BIOCHEMICAL AND MICROBIAL CHANGES DURING
PALM KERNEL MEAL (PKM) FERMENTATION
IKA AYUNINGTYAS
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
DECLARATION
I do hereby declare that the thesis entitled “Biochemical and Microbial Changes during Palm Kernel Meal (PKM) Fermentation” is my original work produced through the guidance of my supervisors and that to the best of my knowledge it has not been presented for the award of any degree in any university. All of the incorporated material originated from other published as well as unpublished papers are stated clearly in the text as well as in the reference.
Bogor, January 2012
Ika Ayuningtyas
ABSTRACT
IKA AYUNINGTYAS. Biochemical and Microbial Changes during Palm Kernel Meal (PKM) Fermentation. Under direction of ANJA MERYANDINI and PASCALE TALAMOND.
Fermentation was the first step of bioconversion process of palm kernel meal (PKM). The fermentation was done in close canister to minimize the environmental effect. The study was conducted to investigate total microorganism and biochemical changes during PKM fermentation in a closed canister. Fourty two kg of PKM were mixed with 84 L of water and put in the big drum (height 81 cm; diameter 48 cm). The fermentation height in drum is 80 cm. The sample was taken in the depth of ±40 cm from the surface. At interval 12 hours during 7 days take a sample for microbial analysis, pH, total acid, temperature, fibre, protein content, D-mannose, and volatile organic acid. The highest number of aerobic bacteria, 17.2 log10 CFU/g, was found at 24 hours of fermentation PKM. The highest total anaerobic bacteria, 17.3 log10 CFU/g, were found at 72 hours of fermentation PKM. No fungi was found before 96 hours of fermentation and the highest number found, 6.4 log10 CFU/g, was at the end of fermentation PKM. The pH of fermentation decreased after 48 hours from 4.56 to 4.28. The decreased of pH followed by increased total acid concentration from 0.19 to 0.22 mMol/mL after 48 hours of fermentation PKM. After 168 hours of fermentation PKM, the protein content increased 4.79%; while the concentration of hemicellulose, cellulose, and lignin decreased 10.79%, 10.77%, and 51.34%, respectively. The highest mannose concentration, 3.297±0.13 g/L, was found after 36 hours of fermentation PKM. The dominant volatile organic acid is acetic acid with a concentration of 87.89 mMol after 72 hours of fermentation PKM.
SUMMARY
IKA AYUNINGTYAS. Biochemical and Microbial Changes during Palm Kernel Meal (PKM) Fermentation. Under direction of ANJA MERYANDINI and PASCALE TALAMOND.
One of the problems that occur in aquaculture is the shortage of fish meal as a source of protein in aquafeed. The solution to feed shortage is to find alternatives protein source that could substitute fish meal. Palm Kernel Meal (PKM) still contains 13-22% of crude protein but also high content of fiber thus its usage is limited to monogastric animals, such as fish. Bioconversion is a method to added value of PKM. Through bioconversion process, nutrient from PKM converted into biomass of Hermetia illucens larvae (maggot). These larvae contained 42% crude protein and 30% crude fat. The first step of bioconversion process is fermentation with mixing PKM and water with 1:2 comparisons. Propose of this process was to degraded polymer complex of PKM and produce some volatile compound that stimulant incest to oviposition. All this time the fermentation process was done spontaneous and naturally. The naturally fermentation influences by environmental factors, thus the result varies. For sustainability of H. illucens larvae mass production, this process should be controlled. Therefore, the degradation process should be conducted in a closed canister to minimize outside environmental effects. Next, a starter containing PKM degrading microorganisms should be made. In order to make starter, the information concerning the biochemical and microbial changes during the fermentation should first be gathered.
Fourty two kg of PKM mixed with 84 L of water and put in the big drum (height 81 cm; diameter 48 cm). The fermentation height in drum is 80 cm. The sample was taken in the depth of ±40 cm from the surface. At interval 12 hours during 7 days take a sample for microbial analysis, pH, total acid, temperature, fibre, protein content, D-mannose, and volatile organic acid. Microbial analysis was done by investigated total microorganism changed in some media. Total aerobic bacteria counts were determined on Trypticase Soy Agar (TSA) incubated at 30 0C for 48 hours in aerobic condition. Total anaerobic bacteria counts were determined on TSA incubated at 30 0C for 48 hours in anaerobic condition. Enumeration of lactic acid bacteria was carried out using De Man, Rogosa and Sharpe (MRS) medium incubated at 30 0C for 4 days. Enumeration of clostridial bacteria was carried out using Reinforced Clostridial Agar (RCA) incubated at 30 0
C for 48 hours in anaerobic condition. Yeast and moulds were enumerated on plates of Sabouraud Glucose Agar (SGA) at 30 0
aerobic bacteria, 17.2 log10 CFU/g, was found at 24 hours of fermentation PKM. Total anaerobic bacteria counts corresponded approximately to the sum of total bacteria from TSA media an-aerobically incubated and from RCA media. The highest total anaerobic bacteria, 17.3 log10 CFU/g, were found at 72 hours of fermentation PKM. No fungi was found before 96 hours of fermentation and the highest number found, 6.4 log10 CFU/g, was at the end of fermentation PKM. The pH of fermentation decreased after 48 hours from 4.56 to 4.28. The decreased of pH followed by increased total acid concentration from 0.19 to 0.22 mMol/mL after 48 hours of fermentation PKM. After 168 hours of fermentation PKM, the protein content increased 4.79%; while the concentration of hemicellulose, cellulose, and lignin decreased 10.79%, 10.77%, and 51.34%, respectively. The highest mannose concentration, 3.297±0.13 g/L, was found after 36 hours of fermentation PKM. The dominant volatile organic acid is acetic acid with a concentration of 87.89 mMol after 72 hours of fermentation PKM.
Copyright © 2012, by Bogor Agricultural University All right reserved
1. No part or all of this thesis may be excerpted without inclusion or mentioning the sources
a. Excerption only for research and education use, writing for scientific papers, reporting, critical writing or reviewing of a problem
b. Excerption does not inflict a financial loss in the proper interest of Bogor Agricultural University
BIOCHEMICAL AND MICROBIAL CHANGES DURING
PALM KERNEL MEAL (PKM) FERMENTATION
IKA AYUNINGTYAS
Thesis
Submitted in partial fulfilment of Master of Science degree in Study Program Biotechnology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Title of Theses : Biochemical and Microbial Changes during Palm Kernel Meal (PKM) Fermentation
Name of Student : Ika Ayuningtyas Registration Number : P051090121
Approved
Dr. Anja Meryandini, MS
1st Advisor
Dr. Pascale Talamond
2nd Advisor
Acknowledged
Head of Major Program Biotechnology
Dean Graduate School
Prof. Dr. Suharsono, DEA Dr. Ir. Dahrul Syah, MSc.Agr
ACKNOWLEDGMENTS
Alhamdulillahirobbil’alamin. Praise ALLAH, the creator of this magnificent universe filled with rainbows which would only increase our awe when un-weaved by science. Shalawat and salam be to the prophet Muhammad S.A.W. who had brought forth the light of Islam.
The author is indebted to Dr. Anja Meryandini, MS and Dr. Pascale Talamond as the author’s advisors for this research project and also for their knowledge. I would like to thank to Prof. Dr. Suharsono, DEA as a head of the major Biotechnology study program. The author would also like to thank to Dr. Aris Tjahjoleksono as this research project’s assessors, for his comments, inputs, and discussions. I would like to thank to Drs. I Wayan Subamia, M.Si as a head of Balai Riset Budidaya Ikan Hias (BRBIH) Depok, who give permission to do my research in this place. I would like to thank to IRD for funding my study and this research. The author would like to thank to all researcher in IRD especially Dr. Saurin Hem for all discussion and gave attention to my study.
The author would like to thank to all my friends in BRBIH, Rina Hirnawati, Nina Meilisza, Irmayanti, Melta Rini Fahmi, Ahmad Musa, Lili Sholichah for all help in laboratory and discussions. To technicians staff Pak Urip, Kang Usman, Kadek, Pak Kuncoro thanks for all your help in my research. For Rachmawati, Arum Krysan Purbawani, and Nugroho Ponco Sumanto, thanks for all discussion and reviewing the early version of this manuscript.To all the staff in PAU and to my entire friend BTK 09, thank you for the countless favours and friendship.
Last but not least, the author’s deepest gratitude and love goes to the author’s mom, Ibu Nining Rohimah, and dad, Bapak Chodim Rusmiaji, without them, and their genes, the author would not be here. To my brother and sister, thanks for making the author’s life at home worthwhile.
The author realized that this thesis would be far from perfect but still hoped that it could be useful to all that read it.
Bogor, January 2012
AUTOBIOGRAPHY
The author was born in Jakarta, on October 31st
After finished her senior high school, in 2001, the author attended in University of Indonesia, majoring Biology and received the Bachelor Degree of Science (S.Si) in 2006. In 2009, she attended in Bogor Agricultural University, in major Biotechnology study program.
