Soil pH is affected by the mineral composition of the soil's parent material and the weathering reactions that parent material undergoes. Therefore, farmers must be encouraged to regulate soil pH for optimal crop performance.
Soil acidification
High rainfall
Crop growth
Use of fertilizers
Acid rain
Oxidative weathering
Soil alkalinity
Amendment of soil acidity and alkalinity
Author details
Soil Fertility and Plant Nutrition
Effects of Acid Soils on Plant Growth and Successful Revegetation in the Case of Mine Site
Provisional chapter
Effects of Acid Soils on Plant Growth and Successful Revegetation in the Case of Mine Site
- Introduction
- Methods
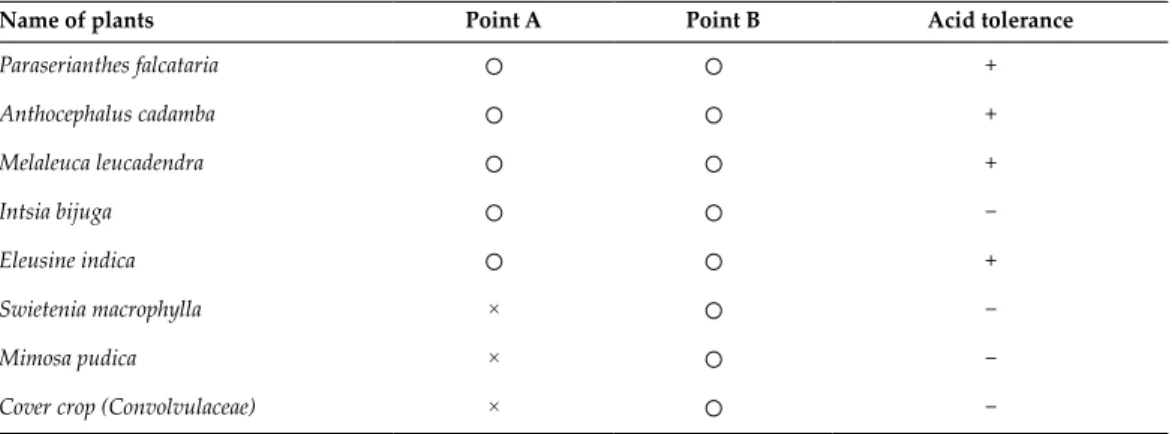
- Vegetation survey
- Soil analysis
- Vegetation test
- Results and discussion
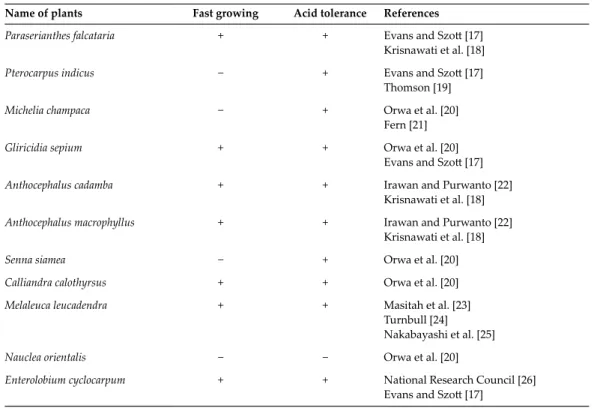
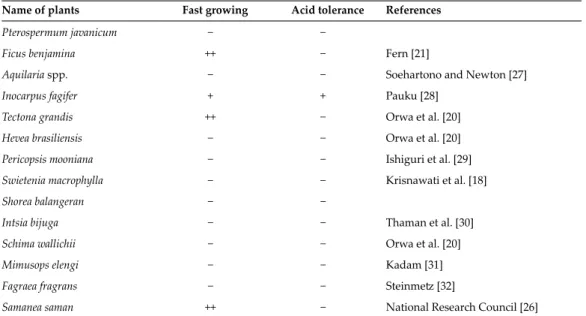
- Effects of acid soils on plant growth in mine site
- Tolerance characteristics of plants to acid soils
- Conclusions
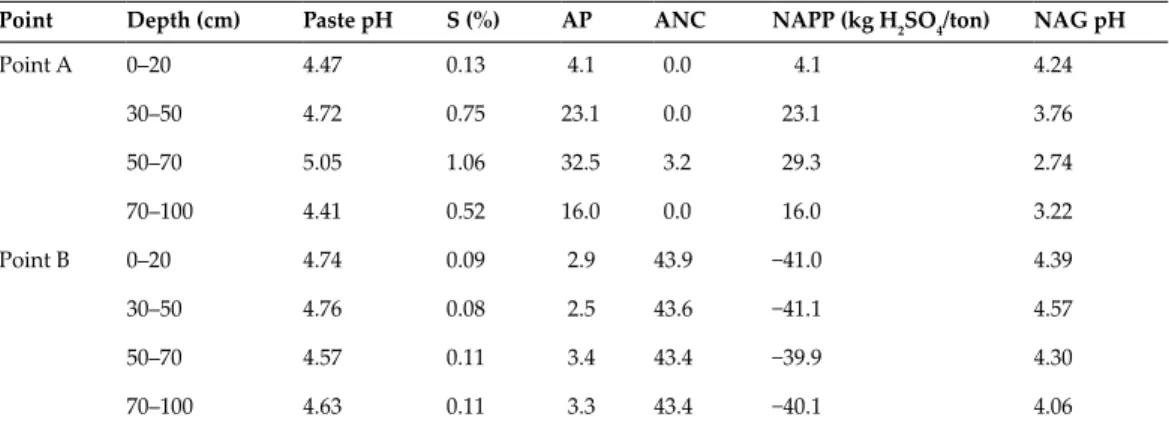
