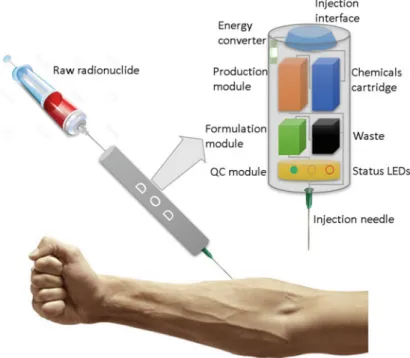
For the production of [18F]fluoride (~1 mCi/min at 5μA), the process is complete once the product is delivered into the specified vial. The previous example of the production of [18F]FLT is the latest in a growing list of radiotracers prepared using the NanoTek system.
Conclusions
A solid phase route to 18F-labeled tracers exemplified by the synthesis of [18F]2-Fluoro-2-deoxy-D-glucose. No carrier added nucleophilic aromatic radiofluorination using solid phase supported arenediazonium sulfonates and 1-(aryldiazenyl)piperazines.
Introduction
Radiosynthesis of Positron Emission Tomography Tracers
Microfluidics for Radiosynthesis
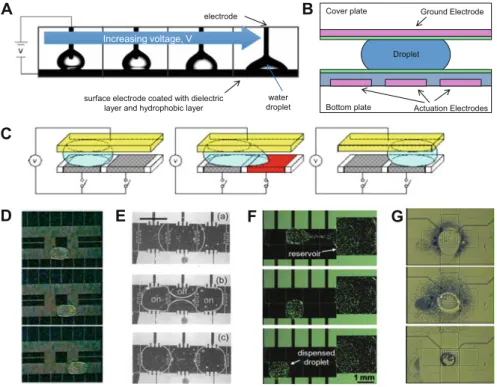
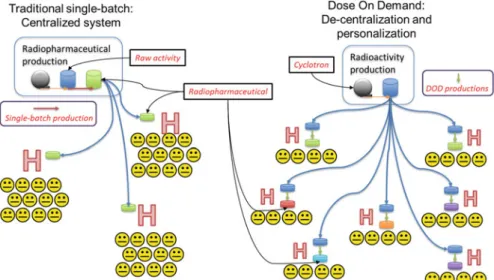
Most commonly in radiochemistry, these devices are based on "flow-through" microreactors, where reactions occur by flowing reagent streams through mixers and preheated capillary tubes or microchannels. Another class of microfluidic device based on small (<50μL) volumes known as “batch” microreactors has also been used for radiochemistry (Fig. 7.1).
Platforms for Microliter Volume Synthesis
These devices are particularly attractive for radiochemistry because all synthetic steps including solvent exchange can be integrated on a single chip and because small volumes can reduce reagent consumption, increase reaction kinetics, improve specific activity, reduce radiolysis and simplify cleaning, as described below. . Because the droplets are surrounded by gas, evaporation and solvent exchange can be easily performed on the chip.
Advantages of Radiosynthesis at the Microliter Scale .1 Miniaturization and Disposability
Reduced Radiolysis
In contrast, in an EWOD microfluidic chip, one dimension of the solvent volume is very thin compared to the positron range. Thus, most positrons will escape the solvent after depositing only a small portion of their energy in the solvent, reducing radiolysis.
Reagent Minimization
The reduced amount of reagents can also simplify the quality control (QC) testing needed before injection into humans. In general, the reduced amount of reagents and solvents will lead to reduced amounts of impurities remaining after the cleaning process.
High Specific Activity
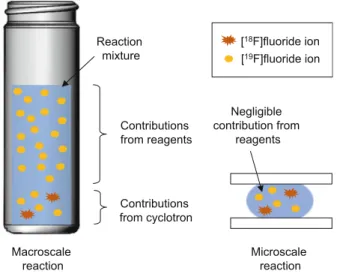
It is therefore desirable to minimize the amount of excess precursor to maintain high SA. On the macroscale, reagents are the dominant source of fluorine-19 contamination in the fluorination reaction mixture. At the microscale, the reagents add negligible fluorine-19 contamination to that already present by the cyclotron.