1983. Her father named Chodim Rusmiaji, and her mother named Nining Rohimah. She was the oldest child from three children.
LIST OF CONTENT
V. CONCLUSION AND RECOMMENDATION ... 29
5.1 Conclusion ... 29
5.2 Recommendation ... 29
REFERENCES ... 30
LIST OF TABLE
Page 1. Proximate analysis (%) and amino acid content (g/16 g N) of PKM .... 5 2. Sugar Composition of PKM ... 6 3. Total bacteria and fungi (log10 CFU/g) during PKM fermentation 20
...
4. The number of bacteria on each sampling and their respective
characterization ... 21 5. pH, total acid (mMol/mL), and temperature (0C) changed during
PKM fermentation ... 22 6. Hemicellulose, cellulose, lignin (% in dry basis) and crude protein (%
LIST OF FIGURE
Page
1. Production of palm oil and it’s by product ... 4
2. Major type of linkages found in lignin ... 8
3. Schematic representation of cellulose enzymes over cellulose structure ... 11
4. Schematic hydrolyzed of mannan by some enzyme ... 11
5. Aromatic degradation pathway of -Aryl ether lignin compound ... 12
6. Aromatic degradation pathway of biphenyl lignin compound ... 13
7. Aromatic degradation pathway of diarypropane lignin compound ... 14
8. Aromatic degradation pathway of phenylcomarane lignin compound . 14 9. Aromatic degradation pathway of pinoresinol lignin compound ... 15
LIST OF APPENDIX
I. INTRODUCTION
1.1 Background
One of the problems that occur in aquaculture is the shortage of fish meal as a source of protein in aquafeed. The production rate of aquafeed today could not keep up with the increased demand of fish meal in aquaculture and competition for its use in livestock and poultry feeds, thus the price becomes
expensive. Data from Indexmundi
fishmeal price since the last 10 years from US$ 545/metric ton on august 2001 up to the level of US$ 1455/metric ton in august 2011. This situation lead to the increase of production costs in aquaculture. The solution to this increased price and feed shortage is to find alternatives protein source that could substitute fish meal.
Some by products from agriculture industry are known to have relatively high protein content, like palm kernel meal (PKM); which is by product from palm oil industry (Sundu & Dingle 2003). Since 2006, Indonesia has become the largest producer of palm oil, surpassing Malaysia. In 2006, it reached 44% (15.9 million tons) of world palm oil production (USDA 2007). In Malaysia palm oil industry, the extraction of palm kernel produces 53.3% of PKM (Ng 2003). Therefore supply of PKM in Indonesia overabundance.
PKM contain 13-22 % of crude protein, but as with most plant-based and oil seed meal ingredients, several factors can limit the incorporation of PKM into fish diets (Sundu & Dingle 2003). PKM contain high fiber content and non-starch polysaccharides (NSPs), thus its usage is limited to monogastric animals, such as fish (Ng 2003). Zahari and Alimon (2005) recommended used of PKM in mixture diet for freshwater fish only 10-20%. Yann (2009 unpublish data) concluded that use PKM in nutritive ingredients of feed for Pangasius djambal (catfish) should be less than 18%.
content of PKM was increased from 16.86% to 31.27%. The fiber content was decreased from 15.12% to 14.51%. Later, Ng et al (2002) used the fermented PKM as a dietary ingredient for red hybrid tilapia (Oreochomis sp.). Their results showed the hybrid tilapia that was fed fermented PKM-based diets at all dietary inclusions tested showed the poorest growth, and this could indicate the presence of anti-nutrients in the resultant fungal biomass.
Hem et al. (2008) tried another method using bioconversion process to added value of PKM. Bioconversions are a process of converting or changing an organic material into other useful products and has added value by taking advantage of events and the biosynthesis or the formation biolysis or solutions (Sukaryana et al. 2010). Through bioconversion process, nutrient from PKM converted into biomass of Hermetia illucens larvae (maggot). These larvae contained 42% crude protein and 30% crude fat (Hem et al. 2007). Septivia (2008) demonstrated that Botia fry (Chromobotia macracanthus Bleeker) showed the best growth by feeding 5 days old larvae, compared to bloodworm and earthworm. From the report of the results of trial production of larvae in Sungai Gelam (Jambi), it is known that the price-based pellets H. illucens larvae sold for Rp.3500 per kilogram (pricing Rp.200/kg PKM), is cheaper than the price of commercial pellets, namely Rp.7000 per kilogram (Hem 2009 unpublished data
The first step of bioconversion process mixes PKM and water (1:2) (Hem
et al. 2008). This process proposes to degrade polymer complex of PKM. PKM is very rich in polymer complex hemicellulose, cellulose and lignin (Düsterhöft et al. 1992). By including NSPs, the substrate consists of about 50% fiber (Knudsen 1997; Atasi & Akinhanmi 2009). The process of degradation polymers complex carried out by some type of microorganisms. Hemicellulose can be degraded by aerobic microorganisms such as genera Aspergillus and Trichoderma. Aerobic bacteria, such as Bacilli and Cellvibrio also have polysaccharides-backbone-degrading enzyme to breakdown hemicellulose. Clostridia, anaerobic bacteria, also have unique multi-enzyme complexes, the cellulosomes, which integrated many cellulolytic and hemicellulolytic enzyme to degrade the hemicellulose (Shallom & Shoham 2003).
The polymer degradation process is important because most of insects are unable to utilize cellulose and other plant polymers. It is because of the lack of the digesting enzyme (Chapman 1998). The process of degradation polymer will produce some volatile compound that stimulant incest to oviposition. Rachmawati (2010 & 2007) demonstrated that H. illucens will only lay their eggs in the PKM that has been fermented for 6 days or longer.
The production of H. illucens larvae for aquaculture development in Indonesia have been initiated in project design between Balai Riset Budidaya Ikan Hias (BRBIH) Depok and IRD (Institut de Recherche pour le Développement) since 2006. The step of degradation of polymer complex in bioconversion of PKM is done spontaneous and naturally. The natural fermentation was influenced by environmental factors, thus the result varies. For sustainability of H. illucens
larvae mass production, this process should be controlled. Therefore, the degradation process should be conducted in a closed canister to minimize outside environmental effects. Next, a starter containing PKM degrading microorganisms should be made. In order to make starter, the information concerning the biochemical and microbial changes during the fermentation should first be gathered.
1.2 Objective
II. LITERATURE REVIEW
2.1 Palm Kernel Meal (PKM)
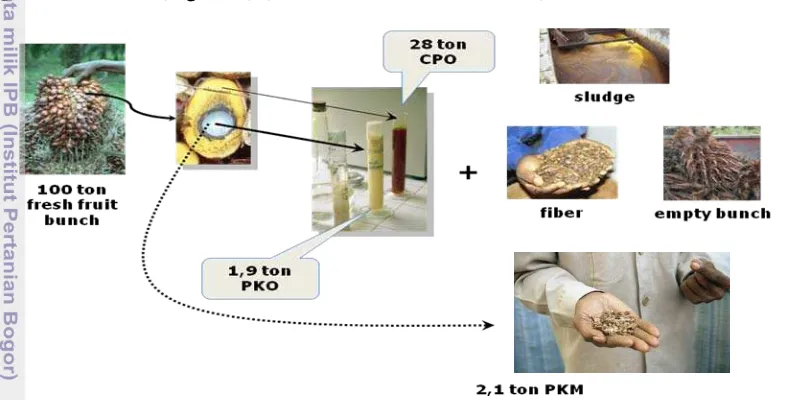
Palm oil (Elaeis guineensis Jacq.) industry has two main products, crude palm oil (CPO) and palm kernel oil (PKO) and several by products, empty fruit bunch, palm fiber, sludge, and palm kernel meal (PKM). CPO is processed from fresh fruit of E. guineensis by extraction process. Fiber and sludge are by product of this process. Palm kernels are processed to produce PKO and by product of this process is PKM (Figure 1) (Hem et al. 2008; Poku 2002).
Figure 1. Production of palm oil and it’s by product (Hem et al. 2008; Poku 2002)
The by product from this industry is more abundant than the main product. Every 100 tons of fresh fruit bunch produced 24-28 ton CPO and 4 tons of palm kernel oil. It means that 72-76% fresh fruit weight will become the by-product of palm oil industry (Poku 2002). In Malaysia, 3 million tons of palm kernels are processed at palm kernel crushing plant into 1.4 million tons of PKO and 1.6 million tons PKM (Ng 2003).
2.1.1The Content of PKM
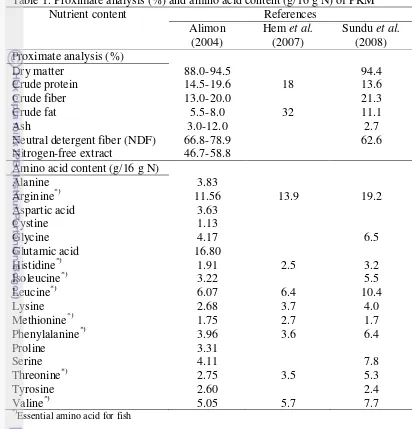
crude fiber (Alimon 2004). PKM is rich in some essential amino acid for fish like arginine and leucine, but deficient in methionine. The complete nutrient content of PKM is shown on Table 1.