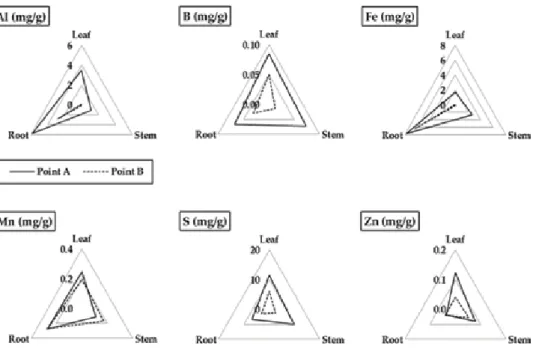
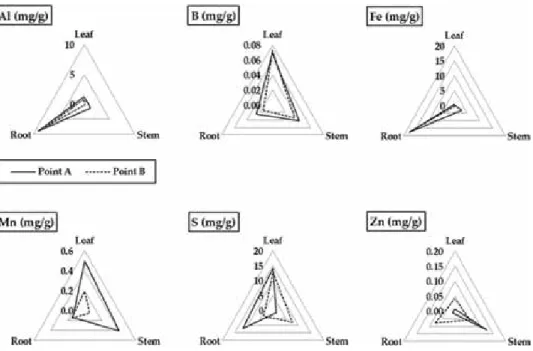
This indicated that the accumulation capacity of the elements in the plant body depends on the species. In contrast, the accumulation of Al in the roots resulted in the inhibition of root elongation and death of A.

Acknowledgements
Al dissolution under acidic conditions in acidic soils, which was attributed to the formation of acid water triggered by sulfide dissolution, affected plant growth in the mine. For successful revegetation, it is necessary to select the appropriate plant in terms of Al tolerance and Al dissolution through the formation of acidic water over time. Not only soil pH, but also assessment of sulfide dissolution over time is critical to successful revegetation, suggesting that net acid potential (NPP) and net acid generation (NAG) pH, used to evaluate acid water generation, are useful for assessing soil conditions for vegetation restoration in addition to soil pH.