Practical Considerations .1 Limits of Volume Reduction
Radioisotope Concentration
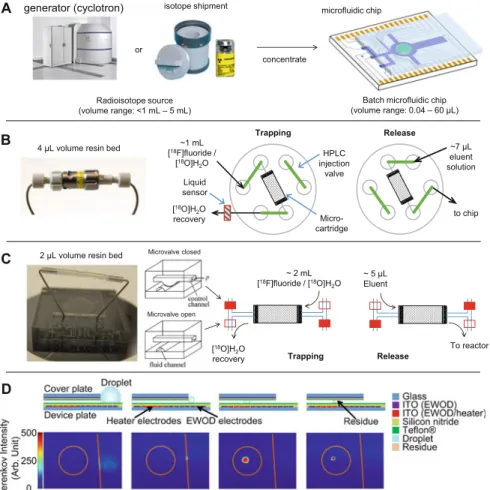
7.5 (a) Since the volume of batch microfluidic chips is much smaller than the volume of the radioisotope source, concentration is required to ensure that a sufficient amount of radioactivity can be loaded onto the chip. By activating the nearby heater, the droplet volume is reduced to ~5 μL and then pulled into the chip by activating the electrode. A special chip was made where the bottom plate extended over the edge of the cover plate to create a platform.
Synthesis Automation
A large droplet of [18F]fluoride solution (with a phase transfer catalyst) was deposited on this platform next to the gap between the two plates of the EWOD chip and then rapidly evaporated to a small amount that could be transferred between the plates (Figure 7.5). d). As some groups have reported that the anion exchange resin can contribute to fluorine-19 contamination [55], this approach may reduce the amount of fluorine-19 in the reaction mixture and thus enable the production of PET tracers with higher specific activity. Either of these approaches can concentrate a significant fraction of the radioactivity from cyclotron bombardment into a volume that can be loaded onto a microfluidic chip, allowing radiochemistry to be performed in microliter volumes with high radioactivity.
Conclusions and Outlook
Towards reliable synthesis of radiotracers for positron emission tomography on PDMS microfluidic chips: study and optimization of the [18F] fluorine drying process. In: Proceedings of the 16th international conference on miniaturized systems for chemistry and life sciences, Okinawa. Proceedings of the 16th International Conference on Solid State Sensors, Actuators and Microsystems (TRANSDUCERS), Beijing.
Introduction
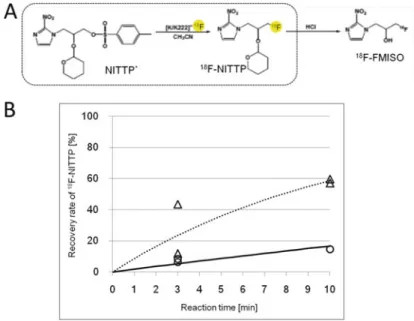
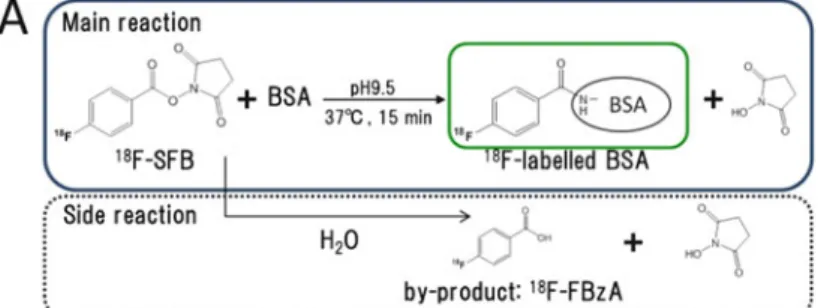
Two 18F labeling reactions (18F labeling of bovine serum albumin (BSA) by N-succinimidyl-4-[18F] fluorobenzoate (SFB) and 1-(20-nitro-10-imidazolyl)-2-O-tetrahydropyranyl-3 -O -toluenesulfonylpropanediol (NITTP) from 18F) were performed using the microreactor. Conversely, the yield of 18F labeling of NITTP obtained using the microreactor was about half that using the batch method. And both a flow microreactor and a batch microreactor have been applied to the radiosynthesis of PET probes for 18F labeling [11].
Materials and Methods
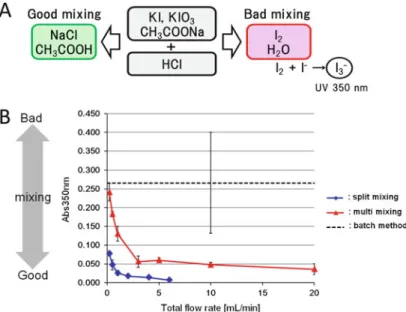
Villermaux-Dushman Method
Trifluoroacetic acid (TFA) was added to the mixture to terminate the reaction at times of 2, 10 and 20 min. The reaction mixture (20 μL) was loaded onto a C8 reversed phase column (CAPCELL PAK C8 SG300, Shiseido, Tokyo, Japan). The solution mixed in the microreactor was then sent to a PTFE tube (φ0.5510 mm, GL science) connected to an outlet of the microreactor, and the reaction in the PTFE tube was allowed to proceed for 3 or 10 minutes at 80C.