Table 1. Proximate analysis (%) and amino acid content (g/16 g N) of PKM
Nutrient content References
Neutral detergent fiber (NDF) 66.8-78.9 62.6
Nitrogen-free extract 46.7-58.8
Amino acid content (g/16 g N)
Alanine 3.83
Essential amino acid for fish
2.1.2 Utilization of PKM
Some researcher tried to utilize PKM through various ways. Yopi et al.
(2006) used PKM as a substrate to produce functional oligosaccharide such as mannose and manno-oligosaccharide. Utilization of PKM for production of
-glucosidase and -mannase by Aspergillus niger FTCC 5003 in solid substrate
fermentation using an aerated column bioreactor was already tried by Abdeshahian et al. (2010a & 2010b). To produce bioethanol, Cerveró et al. (2010) tried to hydrolyze fermented PKM using Saccharomyces cerevisiae.
2.2Polysaccharides in PKM
Polysaccharides are carbohydrate containing many (hundred or even thousands) of monomeric unit called monosaccharides which connected by covalent bond called glycosidic bonds. Cellulose is a linear condensation polymer consisting of glucose units joined together by -1,4-glycosidic bonds. The glycosidic linkage acts as a functional group, and this along with the hydroxyl groups mainly determine the chemical properties of cellulose. The PKM contain 12% of cellulose. Cellulose has a low digestibility or even indigestible in poultry (Sundu & Dingle 2003).
Hemicellulose is structure of polysaccharides of plant cell wall, in close association with cellulose and lignin. Hemicellulose is mixture of polymer made up of sugars (mostly not glucose) and sugar derivative. The monomers of hemicelullose consist of three hexoses (glucose, galactose, and mannose) and two pentoses (xylose and arabinose). Most of the main-chain sugars on hemicellulose
structure are linked together by -1,4-glycosidic bonds (Moreira & Filho 2008). In
PKM the biggest content of hexoses is mannose (Cerveróa et al. 2010 & Düsterhöft et al. 1992). The complete sugar compositions of PKM are shown in Table 2.
Table 2. Sugar composition of PKM
According to the main sugar residue in the backbone, hemicellulose has different classification e.g., xylans, mannans, glucans, glucoronoxylans, arabinoxylans, glucomannans, galactomannans, galactoglucomannans, β-glucans, and xyloglucans. In total NSPs, PKM contain about 78% of mannans. On a dry matter basis, PKM have been estimated to have 30-γ5% of -mannans. Mannans in palm kernel resembles very much cellulose by being crystalline, hard and water insoluble. Mannans are found in the cell wall and impair the digestibility and utilization of nutrients either by direct encapsulation of the nutrients or by increasing the viscosity of the intestinal contents. This causes a reduction of the hydrolysis rate and the absorption of nutrients in the diet (Sundu & Dingle 2003).
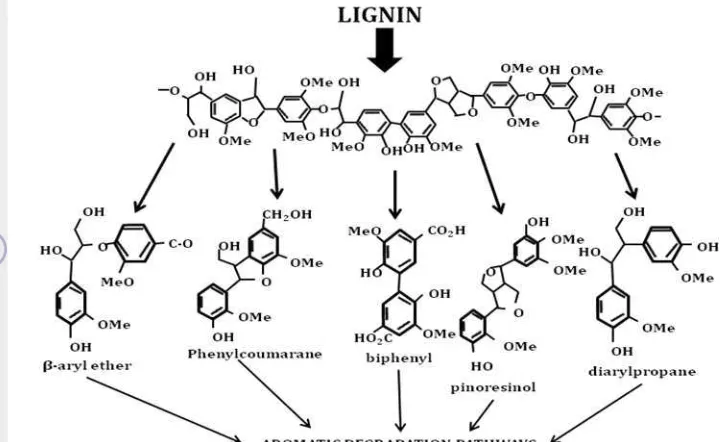
Figure 2. Major type of linkages found in lignin (Bugg et al. 2011)
2.3 Polysaccharide Degrading Microorganisms
The biodegradation of polysaccharide structures involves the concerted action of a variety of hydrolytic enzymes. Some microorganisms have been identified having hydrolytic enzymes for cleavage of almost chemical bonds that found in plant structures.
Streptomyces coalicolor A3(2), Bacillus halodurans C-125, Bacillus subtilis 168, Cellvibrio japonicus, Clostridium acetobutylicum ATT 824, Bifidobacterium longum NCC2705, Xanthomonas axonopodis pv. Citri str. 306, Xanthomonas campestris pv campestris str. ATTC33913, Geobacillus stearothermophillus T-6, Geobacillus stearothermophillus 21 are known as bacteria having ability to produced hemicellulolytic enzymes (Shallom & Shoham 2003).
Enterobacter, Vibrio, Aeromonas, Moraxella, and Bacillus. These bacteria were collected from sea, freshwater, and land environments.
Mannan–degrading enzyme is also produced by some eukaryotic organisms, such as Trichoderma reesei,Aspergillus aculeatus, Agaricus bisporus
(Moreira & Filho 2008; Sundu & Dingle 2003). Cellulolytic fungus such as
Trichoderma koningii has been used to reduce fiber and increase protein content of PKM by solid-state fermentation (Ng 2003). To decrease fiber and increase protein content of palm kernel, Illuyemi et al. (2006) also tried to ferment palm kernel cake using Sclerotium rolfsii, Trichoderma harzianum, Trichoderma longiobrachiatium, Trichoderma koningii, and Aspergillus niger. Yopi et al.
(2006) found that Streptomyces limpanii and Saccharopolyspora flava have a capability to degrade mannan of palm kernel cake.
Pseudomonas spp., P. acidovorans, P. cepacia, P. cruciviae, P. flourescens, P. multivorans, P. ovalis, P. paucimobilis, P. pseudoalcaligenes, P. putida, Xanthomonas, Acinetobacter, Flavobacterium, Achromobacter, Agrobacterium, Beijerinckia, Aeromonas, Erwinia, Actinomadura, Arthrobacter
spp., A. simplex, Corynebacterium, Nocardia, Rhodococcus, Streptomyces spp., S. badius, S. cyaneus, S. rimosus, S. viridosporus, B. megaterium, B. subtilis are known as bacteria that have ability to degrade lignin (Zimmermann 1990).
2.4 Bioconversion using Hermetia illucens larvae
The bioconversion or known as biotransformation is the process that uses live organisms, often microorganisms, to convert a substrate into chemically modified form. One of the types of bioconversion is conversion of organic refuse by saprophages. In this system, live organisms feed on organic matter to reduce and convert organic waste in to live feedstock for livestock farming.
One of organisms that already used in bioconversion process is H. illucens
larvae reduced at least 50% (in depth) the manure accumulation in a caged layer hen manure management system. Hem et al. (2008) used these larvae to reduce PKM. Larvae H. illucens that already used to reduce by product or manure is useful as feed for poultry. Newton et al. (1977) used these larvae as feed for poultry and pig. In aquaculture, these larvae already used as feed for tilapia (Hem
et al. 2008), catfish (Bondari & Sheppard 1981), and botia (Septivia 2008). Prepupae of these species can replace fish meal up to 25% in feed formulation for rainbow fish (St-Hilaire et al. 2007).
2.5 Fermentation
Fermentation is catabolism of organic compound, generally carbohydrate, that carried out by microorganism (Gandjar et al. 2006). Some aspects that influence the fermentation including physiochemical and biochemical parameter are particle size, initial moisture, pH, pre-treatment of the substrate, relative humidity, temperature on incubation, agitation and aeration, age and size of inoculums. The temperature of the substrate is affects the growth of microorganism, spore formation, germination, and product formation. Water content in the fermentation system influences microbial activity. High moistures results in decreased substrate porosity, which in turn prevents oxygen penetration. Otherwise, low moisture may lead poor accessibility of nutrients resulting poor microbial growth (Pandey 2003).
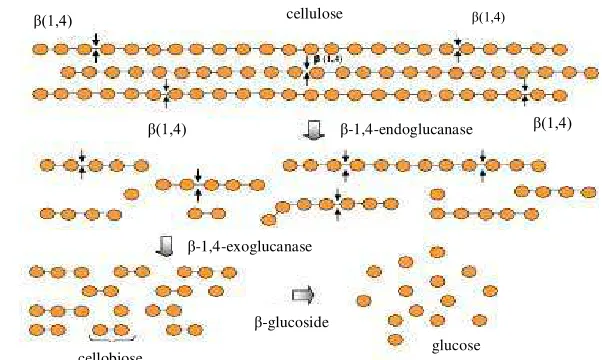
Figure 3. Schematic representation of cellulase enzymes over the cellulose structure (Mussatto & Teixeira 2010)
Hemicellulose is degraded into variety of monosaccharide, including galactose, arabinose, xylose, mannose, and glucose. Mannan, as a biggest content of hemicelluloses in PKM, is degraded by some enzymes i.e. endo- -mannanase (1,4- mannan mannohydrolase, EC 3.2.178), exo- -mannosidase (1,4- mannopyranoside hydrolase, EC 3.2.1.25), and exo- -glucosidase (1,4- -D-glucoside glucohydrolase, EC 3.2.1.21). Endo- -mannanase cleavage -1,4-linked internal linkages of the mannan backbone randomly to produce new chain ends. Exo- -mannosidase cleavage -1,4-linked mannosides and produce mannose from the nonreducing end of mannans and manno-oligosaccharides. Exo- -glucosidase hydrolyze 1,4- -D-glucopyranose at the nonreducing end of the oligosaccharides released from glucomannan and galacto-glucomannan by -mannanase (Figure 4) (Moreira & Filho 2008).