The effects of acidic soils on plant growth vary by plant species because the mechanism of plant tolerance to Al is species dependent. Therefore, revegetation must take into account the time of transplanting plants and the acidification of the soil over time. Tropical legumes: Resources for the future: Report of an ad hoc panel of the Advisory Committee on Technological Innovation, Committee on Science and Technology for International Development, Commission on International Relations / National Research Council (US).
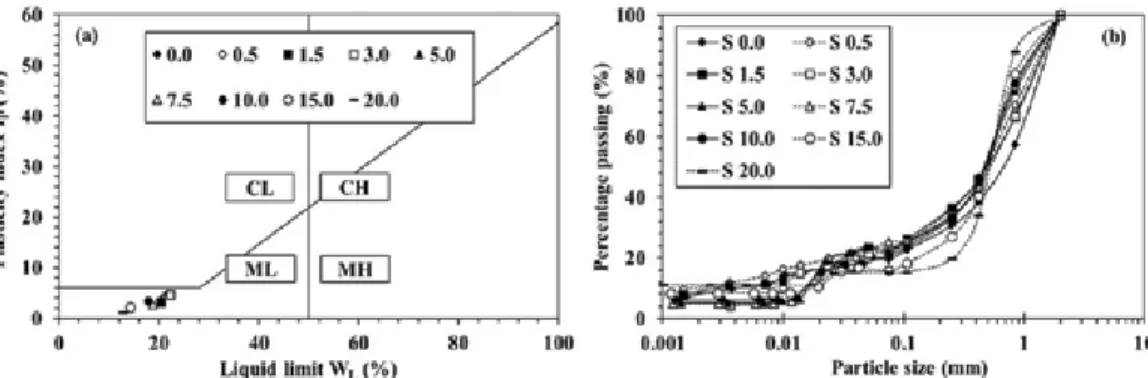
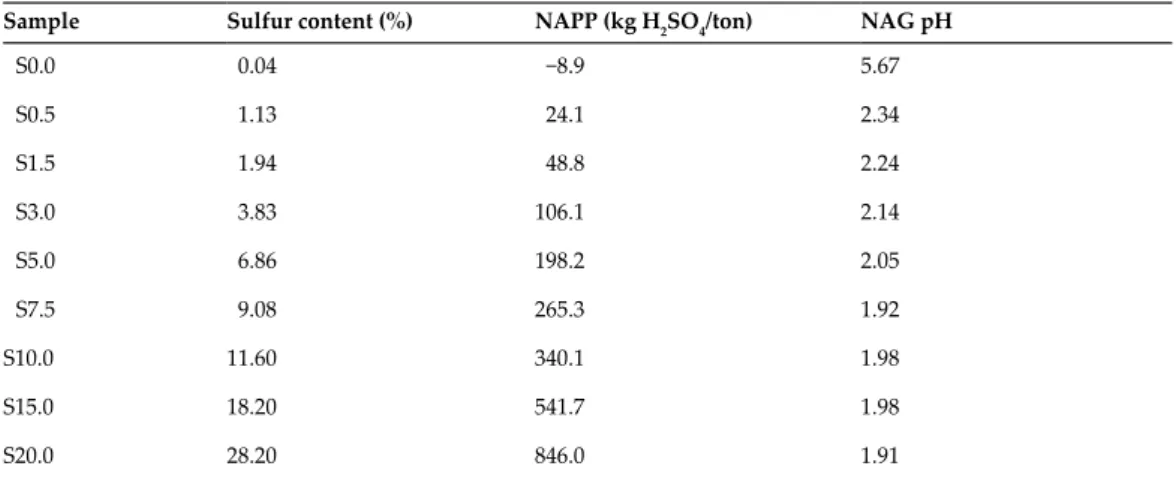
Acid mine drainage (AMD) prevention using sulfur-bearing cap rock in a dry cap system by sulfur form.
