sodium phosphate buffer and Tris-HCl; similar activity at pH 8 in sodium phosphate buffer, Tris-HCl and sodium borate). On the contrary, the relative activity of MTtHGXPRT5 was also high (>80%) in the pH range of 7-8, but was strongly influenced by the nature of the solution and showed high activity values only when incubated in 50 mM sodium phosphate buffer.
Considering all the experimental results (catalyst loading, retained activity, temperature and pH dependence of enzymatic activity, and thermal stability), MtHGXPRT3 was selected as the best derivative for synthetic applications. All other reagents and organic solvents were purchased from Scharlab (Barcelona, Spain) and Symta (Madrid, Spain). All determinations were performed in triplicate, and the maximum error was less than 5%.
Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked
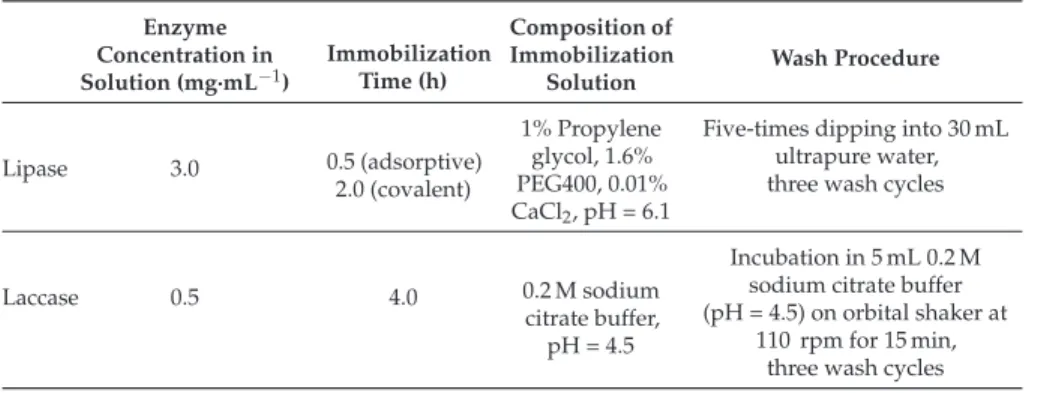
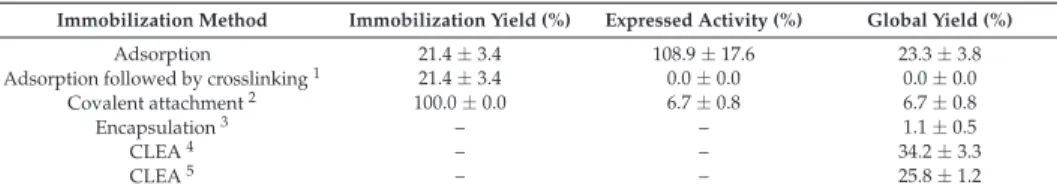
Recently, the effect of pH on the first immobilization step has also been shown [38,39]. Some authors suggest amination of the enzyme (genetically or chemically) [50,51], or coaggregation of the enzyme and aminated polymers [52,53]. By using a nutrient protein, the final activity of the biocatalyst was higher than using the enzyme alone.
This larger size may account for the lower expressed activity of CLEA-β-SPI-12 compared to CLEA-β-BSA-5. The effect of temperature on enzyme activity was similar for allβ-amylase preparations (Figure 2a), expressing maximum activities in the range of 50-55◦C. This may be due to the high size of the starch used, as well as the low DE, possibly causing diffusive limitations in CLEA-β-SPI-12.
7LPHK
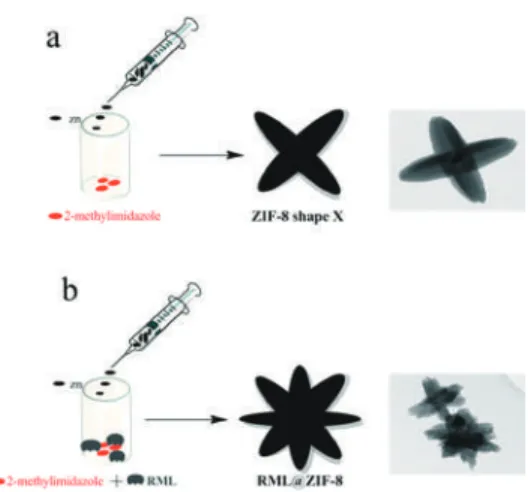
Shaped ZIF-8 for Immobilization
These results were due to the role of the enzyme in modulating the morphological properties of ZIF-8 during the immobilization process. Then, the activity recovery started to decline due to the thermal inactivation of the lipase (Figure 6c). Under these optimal conditions, enzyme loading, encapsulation time, and immobilization temperature led to a 26-fold increase in enzyme activity recovery compared to free enzyme.