Figure 4. Schematic hydrolyzed of mannans by some enzyme (Moreira & Filho 2008)
-glucoside
-1,4-endoglucanase
-1,4-exoglucanase cellulose
cellobiose glucose
(1,4)
(1,4) (1,4)
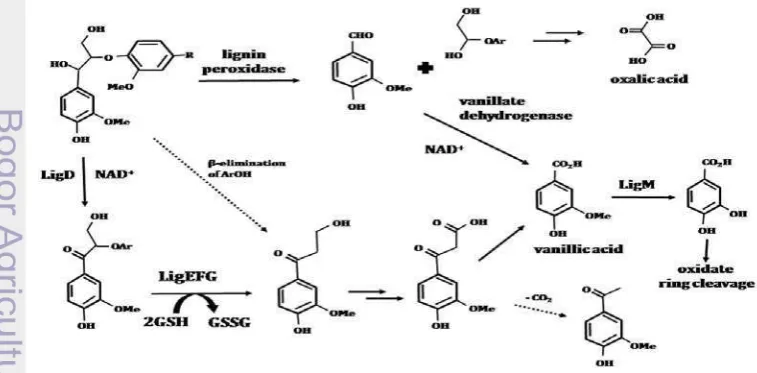
As a complex aromatic heteropolymer, degradation of lignin needs various enzymes. Lignin consists of some components. The catabolic pathway for breakdown lignin components have been identified and characteristics using model substrate. The pathway of degradation lignin components are shows in Figure 5. The pathway of -aryl ether lignin dimer compound is by oxidation of the α-hydroxyl group to the corresponding ketone which catalysed by the NAD-dependent dehydrogenase LigD. Then, an unusual reductive ether cleavage reaction takes place, via a novel glutathione dependent -etherase enzyme. The LigEFG gene products are responsible for this transformation. In Pseudomonas putida and Rhodococcus jostii RHA1, the ketone product has been detected by-product of lignocellulose breakdown via the -etherase cleavage reaction, or via a
-elimination reaction. The ketone product is then metabolised to vanillic acid,
probably via oxidation of the -hydroxyl group to the carboxylic acid. In S.
paucimobilis SYK-6 demethylation of vanillic acid to protocatechuic acid is catalysed by a tetrahydrofolate dependent demethylase LigM. The demethylation reaction in Pseudomonas and Acinetobacter is catalysed by a non heme iron dependent demethylase enzyme. The product, protocatechuic acid, is then a substrate for oxidative ring cleavage. In white-rot fungi, metabolism of -aryl ether model compounds is predominantly via Cα-C oxidative cleavage, to give vanillin as a product. Vanillin is then oxidised to vanillic acid by vanillate dehydrogenase (Figure 5) (Bugg et al. 2011).
Degradation of the biphenyl compound is via oxidation 2,3-dihydroxybiphenyl, followed by oxidative meta-cleavage. The pathway for the degradation of the biphenyl compound of lignin in S. paucimobilis SYK-6 is shown in Figure 6. Demethylation of one methoxy group is catalysed by a non-heme iron-dependent demethylase enzyme LigX. The catecholic product of LigX is then a substrate for oxidative meta-cleavage by the extra dioldioxygenase LigZ, and the ring fission product is then cleaved by C–C hydrolase LigY, to form 5-carboxyvanillic acid and 4-carboxy-2-hydroxypentadienoic acid. Decarboxylase enzymes LigW convert 5-carboxyvanillic acid into the central intermediate vanillic acid. By analogy with other aromatic degradation pathways, where the product of hydrolytic C–C cleavage is often the substrate for a hydratase enzyme, the by product of LigY-catalysed cleavage, 4-carboxy-2-hydroxypentadienoic acid, is probably hydrated to form 4-hydroxy-4-methyl-2-oxoglutarate, for which an aldolase enzyme. Aldolase-catalysed cleavage would then generate two molecules of pyruvic acid (Bugg et al. 2011).
Figure 6. Aromatic degradation pathway of biphenyl lignin compound (Bugg et al. 2011)
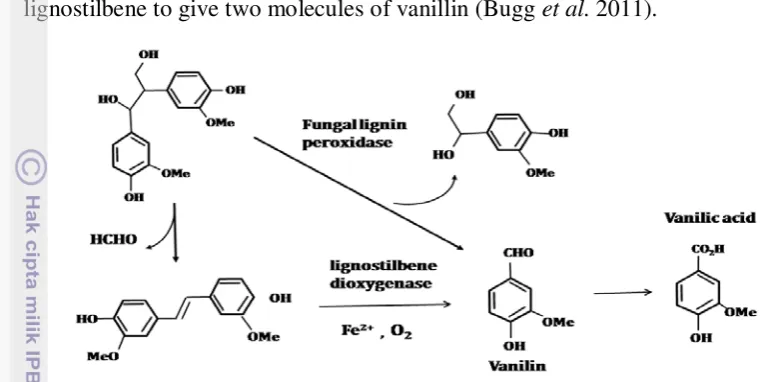
metabolised by lignostilbene. Lignostilbene dioxygenase is a non-heme iron-dependent dioxygenase enzyme that catalyses the oxidative cleavage of lignostilbene to give two molecules of vanillin (Bugg et al. 2011).
Figure 7. Aromatic degradation pathway of diarypropane lignin compound (Bugg et al. 2011)
Breakdown of the phenylcoumarane compound of lignin has been studied in fungus Phanerochaete chrysposporium and Fusarium solani (Figure 8). Degradation of the alkylated phenylcoumarane by P. chrysosporium was initially by oxidation of the sidechain, then oxidation of the heterocyclic ring to a furan,
and finall y oxidative Cα–C bond cleavage. In Fusarium solani M-13-1,
breakdown of the phenolic phenycoumarane proceeded via direct Cα–C bond to give 5-acetylvanillone. Loss of the -hydroxyl group could be rationalised by the formation of an epoxide intermediate, followed by hydrolytic cleavage of the Cα– C bond (Bugg et al. 2011).
The pathway for breakdown of the pinoresinol compound of lignin has also been studied in Fusarium solani M-13-1 (Figure 9). Oxidation of the benzylic position was observed, leading to C–O bond cleavage, to form a monocyclic ketone intermediate. Further aryl–alkyl oxidation yielded a carboxylic acid product, and the corresponding lactone. In bacteria, S. paucimobilis SYK-6 is reported to degrade pinoresinol lignin model compounds, but the genes responsible have not yet been identified. The catabolic pathways for both heterocyclic lignin compounds appear to involve α-hydroxylation as an initial step (Bugg et al. 2011).
Figure 9. Aromatic degradation pathway of pinoresinol lignin compound (Bugg et al. 2011)
III. MATERIALS AND METHODS
3.1 Site of Research
The research was conducted at Balai Riset Budidaya Ikan Hias (BRBIH), Depok from March to September 2011.
3.2 Condition of Fermentation
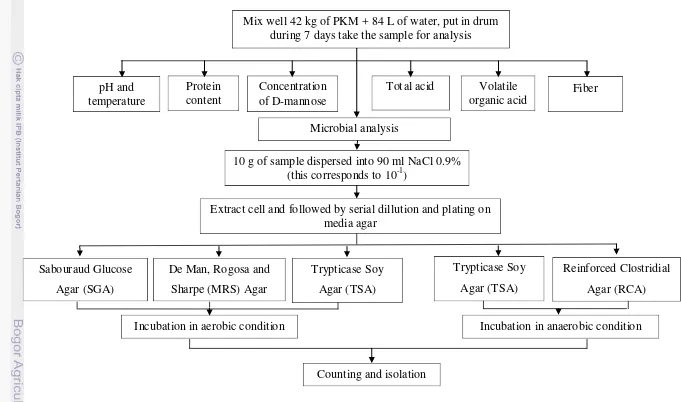
PKM supply has been ordered from PT. Perkebunan Nusantara VII, Bandar Lampung. The fermentation was made by mixing 42 kg of PKM with 84 L of water (1:2) and put it in the big drum (height 81 cm; diameter 48 cm). The fermentation height in drum is 80 cm. The sample was taken from the depth of ±40 cm using pipe at some point. At 12 h intervals during 7 days take the sample for analysis of pH, total acid, temperature, and microbial analysis. Analysis of D-mannose was conducted at 0, 36, 48, 72, 96, and 120 hours. Analysis of concentration of volatile organic acid was conducted at 0, 36, 72 and 168 hour. Analysis of fiber was conducted at 0, 36, and 168 hours. Analysis of protein content was conducted at the beginning and at the end of fermentation (Figure 10).