Soil Chemistry and Mineralogy
Fluoride Adsorption onto Soil Adsorbents: The Role of pH and Other Solution Parameters
Fluoride Adsorption onto Soil Adsorbents: The Role of pH and Other Solution Parameters
Adsorption surface enhancement for soil adsorbents
Based on estimated fluoride adsorption capacities of the minerals often disclosed in the literature, the minerals that have shown the most promising water defluorination potential in the most recent studies include palygorskite (with an average fluoride adsorption capacity of 57.97 mg/g), pumice (18.27 mg/g), zeolites (15.65 mg/g), hydroxyapatite (13.27 mg/g), iron-enriched laterites (9.39 mg/g), bauxite ( 7.53 mg/g) and montmorillonites (4.82 mg/g). The ability of soil media to sorb large amounts of fluoride is determined by the predominant surface chemistry of the soil systems. The primary fluoride sorption sites of clay colloids in the soil minerals mainly include the protonated or unprotonated silanol groups and the cationic positive centers provided by prevailing soil cations such as Fe3+, Al3+ and Si4+.
However, natural soil systems are generally associated with low ion exchange capacities because soil surfaces are normally saturated with exchangeable counterion groups, which mask and neutralize the internal surface charge in order to maintain the stability of the mineral surface. The ion exchange properties of soil minerals, however, can be improved by pretreatments aimed at displacing masking ions from soil surfaces in order to increase the reactivity of soil surfaces to the target adsorbing ion and unblock pores. in the crystal lattice structure of earth systems [16]. Surface charge reversal for negatively charged soil adsorbents, which aims to increase the electroactivity of their colloidal surfaces towards aqueous fluoride particles, can be achieved by impregnating the structure of the absorbent soil with multivalent metal ions or by grafting and intercalating the soil absorbents . with charged reactive groups.
The latter procedure leads to partial dealumination of the clay structure, which increases the proportion of silica and the density of acid silanol groups on soil adsorbing surface leading to increased overall positive charge of the clay surfaces [19, 20].
Effect of selected solution parameters
- Effects of pH
- Adsorption temperature
- Contact time
- Co-existent ions
As with montmorillonite, the typical pH for effective fluoride removal from water using metal-enhanced palygorskite is typically in the range of 2-8. Alumina minerals typically have a narrower fluorine absorption edge in the pH range of 5.5–6.5 as the maximum fluorine absorption in Ca-based minerals occurs within pH values of 5–6 . Likewise, optimal fluoride removal using carbon adsorbents can be achieved at room temperature in the pH range of 4-12.
The usual pH value for effective removal of fluoride from water using metal reinforced palygorskite is in the range of 32-2. However, fluoride adsorption on metal-exchanged zeolites and on synthetic HAps is independent of pH, and high fluoride adsorption occurs in the pH range 4–10. Alumina minerals, on the other hand, usually have a narrow sorption edge in the pH range 5.5-6.5.
In the same way, maximum fluoride adsorption on most of the calcareous minerals occurs within pH values of 5-6.
Agro-ecology
Removal of fluoride from water by metal ions (Al3+, La3+ and ZrO2+) charged by natural zeolite. A calcite permeable reactive barrier for fluoride remediation from spent potash (SPL) contaminated groundwater.
Control of Soil pH, Its Ecological and Agronomic Assessment in an Agroecosystem
Control of Soil pH, Its Ecological and Agronomic Assessment in an Agroecosystem
Description of the experiment
- Site
- Soil
- Climate
- Experimental design
- Object of investigation
- Trial history
According to its profile differentiation and all acidity parameters, the soil under study has preference in terms of the need for liming. This leads to the disturbance of the essential ecological functions of the soil, including chemical buffer capacity, nutrient cycles, biodiversity, organic matter decomposition, as well as aesthetic functions. Soil acidification management includes both neutralization of soil acidity and regulation of the acidification of limed soil.
A key aspect in managing acidic soils for plant growth is the pH change of the soil. The soil bioavailability of many nutrients and toxic elements and the physiology of the roots and rhizosphere microorganisms are affected by soil pH [9]. Soil pH affects the changes in the species composition, structure, and plant and environment interaction of the agrophytocenoses.