We attribute this result to the characteristics of the carrier and the immobilization method, as well as the unique characteristics of lipase. To prevent alcohol-induced inactivation of the enzyme, EtOH was gradually added to the system. Effect of reaction conditions on enzyme-catalyzed transesterification of castor oil: a possible step in biodiesel production. Bioresour.
Immobilization Effects on the Catalytic Properties of Two Fusarium Verticillioides Lipases: Stability,
Hydrolysis, Transesterification and Enantioselectivity Improvement
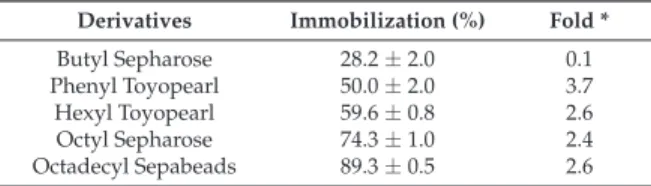
Folding degree of activation and/or inhibition of the lipases when immobilized on hydrophobic supports, compared to their free form. Initially, 70% of the active lipase (confirmed by pNPB assay) was adsorbed on octyl Sepharose (Lip1) after 3 h (Supplementary Figure S1A) and the supernatant containing the remaining lipase activity (Lip2) was further incorporated into octadecyl Sepabeads, resulting in total Slipase or Slipase uptake (S1A). On the other hand, Ba2+ increased the activity of the same enzyme by 45% when 10 mM (final concentration) was added.
The conversion rate of the transesterification reaction was also checked by gas chromatography coupled to a mass spectrometer (Table 5). One gram of each support was added to 5 mL of 10 mM sodium phosphate buffer, at pH 7, and to 5 mL of the crude extract. Modulation of selectivity of immobilized lipases by chemical and physical modifications: Release of Omega-3 fatty acids from fish oil.
Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl
Hexanoate Synthesis
The changes in the reaction temperature can affect the activity and stability of the enzyme and therefore the reaction rate. The decrease in flavor ester synthesis observed above 20◦C was due to the inactivation of the enzyme at high temperatures. As the reaction proceeded, the substrate concentration decreased resulting in a drop in the degree of substrate saturation of the enzyme [28].
The effect of substrate molar ratio (acid to alcohol) was investigated with lyophilized lipase AMS8. As the enzyme concentration increased, the percent ester yield decreased [27]. The rate of ester synthesis was calculated based on the conversion of acid to ester.
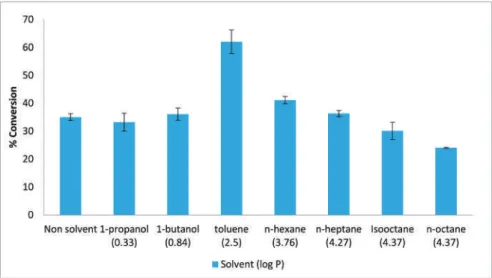
Heterogeneous Biocatalysts Prepared by Immuring Enzymatic Active Components inside Silica Xerogel
Texture of the Biocatalysts
The specific surface area (Ssp.BET) depended both on the taxonomy of the microorganisms and the composition of the multi-component biocatalysts (Table 2). When the content of the microbial biomass was less than 15w/w%, the specific surface area of the biocatalysts was 250 m2/g which was close to Ssp.BET of the mother SiO2-xerogel (without biomass), 260 m2/g. The porosity of the dried biocatalysts was mainly studied by Hg intrusion method; and pore size distribution curves were analyzed.
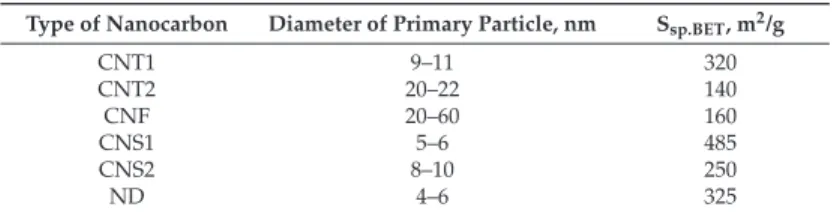
Ssp.BET of the composite lysate-based biocatalysts decreased by a factor of 1.1–1.8 compared to that of the biocatalysts without nanocarbons (Table 3). It was shown that the greater the specific surface area of incorporated nanocarbon, the greater the specific surface area of the composite biocatalyst (Table 3). Therefore, the mesoporous texture was characteristic of the lysate-based biocatalysts and their nanocarbons-in-silica composites (Table 3, Figure 2).