3.3 Microbial analysis
representative colonies were picked up from the higher dilution plate, purified and identified using gram stain and microscopic observation. Yeast and moulds were enumerated on plates of Sabouraud Glucose Agar (SGA) (Fluka) at 30 0C for 4 days. The acceptable range of colonies for counting is 30-300 colonies on the plate (Josephson et.al 2000).
3.4 Analysis of pH and temperature
The pH of PKM fermentation analyzed using microcomputer ion meter (Consort). The temperature of PKM fermentation was analyzed using digital thermometer.
3.5 Analysis of total acid
Total acid value deduced by titrating 5 mL of aliquot of sample with 0.1 M NaOH, using 1% phenolphthalein as an indicator. The volume of titrant that causes a permanent colour change of the sample is recorded and used to calculate the total acid value.
3.6 Analysis of fiber
The fiber of fermented PKM conducted by sending the sample to Laboratory of Indonesian Research Institute for Animal Production Bogor, using Van Soeth technique. The percentage of hemicellulose was calculated as the difference between NDF (Neutral Detergent Fiber) and ADF (Acid Detergent Fiber), and percentage of cellulose as the difference between ADF and lignin.
3.7 Analysis of protein content
18
Figure 10. Schema of the research
Mix well 42 kg of PKM + 84 L of water, put in drum during 7 days take the sample for analysis
pH and
10 g of sample dispersed into 90 ml NaCl 0.9% (this corresponds to 10-1)
Extract cell and followed by serial dillution and plating on media agar
Incubation in aerobic condition Incubation in anaerobic condition
Counting and isolation Sabouraud Glucose
Agar (SGA)
3.8 D-mannose Detection Assay
D-mannose concentration of fermented PKM was determined using a four enzyme coupled assay based on the Megazyme International kit (Megazyme International Ireland Ltd.). Mannose was phosphorylated to mannose-6-phosphate by hexokinase (HK) which was subsequently converted to fructose-6-phosphate through the action of phosphomannose isomerase (PMI). Fructose-6-phosphate was then isomerized to glucose-6-phosphate by phosphoglucose isomerase (PGI) and finally, oxidized to gluconate-6-phosphate by glucose-6-phosphate dehydrogenase (G6P-DH). The G6PDH NADP+
Before analysis, the pH of liquid sample (0.1 ml) was adjusted to 7.6 using 1 M NaOH and then the solution was incubated at room temperature for 30 minutes. Concentration of D-mannose was calculated as follows:
cofactor is concurrently reduced to NADPH. Each reaction of enzyme was monitored at 340 nm.
Where:
c = concentration of D-mannose (g/L) V = final volume (mL)
MW = molecular weight of D glucose or D-mannose (180.16 g/mol) = extinction coefficient of NADPH at 340 nm (6300 l/mol/cm)
d = light path (1 cm) v = sample volume
ΔA = the absorbance difference between added reagent (Source: Megazyme International 2005)
3.9 Volatile organic acid analysis
IV. RESULT DAN DISCUSSION
4.1 Result
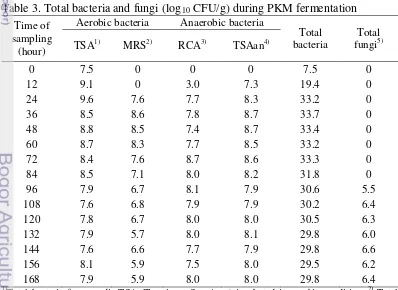
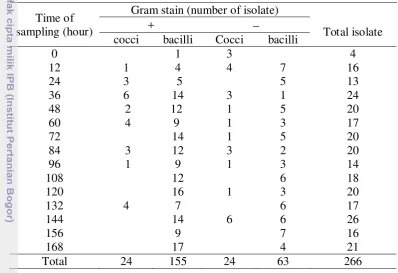
Total microorganism was changed during PKM fermentation (Table 3). Total aerobic bacteria corresponded approximately to the sum of total bacteria from TSA media and from MRS media. The highest number of aerobic bacteria, 17.2 log10 CFU/g, was found at 24 hours of fermentation. The highest total bacteria from TSA media, 9.6 log10 CFU/g, was found at 24 hour and decreased later happened until 7.9 log10 CFU/g at the end of the fermentation process; while lactic acid bacteria, isolated from MRS media, were most found, 8.6 log10 CFU/g, at 36 hours of fermentation. Total anaerobic bacteria corresponded approximately to the sum of total bacteria from TSA media anaerobically incubated and from RCA media. Total anaerobic bacteria were not found until 12 hours of fermentation and the highest number, 17.3 log10 CFU/g, were found at 72 hours. No fungi were found before 96 hours of fermentation.
Table 3. Total bacteria and fungi (log10 Time of
sampling (hour)
CFU/g) during PKM fermentation Aerobic bacteria Anaerobic bacteria
Total
(Reinforce Clostridial Agar); 4) Total bacteria from media TSA (Trypticase Soy Agar) incubated in anaerobic condition; 5)
During fermentation, 266 types of bacteria were successfully isolated. From those isolates, 179 were gram (+) bacteria and 87 gram (−) bacteria. The number of isolates on each sampling and their respective characterization could be seen on Table 4.
Total fungi from media SGA (Sabouraud Glucose Agar).
Tabel 4. The number of bacteria on each sampling and their respective characterization
Time of sampling (hour)
Gram stain (number of isolate)
+ −
Total isolate cocci bacilli Cocci bacilli
0 1 3 4
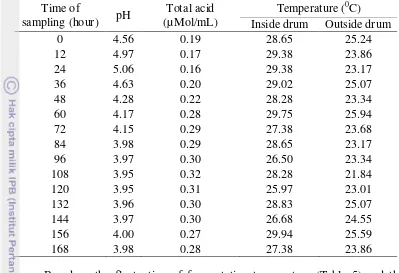
Table 5. pH, total acid (µMol/mL), and temperature (0 Inside drum Outside drum
0 4.56 0.19 28.65 25.24
Figure 11. Relationship between temperature fermentation and microbial activity
Percentage fiber and protein of PKM changed during the fermentation process (Table 6). The lignin content of PKM decreased drastically (51.34%) after 168 hours fermentation. Hemicelluloses and cellulose content of PKM decreased 10.79% and 10.77%, respectively during fermentation process. The protein content of PKM only increased 4.79% after 168 hours of fermentation.
Table 6. Hemicellulose, cellulose, lignin (% in dry basis) and crude protein (% in dry basis) content of PKM during fermentation
Component of PKM (%) Time of sampling (hours)
0 36 168m
Water content*) 69.17±0.25 71.68±0.39 74.82±0.36
NDF 22.57 20.96 17.69
ADF 14.84 12.82 10.79
Lignin 6.02 3.21 2.93
Hemicelullose 7.73 8.14 6.90
Celullose 8.81 9.61 7.86
Crude Protein*) 17.53±0.24 18.37±0.31
*)
average value±SD; NDF = Neutral Detergent Fiber; ADF = Acid Detergent Fiber
Table 7. Concentration of soluble sugar during PKM fermentation
Volatile organic acid concentrations along the process of fermentation could be seen in Table 8. During fermentation, acetic acid dominates the composition of volatile organic acids. After 72 hours the concentration of acetic acid raised, up to 87.89 mMol, compared to the acid’s concentration on the 36 hour.
Table 8. Concentration of volatile organic acid (mMol) during PKM fermentation Type of volatile
lactic acid bacteria lead to the increase of titratable acidity and decreased the pH of fermentation. However, the total lactic acid bacteria decreased when the pH of fermentation below 4 (Kakou et al. 2010). This was caused by the fact that pH level is a limiting factor to microorganism growth (Madigan et al. 2009).
The highest hemicellulose content in PKM is mannan. Clostridium is known to have the ability to degrade mannan; therefore a specific media, RCA, was used to isolate and observe the total Clostridium colony change along the fermentation process of PKM. Bacteria from the RCA media were not discovered until 12 hour of fermentation and the number continue to increase when the pH of fermentation around 4
The cell wall of PKM consists of cellulose, lignin, and hemicelluloses mannan that hard, crystalline, and water insoluble. In degradation of cell walls, enzymes produced by microorganism must be able to penetrate into the cell. The enzymes produced by bacteria generally smaller than the enzymes produced by fungi, making it easier to penetrate into the cell. Therefore, in the fermentation of PKM, fungi were found at 96 hours of fermentation.
. This indicates that Clostridium is capable of living in an acidic environment with a pH level below 5. Similar thing happens in the fermentation of cassava by Brauman et al. (1996), Clostridium spp. was found when the pH below 5 which characterized by the presence of butyric acid.