The naturally acidic soil and the same soil exposed to different lime intensity were the subject of the research.
Management of soil acidity
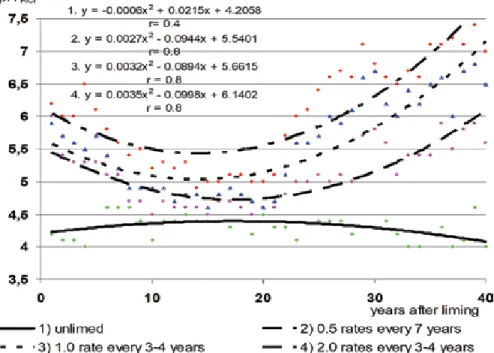
The application of the long-term liming system (primary, repeated and periodic liming) in the period 1949-2005 formed different soil pH levels (Table 1). Calcareous materials change the mobility of some biogenic elements and their accumulation in the soil. Long-term intensive liming causes significant morphological changes in the soil profile and increases the surface area of podzolic veins in the profile wall.
Neutralization of soil acidity in the upper layer and subsoil up to a depth of 60 cm was achieved by periodic liming at a rate of 1.0 every 7 years in combination with application. The effects of long-term liming combined with the introduction of cow manure on soil pH and mobile aluminum were investigated in the entire soil profile up to a depth of 100 cm. In the upper and lower layers of the soil up to a depth of 60 cm, soil acidity was neutralized by systematic liming at a dose of 1.0 every 7 years in combination with the application of 60 t ha-1 of manure every 3–4 years.
Long-term intermittent liming in combination with fertilization improved the chemical properties of the acidic soil profile in the ElB and ElBt horizons.
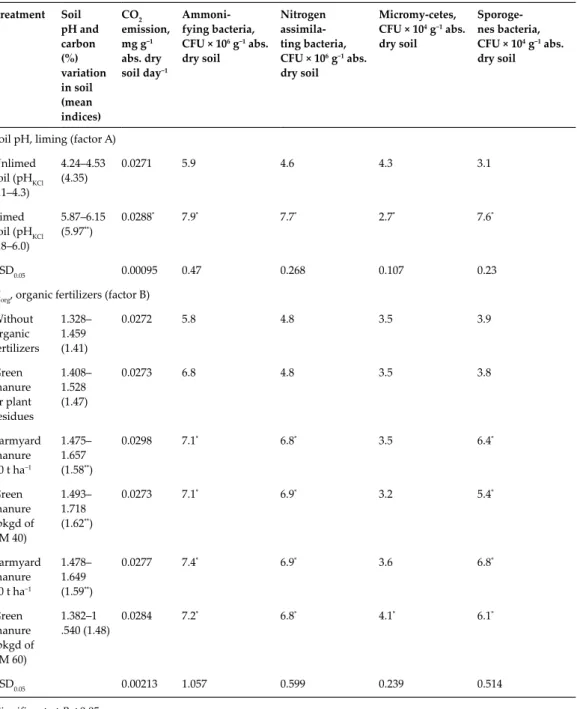
Soil pH and microbial prevalence
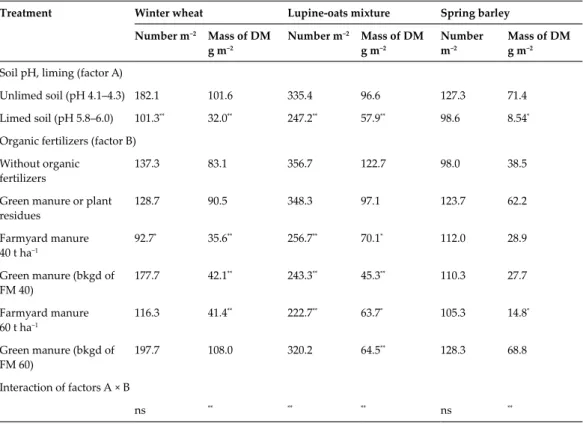
Most soil systems tend to fall in the soil pH range falls below 5.5 in the agricultural ecosystem. Soil liming and feeding with various organic fertilizers: action of plant residues (treatments 2, 4, 6) and 40 t ha−1 (treatments 3, 4), or 60 t ha−1 (treatments 5, 6) of farmyard manure have different conditions created for the functioning of soil microorganisms and carbon sequestration in the soil. The experimental findings indicate that a significant increase in the pH value was achieved due to
However, the prevalence of fungal populations was only significantly higher with the integrated incorporation of manure and plant residues into the soil. Symbiotic nitrogen fixation depends on the presence and survival of rhizobia in the soil and also on their efficiency. Such differences are primarily determined by the presence of the specific rhizobia and the activity of their enzymes in the soil.