Solvent Resistance Test
By batch method, [K/K222]+18 Fand NITTP solutions were mixed in equal amounts in a 1.5 mL microtube.
Results and Discussion
Microreactor with Novel Mixing System
On the multimixing microreactor, the first liquid (solution A) flows from each nozzle in a layer on top of the chip. The flow of liquids in the microchannel for mixing is shown in the cross-section of the microchannel. Then, both liquids form a multilayer flow at a contraction flow part on the bottom of the chip to produce the thin flow of liquids (Fig.8.1b).
Evaluation of Mixing Performance of Prototype Microreactors
The 18F labeling reaction of BSA by 18F-SFB was investigated to evaluate the 18F labeling performance of the split mixing microreactor. As shown in Fig.8.3b, the 18F labeling yield of BSA obtained using the split mixing microreactor was almost the same as that using the conventional batch method (Fig.8.3b). To further evaluate the 18F labeling performance of the split mixing microreactor, the 18F labeling reaction of NITTP was investigated (Fig. 8.4a).
Screening of Material for the Split Mixing Microreactor
To improve the 18F labeling yield of NITTP obtained by naked18F, the material used for the microreactor should be re-examined.
Conclusion
Here we will introduce our research, including the synthesis of [123I]IIMU and its efficacy and safety evaluation, towards its clinical FIH study. Central Institute of Isotope Science, Hokkaido University, Sapporo, Japan Graduate School of Medicine, Hokkaido University, Sapporo, Japan S. Central Institute of Isotope Science, Hokkaido University Department of Integrated Molecular Imaging, Graduate School of Medicine, Hokkaido University, Sapporo, Japan .
Introduction
In vitro and in vivo studies: The in vitro and in vivo uptake of [125I]IIMU by the A431 tumor was attributable to the binding of the radiotracer to its target enzyme, i.e. TP.
Radiosynthesis of [ 123 I]IIMU [2]
The observed adverse effect level (NOAEL) for intravenous administration of non-radiolabeled IIMU to mice was greater than 1.8 mg/kg. After removal of the solvent, the crude product was converted to [123I]IIMU-HCl and simultaneously purified by reverse-phase HPLC using a solvent system containing HCl. The results of the quality control tests prove that the [123I]IIMU preparation is suitable for clinical studies.
In Vitro Study : Uptake of [ 125 I]IIMU in Cultured A431 and AZ521 Cells [4]
When the radiotracer was incubated with A431 cells, the uptake level of the radiotracer increased with an increase in incubation time by 5.3% dose/mg protein after 2 hours of incubation. In the case of AZ521 cells, the uptake of radioactivity was extremely low, at most 0.68% dose/mg protein, regardless of incubation time. To confirm whether the observed uptake is due to the binding of the tracer to TP, similar experiments with A431 tumors were performed in the presence of different concentrations of unlabeled IIMU.
In Vivo Study : Biodistribution of [ 125 I]IIMU in A431 and AZ521 Tumor-Bearing Nude Mice [4]
SPECT/CT Imaging Study [2]
Safety Assessment
Conclusions
Summary The use of biomarkers that reflect the progression of atherosclerosis is important for the prevention of serious cardiovascular events. Clinical plasma samples were collected from patients with acute myocardial infarction (AMI), stable angina pectoris (SA), and healthy low-risk individuals (H-LR). Differential proteomics of clinical plasma samples revealed that more than 10 proteins appeared to be up-regulated (relative abundance of AMI/H-LR or SA/H-LR>1.5) and 5 proteins were down-regulated (AMI/H-LR or SA/H-LR< 1/1.5).
Introduction
Disease proteomics is a profiling technology using mass spectrometry that allows to comprehensively identify disease-related change of protein expression in a wide variety of biological samples, including serum or plasma or extracts from tissues of interest [3]. The ratio of protein amount between these conditions is estimated by comparing MS signal intensity of the corresponding proteolytic peptide fragments [4]. To elucidate clinically relevant molecular determinants in atherosclerotic development, we took a comprehensive approach using mass spectrometry-based differential proteomics.