In the fermentation process of PKM, polysaccharides degraded into monosaccharides. The concentration of mannose increased after 36 hours of fermentation. The results of NDF analysis (hemicelluloses, cellulose, and lignin) also showed a decrease. However, if specified, the lignin content decreased drastically at 36 hours and seen that the value of hemicellulose and cellulose increased. This is because the measuring unit used is percentage (%), so when one of decline, the others will look like an increase, to fulfill the composition of 100%. Therefore, to better illustrate the changes that occur during this process, the calculation of the polysaccharide content should be in weight/volume (w/v).
(Ahmed et al. 2001). Mannan as the biggest content of hemicellulose in PKM will degrade into mannose and manno-oligosaccharides by some microorganism’s enzyme. The degradation of mannan was done by some enzymes i.e. endo
-mannanase which cleavage -1,4-linked internal linkages of the mannan backbone
randomly; exo- -mannosidase which cleavege -1,4-linked mannosides and produce mannose from the nonreducing end of mannans and manno-oligosaccharides; and exo- -glucosidase which will hydrolyze 1,4- -D-glucopyranose at the nonreducing end of the oligosaccharides released from glucomannan and galacto-glucomannan by -mannanase (Moreira & Filho 2008).
Degradation process of mannan into mannose and mano-oligosaccharides can be performed by aerobic and anaerobic bacteria (Moreira & Filho 2008). Concentration of mannose in fermentation of PKM has increased twice, at 36 and at 72 hours fermentation. The increased of mannose concentration at 36 hours of fermentations could be the result of degradation mannan by aerobic bacteria such as Bacillus circulans, Bacillus subtilis, Pseudomonas flourescens, Vibrio sp. Meanwhile, at 72 hours degradation of mannan into mannose was done by anaerobic bacteria such as Clostridium thermocellum dan Clostridium cellulolytic
(Moreira & Filho 2008; Sundu & Dingle 2003).
Production of mannan-degrading enzymes by fungi and bacteria was found to be inducible. Monomer and oligosaccharides, which are the degradation product of mannan by the action of a basal level of hydrolytic enzymes present in the cell, act as inducers and play a key role in the regulation of mannan biosynthesis (Moreira & Filho 2008). In the fermentation of PKM, the existing mannose at the beginning of fermentation (at 0 hours) acts as an enzyme inducer
of -mannanase.
Lignin was the most degraded polysaccharide after 168 hours of fermentation. However, the greatest lignin degradation process occurs in 36 hours of fermentation. Probably, after 36 hours of fermentation the amounts of oxygen in the fermentation system dwindle. This is indicated the by increase of the total anaerobic bacteria. Banner et al. reported that conversion of lignin into a product gas slower in anaerobic than in aerobic condition (see Vicuna 1988). This result also indicates that the fermenting microorganism has the ability to degrade lignin. Martani et al. (2003) showed that Micrococcus and Bacillus are able to degraded lignin from black liquor, by product of pulp industry, about 75 and 78% respectively within 14 days of incubation.
The degradation of polysaccharides caused the increase of protein content of PKM. After 168 hours fermentation, the protein content was increased only 4.79%. The increased in crude protein value of degraded PKM was partly due to ability of enzyme to increase the bioavailability of the protein hitherto encapsulated by cell walls.
Glucose, fructose, and mannose, product of degradation polysaccharide, converted to pyruvate via the glycolysis pathway and pyruvate is split to yield acetyl-CoenzimA. In aerobic condition, acetyl-CoA will enter the Krebs cycle. In anaerobic condition, acetyl-CoA is reduced into acetic acid or other fermentation products using NADH derived from glycolysis reactions as electron donor (Madigan et al. 2009). The increasing acetic acid concentration at 72 hours of fermentation showed the accumulation of acetic acid as a product of anaerobic fermentation.
Butyric acid was a major end product of fermentation by Clostridium. The fermentation product was influenced by the duration and the condition of the fermentation. In fermentation of PKM, the concentration of butyric acid is very few. It is because during fermentation of PKM, the pH was acid (around 4). The same result also showed by Zu and Yang (2004), who tried to fermente xylose by
activities of several key enzymes. The activities of phosphotransbutyrylase (PTB), which is the key enzyme controlling butyrate formation, and NAD-independent lactate dehydrogenase(iLDH), which catalyzes the conversion of lactate to pyruvate, were higher in cells producing mainly butyrate at pH 6.3. In contrast, cells at pH 5.0 had higher activities of phosphotransacetylase (PTA), which is the key enzyme controlling acetate formation, and lactate dehydrogenase (LDH), which catalyzes the conversion of pyruvate to lactate. Also, PTA was very sensitive to the inhibition by butyric acid. Some species Clostridium also produce acetone and butanol as fermentation product. The synthesis of acid will ceases when the pH of medium drop and the neutral product such as butanol and acetone begin to accumulate (Madigan et al. 2009).
V. CONCLUSIONS AND RECOMENDATION
5.1 Conclusions
The highest total aerobic bacteria were found at 24 hours of fermentation. The highest total anaerobic bacteria were found at 72 hours of fermentation. No fungi were found before 96 hours of fermentation. The fermentation pH level decreases after 48 hours. The total acid concentration increased after 48 hours of fermentation. The dominant organic acid during fermentation of PKM is acetic acid. The mannose concentration was increased after 36 hours of fermentation. After 168 hours of fermentation, the protein concentration increases 4.79%; while the concentration of hemicellulose, cellulose, and lignin decreases 10.79%, 10.77%, and 51.34% respectively.
5.2 Recommendations
1. Further identification of all obtained isolates is needed to be conducted to avoid pathogenic microorganism.
REFERENCES
Abdeshahian P. Samat N, Wan Yusoff WM. 2010a. Utilization of palm kernel
cake for production of -glucosicade by Aspergillus niger FTCC 5003 in
solid substrate ferementation using an aerated column bioreactor.
Biotechnology 9(1): 17–24.
Abdeshahian P. Samat N, Wan Yusoff WM. 2010b. Utilization of palm kernel
cake for production of -mannanase by Aspergillus niger FTCC 5003 in
solid substrate ferementation using an aerated column bioreactor
Ahmed Z, Banu H, Rahman MM, Akhter F, Haque MS. 2001. Microbial activity on the degradation lignocellulosic polysaccharides. Online Journal of Biological Science 1(10): 993–997.
Alimon AR. 2004. The nutritive value of Palm Kernel Cake for Animal Feed.
Palm Oil Development 40: 12–14
Atasie VN, Akinhanmi TF. 2009. Extraction, compositional studies and physico-chemical characteristics of palm kernel oil. Pakistan Journal of Nutrition
8(6): 800–803.
Araki T, Kitamikado M. 1978. Distribution of mannan-degrading bacteria in aquatic environments. Bulletin of Japanese Society of Science Fisheries
44(10): 1135–1139.
Bondari K, Sheppard DC. 1981. Soldier fly larvae as feed in commercial fish production. Aquaculture 24: 103–109.
Brauman A, Kéléké S, Malonga M, Miambi E, Ampe F. 1996. Microbiological and biochemical characterization of cassava retting, a traditional lactic acid fermentation for foo-foo (cassava flour) production. Applied and Environmental Microbiology: 2854–2858.
Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R. 2011. Review: Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep 28: 1883– 1896.
Chapman RF. 1998. The Insects: Structure and Function. 4th edition. Cambridge: Cambridge University Press.
Düsterhöft EM, Posthumus MA, Voragen AGJ. 1992. Non-starch polysaccharides from sunflower (Helianthus annuus) meal and palm kernel (Elaeis Guineensis) meal investigation of the structure of major polysaccharides.
Journal of the Science of Food and Agriculture 59(2): 151–160.
Gandjar I, Oetari A, Sjamsurizal W. 2006. Mikologi Dasar dan Terapan. Yayasan Obor Indonesia, Jakarta.
Hart FL, Fisher HJ. 1971. ModernFood Analyses. New York: Springer-Verlag.
Hem S, Fahmi MR, Chumaidi, Maskur, Hadadi A, Supriyadi, Ediwarman, Larue M, Pouyaud L. 2007. Valorization of Palm Kernel Meal (PKM), a by-product from palm oil agro industry, via Bioconversion: a natural process of particular interest for the development of aquaculture in Indonesia. International Conference on Oil Palm and Environment ICOPE 2007 (Posters session) 15–16 November 2007, Bali (Indonesia).
Hem S, Toure S, Sagbla C, Legendre M. 2008. Bioconversion of palm kernel meal for aquaculture: Experiences from the forest region (Republic of Guinea). African Journal of Biotechnology 7(8): 1192–1198.
Indexmundi. Fishmeal Monthly Price-US Dollars per Metric Ton.
Iluyemi FB, Hanafi MM, Radziah O, Kamarudin MS. 2006. Fungal solid state culture of palm kernel cake. Bioresource Technology 97: 477–482.
Josephson KL, Gerba CP, Pepper IL. 2000. Cultural methods. In: RM. Maier, IL. Pepper, CP. Gerba (editors). Environmental Microbiology. Academic Press, San Diego: 213–234.