The gas chromatography assay, used to determine the activity of the nitrogenase enzyme responsible for biological nitrogen fixation in the red clover and Rh.
Soil pH effect on organic carbon and water stable aggregate content
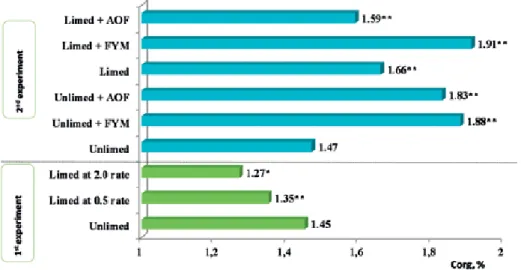
A positive statistically significant effect of fertilization on soil organic carbon content was established. The highest amount of soil organic carbon (1.91%) was obtained in the limed soil applied with FYM. Effect of soil liming and fertilization on the amount of organic carbon in the topsoil average data.
A 28-34% increase in the content of water-resistant aggregates was recorded in calcareous soil compared to non-calcareous soil. The content of water-resistant aggregates in both naturally acidic and calcareous soils was 30.3%. Effects of long-term liming, fertilization and their combination on the change of water-resistant aggregates in the soil profile.
The highest content of water-stable additives (59.4%) was found in the limed and fertilized soil.
Soil pH effect on crop productivity and weediness
Liming and its combination with the application of organic manure had a positive effect on the formation of water-stable aggregates even in the deepest soil layer of 30-60 cm. The highest weed number (356.7 m−2) and weed mass (122.7 g m−2) were established in the treatment where the mixture was grown without organic fertilizers. However, the use of green manure in the background of different levels of farmyard manure increased the number of weeds in the cereal crops of the rotation system.
The effect of organic manure was weaker in the winter wheat crop, where green manure was incorporated against the background of different amounts of farmyard manure. However, a reduction in the number of weeds in the farm's manure soil was observed. In all experimental years, the total number of weeds was also significantly dependent on the mobile aluminum content in the soil: r = 0.92** in the winter wheat crop, r = 0.86** in the lupine-oat mixture and r = 0.84** in the spring barley crop.
In the total weed seed bank of calcareous soils (pH 6.4–6.8), the nitrophilous weed Chenopodium album L represented 72.8%.
Conclusions
In all years of the experiment, short-lived weeds dominated the crops and accounted for 96.4% of the total number of weeds. Statistically significant correlations were determined between soil agrochemical indicators and total number and mass of weeds in rotational crops. However, fertilization with different percentages of farmyard manure or incorporation of green manure against the background of different rates of farmyard manure resulted in reduction of the weed seed bank in the soil.
The effect of soil acidity and organic fertilizers on weed infestation in crop rotation during maturity. Changes in nutrients due to long-term liming and fertilization were also observed in the deeper horizons (ElB and ElBt) of the soil profile. Soil pH determines the activity of biological processes taking place in the soil (CO2 emission, atmospheric nitrogen fixation intensity, mineralization rate of organic matter, distribution of beneficial microorganisms).
Only the application of organic manure did not have any effect on the formation of water-stable aggregates in the topsoil.