Materials and Methods
A decade of progress to determine changes in disease-related protein expression has achieved accurate and high-throughput quantification of the individual proteins within mixtures using differential stable isotopic labeling, such as cleavable isotope-encoded affinity tags (cICATs) [4] . Light or heavy cICATs are labeled at the free thiol groups of cysteine residues from which proteins were isolated from two different samples (i.e., disease and healthy conditions). First, we performed differential human plasma proteomic studies using clinical samples from patients with atherosclerosis-related cardiovascular disease and healthy (or low-risk) volunteers.
Results and Discussion
- Disease Stage-Dependent Differential Proteome in Human Plasma
- Disease Stage-Dependent Differential Proteome in Atherosclerosis Mouse Model
- Comparison Between Human and Mouse Plasma Proteome
- Limitation
As shown in Figure 10.1, we performed disease stage-dependent differential proteomic experiments on human plasma using clinical samples. 10.1, we performed differential proteome experiments using plasma and arterial tissues from WT and apolipoprotein E-deficient (apoED) mice. Importantly, we found that the changes associated with disease progression in the amount of proteins identified did not always coincide.
Conclusion
In the case of CFD, the plasma level of CFD in humans determined by proteomic study or ELISA decreased in accordance with the severity of clinical conditions. In our previous proteomic studies of atherosclerosis mice, the protein level of thrombospondin-4 (TSP4) in aorta, but not in plasma, increased relatively with the formation of atherosclerotic plaques. IgG is also known to accumulate nonspecifically in immunological diseases such as inflammatory arthritis.
Introduction
In this chapter, we would like to introduce recent topics on atherosclerotic imaging, focused on our work to explore the accumulation mechanisms of IgG in atherosclerotic lesions and elucidate the utility of radiolabeled IgG images for the diagnosis of atherosclerosis.
Materials and Methods .1 Materials
Preparation of Radiolabeled IgG
The mixture was purified by size exclusion filtration using a diafiltration membrane (Amicon Ultra 4 [molecular weight cutoff, 30,000]; Millipore Co., Billerica, MA). It was added to the purified solution of HYNIC-TSP4 mAb or NC mAb solution in 10 mM citrate buffer (pH 5.2) (30 μl, 1 mg/ml) and the mixture was incubated at room temperature for 1 h. The radiochemical purity of 99mTc-TSP4-mAb or 99mTc-NC-mAb was measured by size exclusion filtration of the PD-10 column and size exclusion high performance liquid chromatography (HPLC).
Animal Study
The stability of 99mTc-TSP4-mAb or 99mTc-NC-mAb in plasma was assessed by the method described below.
Histochemical Study
Results
Probe Preparation
In Vivo Study
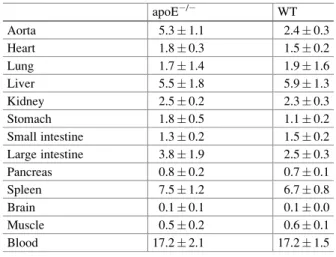
On the other hand, the 99mTc-NC-mAb accumulation levels in the aorta of apoE/ mice were also significantly higher than those of WT mice (5.11.4 vs. 2.80.5% ID/g,p<0.05) , whereas the radioactivity in other organs did not differ between apoE/mice and wild-type mice. The radioactive distribution in the aortic roots of apoE/mice injected with 99mTc-TSP4-mAb measured by ARG was within the atherosclerotic plaque lesions and coincided with the Mac-2 positive areas (Fig. 11.3). These results suggest that 99mTc-TSP4-mAb accumulated in the macrophage-infiltrated area of plaque lesions.
Discussion
These findings suggest that IgG itself is accumulated in M1-polarized macrophages via Fcγ receptors in the atherosclerotic plaques. In nuclear imaging for the diagnosis of atherosclerosis, 18-fluoro-deoxyglucose (18F-FDG) is widely used [22]. The third-party images or other materials in this chapter are included in the Creative Commons license of the work, unless otherwise noted in the credit line; if such material is not included in the Creative Commons license of the work and the act in question is not permitted by law, users will need to obtain permission from the licensee to duplicate, modify or reproduce the material.
![Fig. 6.2 TAD residue in reversible click-chemistry transformations (Taken from [45])](https://thumb-ap.123doks.com/thumbv2/1libvncom/10207172.0/11.659.122.544.419.869/fig-tad-residue-reversible-click-chemistry-transformations-taken.webp)