Kakou AC, Guehi TS, Olo K, Kouame AF, Nevry KR, Koussemon MC. 2010. Biochemical and microbial changes during traditional spontaneous lactic acid fermentation process using two varieties of cassava for production of a “Alladjan” starter. International Food Research Journal
17: 563–573.
Knudsen KEB. 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science Technology 67: 319–338.
Madigan MT, Martinko JM, Dunlap PV, Clark DP. 2009. Brock Biology of Microorganisms. 12th edition. San Francisco: Pearsons Education Inc.
Martani E, Haedar N, Margino S. 2003. Isolasi dan karakteristik bakteri pendegradasi lignin dari beberapa substrat alami. Gama Sains V (2): 97– 107.
Megazyme International. 2005. Megazyme: Assay Procedure for analysis D-Mannose, D-fructose, and D-glucose. Megazyme International Ireland Ltd., Co. Wicklow: 11 hlm.
Moreira LRS, Filho EXF. 2008. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79: 165–178.
Mussatto SI, Teixeira JA. 2010. Lignocellulose as raw material in fermentation processes. In: Méndez-Vilas A (Editor). Current Research, Technology and Education Topics in Applied Microbiology and Microbial
Biotechnology: 897–907
Newton GL, Booram CV, Barker RW, Hale OM. 1977. Dried Hermetia illucens
larvae meal as a supplement for swine. Journal of Animal Science 44(3): 395–400.
Ng WK, Chong KK. 2002. The nutritive value of palm kernel meal and the effects of enzyme supplementation in practical diets for red hybrid tilapia (Oreochromis sp.). Asian Fisheries Science 15: 167–176.
Ng WK. 2003. The potential use of palm kernel meal in aquaculture feeds.
Aquaculture Asia7(1): 38–39.
Olivier P. 2001. Larval bio-conversion. Electronic Forum on Area-Wide Integration of Specialized Crop and Livestock Production. Livestock Environment and Development (LEAD) Initiative. http://lead.virtualcentre.org/en/ele/awi_2000/particip/oliver.pdf [8 Okt 2006].
Pandey A. 2003. Solid-state fermentation. Biochemical Engineering Journal 13: 81–84.
Phothichitto K, Nitisinprasert S, Keawsompong S. 2006. Isolation, screening and identification of mannanase producing microorganisms. Kasetsart J. (Nat. Sci.) 40 (Suppl.): 26–38.
Rachmawati. 2007. Pola suksesi dan interaksi antara larva Hermetia illucens
(Diptera, Stratiomyidae) dan Musca domestica (Diptera, Muscidae) pada bungkil kelapa sawit [skripsi]. Depok: Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Indonesia.
Rachmawati. 2010. Sejarah kehidupan Hermetia illucens (Linnaeus) (Diptera: Stratiomydae) pada bungkil kelapa sawit [Thesis]. Sekolah Pascasarjana, Institut Pertanian Bogor.
Septivia SA. 2008. Pertumbuhan benih ikan botia (Chromobotia macracanthus
Bleeker) yang diberi pakan alami maggot, cacing darah, dan cacing tanah [skripsi]. Jakarta: Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Negeri Jakarta.
Shallom D, Shoham Y. 2003. Microbial hemicellulases. Current Opinion in Microbiology 6: 219–228.
Sheppard DC, Newton GL, Thompson SA, Savage S. 1994. A value added manure management system using the black soldier fly. Bioresource Technology 50: 275–279.
St-Hilaire S, Sheppard C, Tomberlin JK, Irving S, Newton L, Mcguire MA, Mosley EE, Hardy RW, Sealey W. 2007. Fly prepupae as a feedstuff for Rainbow Trout, Oncorhynchus mykiss. Journal of the World Aquaculture Society 38(1): 59–67.
Sukaryana Y, Atmomarsono U, Yunianto VD, Supriyatna E. 2010. Bioconversion of palm kernel cake and rice bran mixtures by Trichoderma viride toward nutritional contents. Internat. J. Of Sci and Eng 1(2): 27–32.
Sundu B, Dingle J. 2003. Use of enzymes to improve the nutritional value of palm kernel meal and copra meal. Proc. Queensland Poult. Sci. Symp. Australia
11(14): 1–15.
Sundu B, Kumar A, Dingle J. 2008. Amino Acid Digestibilities of Palm Kernel in Poultry. J.Indon.Trop.Anim.Agric 3(2): 139–144.
[USDA] United States Department of Agriculture. 2007. Indonesia: Palm oil production prospects continue to grow. Commodity Intelligence Report. www.pecad.fas.usda.gov
Vicuna R. 1988. Bacterial degradation of lignin. Enzyme Microb. Technol. 10: 646–655
[5 Des 2008].
Yopi, Purnawan A, Thontowi A, Hermansyah H, Wijanarko A. 2006. Preparasi mannan dan mannanase kasar dari bungkil kelapa sawit. Jurnal Teknologi
Zahari MW, Alimon AR. 2005. Use of palm kernel cake and oil palm by-products in compound feed. Palmas Journal 26(1): 5–9.
Zhu Y, Yang ST. 2004. Effect of pH on metabolic pathway shift in fermentation of xylose by Clostridium tyrobutyricum. Journal of Biotechnology 110: 143–157.
Appendix I. Crude protein analysis (Kjeldahl method)
Procedure
Weigh 0.1 g dry sample in a piece of alumunium foil (MS, sample mass) and transfer in glass tube kjeldahl.
Mineralization
1. Add half of kjeltabs®, 3 pieces boiling stone and 10 mL H2SO4
2. Transfer to mineralization system with neutralization system, cover and heat on (temperature 375
96% (1).
0
3. Open the cover and remove kjeldahl tube, than allow cooling. C) for 60 minutes.
Distillation
1. Heat up on distillation apparatus. Repeat blank distillation until output reaches at least 80 mL.
2. Transfer glass tube kjeldahl to distillation apparatus. Add 30 mL of water and NaOH 40% until sample solution in tube turn to brown or dark blue solution. 3. Place down 100 mL erlenmeyer containing 10 mL indicator solution and a
magnetic stirrer in output system. 4. Start distillation during 5-6 minutes.
5. Do blanko and standard to the same procedure distillation. For blank, use a clean tube and add 30 mL of water plus 20 mL NaOH 40%. For standard, add 5 mL (5 mg N) standard solution 1/28 M (NH4)2SO4 in clean tube, then add 30 mL of water plus 20 mL NaOH 40%.
Titration
1. Remove the 100 mL erlenmeyer containing ammonia extracted from output system to titration apparatus.
Calculation
Standard measurement:
ABSTRACT
IKA AYUNINGTYAS. Biochemical and Microbial Changes during Palm Kernel Meal (PKM) Fermentation. Under direction of ANJA MERYANDINI and PASCALE TALAMOND.
Fermentation was the first step of bioconversion process of palm kernel meal (PKM). The fermentation was done in close canister to minimize the environmental effect. The study was conducted to investigate total microorganism and biochemical changes during PKM fermentation in a closed canister. Fourty two kg of PKM were mixed with 84 L of water and put in the big drum (height 81 cm; diameter 48 cm). The fermentation height in drum is 80 cm. The sample was taken in the depth of ±40 cm from the surface. At interval 12 hours during 7 days take a sample for microbial analysis, pH, total acid, temperature, fibre, protein content, D-mannose, and volatile organic acid. The highest number of aerobic bacteria, 17.2 log10 CFU/g, was found at 24 hours of fermentation PKM. The highest total anaerobic bacteria, 17.3 log10 CFU/g, were found at 72 hours of fermentation PKM. No fungi was found before 96 hours of fermentation and the highest number found, 6.4 log10 CFU/g, was at the end of fermentation PKM. The pH of fermentation decreased after 48 hours from 4.56 to 4.28. The decreased of pH followed by increased total acid concentration from 0.19 to 0.22 mMol/mL after 48 hours of fermentation PKM. After 168 hours of fermentation PKM, the protein content increased 4.79%; while the concentration of hemicellulose, cellulose, and lignin decreased 10.79%, 10.77%, and 51.34%, respectively. The highest mannose concentration, 3.297±0.13 g/L, was found after 36 hours of fermentation PKM. The dominant volatile organic acid is acetic acid with a concentration of 87.89 mMol after 72 hours of fermentation PKM.
I. INTRODUCTION
1.1 Background
One of the problems that occur in aquaculture is the shortage of fish meal as a source of protein in aquafeed. The production rate of aquafeed today could not keep up with the increased demand of fish meal in aquaculture and competition for its use in livestock and poultry feeds, thus the price becomes
expensive. Data from Indexmundi
fishmeal price since the last 10 years from US$ 545/metric ton on august 2001 up to the level of US$ 1455/metric ton in august 2011. This situation lead to the increase of production costs in aquaculture. The solution to this increased price and feed shortage is to find alternatives protein source that could substitute fish meal.