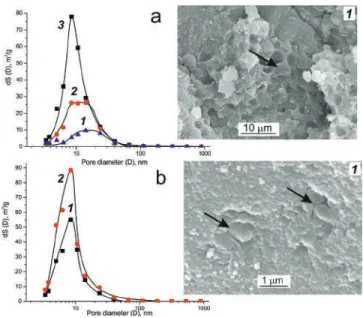
Heterogeneous Whole-Cell and Lysates-Based Biocatalysts
A negligible effect of nanohydrocarbon inclusions on the activity and stability of GI-active biocatalysts was observed and described in [18]. The invertase activity of the whole-cell biocatalysts was found to be linearly dependent on the baker's yeast content from 5 to 80 m/m%. Here, baker's yeast autolysates were used as the active component of biocatalysts prepared by embedding within silica xerogel and nanocarbon dioxide composites in silica.
The internal morphology of the composite biocatalysts based on the lysate of nanocarbon dioxides in silica, examined with a scanning electron microscope, is shown in Figure 5. The internal morphology of the composite biocatalysts based on the lysate was examined with a scanning electron microscope (Figure 7). The stability of lipase-active biocatalysts also depended on the type of catalyzed reaction.
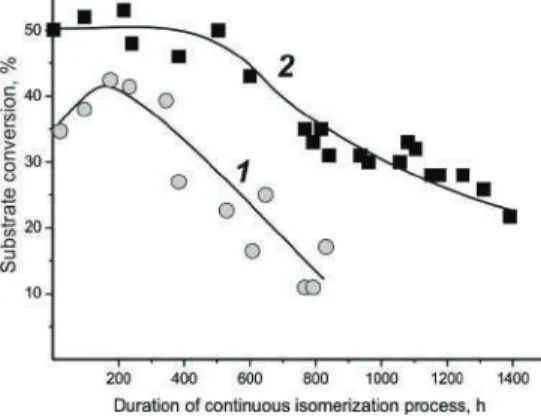
Materials and Methods 1. Enzymatic Active Substances
The thermostat was maintained at the given temperature (50–60 ◦C) of the reaction medium and in the biocatalyst layer. A peristaltic pump ensured circulation of the substrate solution through the biocatalyst layer with a flow rate of 1–35 mL·min−1. Steady-state biocatalyst activity was determined after conditioning the biocatalysts as described elsewhere [18].
A stainless column reactor was filled with 0.5-2 mm granules of the rE.coli/lip lysate-based biocatalyst, uniformly mixed with the inert filler, as well as 1-2 mm commercial silica granules in a 3:1 volume ratio. Depending on the activity of the prepared biocatalyst, the duration of one reaction cycle varied from 5 to 144 hours. The degree of esterification was determined by the consumption of the organic acid involved in the synthesis of the ester.
Main Equipment
Conclusions
Immobilization of glucoamylase by adsorption on carbon supports and its use for heterogeneous hydrolysis of dextrin. Carbohydr. Glucose isomerase activity in suspensions of Arthrobacter nicotianae cells and adsorption immobilization of the microorganisms on inorganic supports. Immobilization of a recombinant strain producing glucose isomerase inside SiO2 xerogel and properties of prepared biocatalysts. Appl.
Immobilization of baker's yeast on jute fabric by polyethyleneimine adhesion: application in an annular column reactor for sucrose inversion. Process. Inorganic gels for immobilization of biocatalysts: Incorporation of invertase-active whole yeast cells (Saccharomyces cerevisiae) into thin layers of SiO2gel applied to glass plates.J. Immobilization of oxidoreductases on alumina-based inorganic supports: Role of mutual correspondence of hydrophobic-hydrophilic features of the enzyme support.
Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus
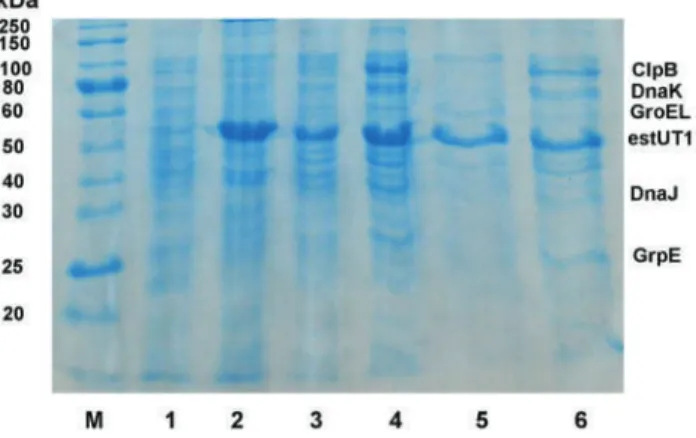
Biocatalyst preparation: 0.5 mL enzyme solution (1 mg mL−1), precipitant-protein ratio 3:1 (v/v), 75 mM glutaraldehyde, 4 h cross-linking. In this study, RSM with FCCCD was used to optimize the conditions for the preparation of CLEA-estUT1 biocatalyst (Table 3). In this study, the effect of BSA concentration on CLEA activity was evaluated.
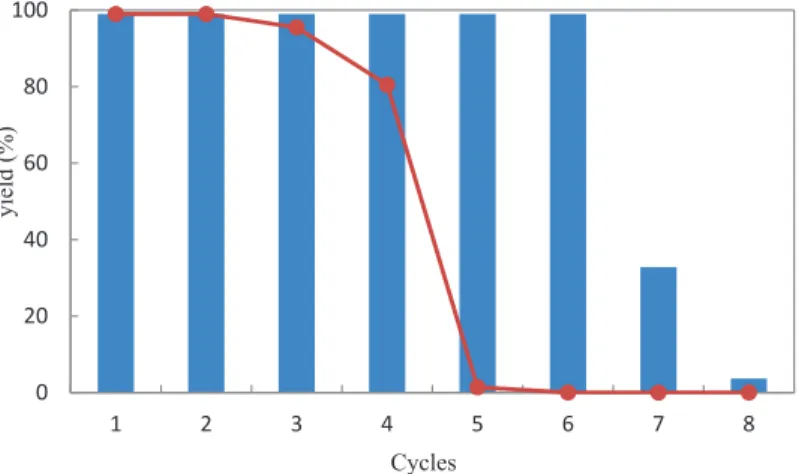
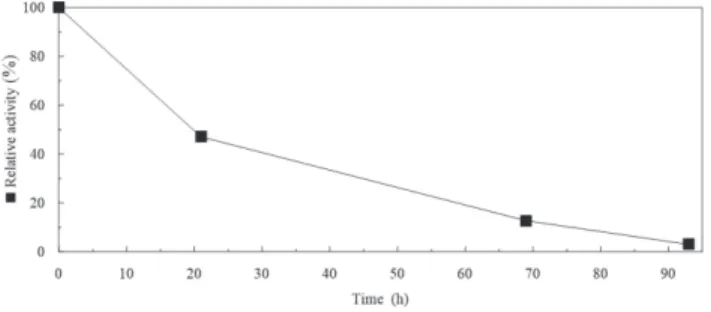
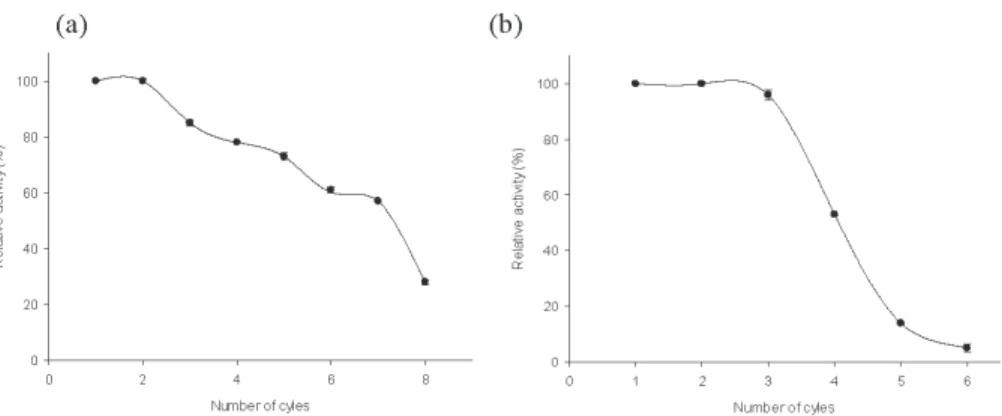
Comparative study of the thermostability of free and immobilized enzyme was performed at 50-80◦C for 1-8 hours (Figure 5). During the operation, the biocatalyst activity gradually decreased during all cycles of malathion hydrolysis. However, such a high activity of the carboxylesterase D1CarE5 may be due to a low initial concentration of malathion in the solution. Bacillus sp.