Some by products from agriculture industry are known to have relatively high protein content, like palm kernel meal (PKM); which is by product from palm oil industry (Sundu & Dingle 2003). Since 2006, Indonesia has become the largest producer of palm oil, surpassing Malaysia. In 2006, it reached 44% (15.9 million tons) of world palm oil production (USDA 2007). In Malaysia palm oil industry, the extraction of palm kernel produces 53.3% of PKM (Ng 2003). Therefore supply of PKM in Indonesia overabundance.
PKM contain 13-22 % of crude protein, but as with most plant-based and oil seed meal ingredients, several factors can limit the incorporation of PKM into fish diets (Sundu & Dingle 2003). PKM contain high fiber content and non-starch polysaccharides (NSPs), thus its usage is limited to monogastric animals, such as fish (Ng 2003). Zahari and Alimon (2005) recommended used of PKM in mixture diet for freshwater fish only 10-20%. Yann (2009 unpublish data) concluded that use PKM in nutritive ingredients of feed for Pangasius djambal (catfish) should be less than 18%.
content of PKM was increased from 16.86% to 31.27%. The fiber content was decreased from 15.12% to 14.51%. Later, Ng et al (2002) used the fermented PKM as a dietary ingredient for red hybrid tilapia (Oreochomis sp.). Their results showed the hybrid tilapia that was fed fermented PKM-based diets at all dietary inclusions tested showed the poorest growth, and this could indicate the presence of anti-nutrients in the resultant fungal biomass.
Hem et al. (2008) tried another method using bioconversion process to added value of PKM. Bioconversions are a process of converting or changing an organic material into other useful products and has added value by taking advantage of events and the biosynthesis or the formation biolysis or solutions (Sukaryana et al. 2010). Through bioconversion process, nutrient from PKM converted into biomass of Hermetia illucens larvae (maggot). These larvae contained 42% crude protein and 30% crude fat (Hem et al. 2007). Septivia (2008) demonstrated that Botia fry (Chromobotia macracanthus Bleeker) showed the best growth by feeding 5 days old larvae, compared to bloodworm and earthworm. From the report of the results of trial production of larvae in Sungai Gelam (Jambi), it is known that the price-based pellets H. illucens larvae sold for Rp.3500 per kilogram (pricing Rp.200/kg PKM), is cheaper than the price of commercial pellets, namely Rp.7000 per kilogram (Hem 2009 unpublished data
The first step of bioconversion process mixes PKM and water (1:2) (Hem
et al. 2008). This process proposes to degrade polymer complex of PKM. PKM is very rich in polymer complex hemicellulose, cellulose and lignin (Düsterhöft et al. 1992). By including NSPs, the substrate consists of about 50% fiber (Knudsen 1997; Atasi & Akinhanmi 2009). The process of degradation polymers complex carried out by some type of microorganisms. Hemicellulose can be degraded by aerobic microorganisms such as genera Aspergillus and Trichoderma. Aerobic bacteria, such as Bacilli and Cellvibrio also have polysaccharides-backbone-degrading enzyme to breakdown hemicellulose. Clostridia, anaerobic bacteria, also have unique multi-enzyme complexes, the cellulosomes, which integrated many cellulolytic and hemicellulolytic enzyme to degrade the hemicellulose (Shallom & Shoham 2003).
The polymer degradation process is important because most of insects are unable to utilize cellulose and other plant polymers. It is because of the lack of the digesting enzyme (Chapman 1998). The process of degradation polymer will produce some volatile compound that stimulant incest to oviposition. Rachmawati (2010 & 2007) demonstrated that H. illucens will only lay their eggs in the PKM that has been fermented for 6 days or longer.
The production of H. illucens larvae for aquaculture development in Indonesia have been initiated in project design between Balai Riset Budidaya Ikan Hias (BRBIH) Depok and IRD (Institut de Recherche pour le Développement) since 2006. The step of degradation of polymer complex in bioconversion of PKM is done spontaneous and naturally. The natural fermentation was influenced by environmental factors, thus the result varies. For sustainability of H. illucens
larvae mass production, this process should be controlled. Therefore, the degradation process should be conducted in a closed canister to minimize outside environmental effects. Next, a starter containing PKM degrading microorganisms should be made. In order to make starter, the information concerning the biochemical and microbial changes during the fermentation should first be gathered.
1.2 Objective
II. LITERATURE REVIEW
2.1 Palm Kernel Meal (PKM)
Palm oil (Elaeis guineensis Jacq.) industry has two main products, crude palm oil (CPO) and palm kernel oil (PKO) and several by products, empty fruit bunch, palm fiber, sludge, and palm kernel meal (PKM). CPO is processed from fresh fruit of E. guineensis by extraction process. Fiber and sludge are by product of this process. Palm kernels are processed to produce PKO and by product of this process is PKM (Figure 1) (Hem et al. 2008; Poku 2002).
Figure 1. Production of palm oil and it’s by product (Hem et al. 2008; Poku 2002)
The by product from this industry is more abundant than the main product. Every 100 tons of fresh fruit bunch produced 24-28 ton CPO and 4 tons of palm kernel oil. It means that 72-76% fresh fruit weight will become the by-product of palm oil industry (Poku 2002). In Malaysia, 3 million tons of palm kernels are processed at palm kernel crushing plant into 1.4 million tons of PKO and 1.6 million tons PKM (Ng 2003).
2.1.1The Content of PKM
crude fiber (Alimon 2004). PKM is rich in some essential amino acid for fish like arginine and leucine, but deficient in methionine. The complete nutrient content of PKM is shown on Table 1.
Table 1. Proximate analysis (%) and amino acid content (g/16 g N) of PKM
Nutrient content References
Neutral detergent fiber (NDF) 66.8-78.9 62.6
Nitrogen-free extract 46.7-58.8
Amino acid content (g/16 g N)
Alanine 3.83
Essential amino acid for fish
2.1.2 Utilization of PKM
Some researcher tried to utilize PKM through various ways. Yopi et al.
(2006) used PKM as a substrate to produce functional oligosaccharide such as mannose and manno-oligosaccharide. Utilization of PKM for production of
-glucosidase and -mannase by Aspergillus niger FTCC 5003 in solid substrate
fermentation using an aerated column bioreactor was already tried by Abdeshahian et al. (2010a & 2010b). To produce bioethanol, Cerveró et al. (2010) tried to hydrolyze fermented PKM using Saccharomyces cerevisiae.
2.2Polysaccharides in PKM
Polysaccharides are carbohydrate containing many (hundred or even thousands) of monomeric unit called monosaccharides which connected by covalent bond called glycosidic bonds. Cellulose is a linear condensation polymer consisting of glucose units joined together by -1,4-glycosidic bonds. The glycosidic linkage acts as a functional group, and this along with the hydroxyl groups mainly determine the chemical properties of cellulose. The PKM contain 12% of cellulose. Cellulose has a low digestibility or even indigestible in poultry (Sundu & Dingle 2003).
Hemicellulose is structure of polysaccharides of plant cell wall, in close association with cellulose and lignin. Hemicellulose is mixture of polymer made up of sugars (mostly not glucose) and sugar derivative. The monomers of hemicelullose consist of three hexoses (glucose, galactose, and mannose) and two pentoses (xylose and arabinose). Most of the main-chain sugars on hemicellulose
structure are linked together by -1,4-glycosidic bonds (Moreira & Filho 2008). In
PKM the biggest content of hexoses is mannose (Cerveróa et al. 2010 & Düsterhöft et al. 1992). The complete sugar compositions of PKM are shown in Table 2.
Table 2. Sugar composition of PKM
According to the main sugar residue in the backbone, hemicellulose has different classification e.g., xylans, mannans, glucans, glucoronoxylans, arabinoxylans, glucomannans, galactomannans, galactoglucomannans, β-glucans, and xyloglucans. In total NSPs, PKM contain about 78% of mannans. On a dry matter basis, PKM have been estimated to have 30-γ5% of -mannans. Mannans in palm kernel resembles very much cellulose by being crystalline, hard and water insoluble. Mannans are found in the cell wall and impair the digestibility and utilization of nutrients either by direct encapsulation of the nutrients or by increasing the viscosity of the intestinal contents. This causes a reduction of the hydrolysis rate and the absorption of nutrients in the diet (Sundu & Dingle 2003).
Figure 2. Major type of linkages found in lignin (Bugg et al. 2011)
2.3 Polysaccharide Degrading Microorganisms
The biodegradation of polysaccharide structures involves the concerted action of a variety of hydrolytic enzymes. Some microorganisms have been identified having hydrolytic enzymes for cleavage of almost chemical bonds that found in plant structures.
Streptomyces coalicolor A3(2), Bacillus halodurans C-125, Bacillus subtilis 168, Cellvibrio japonicus, Clostridium acetobutylicum ATT 824, Bifidobacterium longum NCC2705, Xanthomonas axonopodis pv. Citri str. 306, Xanthomonas campestris pv campestris str. ATTC33913, Geobacillus stearothermophillus T-6, Geobacillus stearothermophillus 21 are known as bacteria having ability to produced hemicellulolytic enzymes (Shallom & Shoham 2003).