/
ISSN 0215 - 9318
JURNAL PENELITIAN BIOTEKNOLOGI PERKEBUNAN
Menara Perkebunan. 2000. 68(2) 37 47
Solubilization of insoluble phosphates by
Aspergillus niger
Pelarutanfosfat sukar larUE oleh Aspergillus niger LAKSMITA P. SANTII), D. H. GOENADrI), SISWANTOi),
I. SAILAI-e) & ISR0I3 )
I) Biotechnelogy Research Unit for Estate Crops, Bogor 1MSI, Indonesia
2) Bogor Agricultural University, Bogor, Indonp.sia 3) Graduate School, Boger Agricultural University, Bogor, Indonesia
Ringkasan
Penggunaan /angsung fosfat a/am (FA) ke da/am tanah sebagai sumber pupuk P te/ah dila-kukan se/ama bertahun-tahun melalui beberapa macam cara penggunaan. Kualitas FA di Indo-nesia umumnya rendah dan ketersediaan bahan baku yang berkualitas untuk produksi pupllk fosfat ter/arut relatif terbatas. Beberapa mikroba asal tanah yang dapat melarutkan fosfat anorganik lelah banyak dilaporkan. Nanwl1, informasi yang tersedia ten tang mekanisme peiarlltan P dari FA lokal asal Indonesia don P anorganik o/ell Aspergillus niger BCC FI94 belum banyak dite/iti. Satu seri penelitia."l laboralorium lelah dilaksana-kan untuk mengetahlli kelllamplian A. niger BCC FI94 melarutkan P. Evaillasi agronomi FA lokal (FA Cilellngsi dan Madura) di rllmah kaea juga telah diiakukan. A. niger BCC F 194 dapal melarutkan sumber P Sltkar larut, yailu FA Cileungsi dan Madura. serta senyawa CaJ(PO.J2 dan AIP04. Kelarutan P anorganik lersebut ber-hubungan dengan peningkatan aktivitas proton (ff) yang menyebabkan penurunan pH medium dan produksi asam organik. Asam organik ulama yang dihasilkan oleh A. niger BCC FI94 dalam medium cair Pikovskaya yang dimodiflkasi adalah asam oksa/at (3.75 mM). asam silral (2.0 mM). dan asam glukonat (O.Q mM). Kelarulan FA CilelIngsi I"bih besar dibandingkan dengan FA Madura. dan kelarulan CalPO)2 lebih besar dibandingkan kelarlltan AIP04• Tidak ada korelasi anlara kdarutan P anorganik dengan aktivitas enzim fosfatase. walaupun aktivitas enzim fosfa-lase cukup linggi terdeteksi dalam medium. Salu formula biosuperfosfat lelah berhasil dirakit
dengan mereaksikan FA lokal dengan supernatan kultur eair (SKC) pengganti Qsam sulfat. Hasil percobaan pada bibit kakao. kpret dan kelapa sawil di rumah kaea menunjukkan bahwa proto-lipe pupuk biosuperfosfa! dengan bahan baku FA Cileungsi dan Madura bentllk granul ma/lplln serbllk. memiliki ni/ai keefektifan agronomi yang relatif menyamai SP-konvensional.
Summary
SantI ct al.
decrcase and production of organic acid. The
major acidic mctabolites produced by A. niger
BCC F 194 in modified liquid Pikovskaya medium were oxalic acid (3.75 mM), citric acid (2.0 mM), and gluconic acid (0.9 mM). The solubi!ization of
Cileungsi RP was higher than that of Madura RP,
and the solubilization of C"3(P04h \\'flS better than
that of AIP04 . There is no correlation between
solubilization of inorganic
P
and ・ョNセケュ・@activities, although high level of activity of
phosphatase enzyme \Vas detectaJ!e in the
medium. A biosuperphosphate formula had heen
constructed by reac.ting local RP with liquid
culture supernatant (LCS) rcplacing sulfuric acid. In the green housc experiments using cocoa, rubber, and oil palm seedlings, both granular and powder biosuperphosphate prototypes showed a comparable relative agronomic effectiveness value to that of the conventional SP.
[Keywords: P solubilization, Aspergillus niger,
rock phosphate]
Introduction
Most rock phosphate deposits found in the world is classified as low reactive RPs and therefore, it cannot be used successfully as phosphorus (P) sources for crop produc-tion. The composition of these rock phos-phates varies from one deposit to another. Most of the world rock phosphates are-of sedimentary origin. Large rock phosphate
deposits estimated 1-2 million tonnes, are
distributed in different pans of Indonesia
(Moersidi, 1999). Since these deposits are
low in phosphorous content «25% P20 S) and
contain some impurities like oxides of Si, Fe, AI, and Ca, they are unsuitable for the
manufacture of superphosphate (Narsian el
al., 1993). The conventional method for en-hancing the rock phosphate availability is to increase its solubility by treating with inor-ganic acids, mainly sulphuric acid and phos-phoric acid but, this approach is not appli-cable because of high capital production
(Hammond et al., 1989, Goenadi el al.,
2000). A very attractive approach for rock phosphate solubilization is the appiication of microbes capable of excreting organic acids
(Gerke, 1992).
Many soil microbes are known to solu-biliL:e insoluble forms of inorganic phosphatic
compounds. [n vitrc studies with microbial
isolates from セッゥャ@ indicated that fungi were
more efficient in thc solubilization of
inor-ganic phosphate as compared 10 bacteria
(Thomas et ai., \985; Nahas 1996; Goenadi
el 01., 199LJ). Filamentous fungi are widdy
used as producers of organic acid:;, particular-ly A. niger and some Penicillium sp., which
have been tested i!1 fermentation systems or
inoculated directly into soil in order to
solu-bilize rock phosphate (Vassilev et al.. \997;
V asi leva et al., 1998).
Acidification of soil is a consequence of natural and anthropogenic processes (Ulrich
& Sumner, 1991). One of the outstanding changes in soil due to acidification is the
mobilization of Ae+ ions, which are toxic to
plants on one hand and cause chemical fixa-tion of plant-available P on the other hand. Hardly-soluble aluminum phosphates are for-med and become the largest P-fraction in
many acidic soils (McLean, 1976). AIP04• i:1
the contrary to calsiui7l phosphate, will never play an important role il1 ameliorating soils. Leaving that out of consideration, the
impor-tance of Alr04 solubilization fOf soil
forma-tion, mineral transformation and AI-toxicity
is obvious (lllmer et al., 1995). In this
inves-tigation, pure insoluble inorganic phosrhates
i. e. aluminum phosphate (A IP04 ) and ca
1-cium phosphate (CaJ(P04)2) have been used
for expressing phosphate :,olubi!izillg activity ofA. niger BeC f194.
Natural RPs has been recognized as a valuable alternative source for P fertilizer, especially for acid soils. The economic value of the rocks increases considerably along with the increasing costs of SP production
(Goenadi et al., 2000). Consequently, there is
Soiubilizatiol: if insoluble phosphates by Aspergillus niger
a growing interest in ways of manipulating such rock to obtaIn a more valuable product,
i.e. partially acidulating RPs (Goenadi, 1990; Rajan & Ghani, 1997), reacting with
syn-thetic organic acids (Sagoe et al., 1998) or
natural organic acids (Singh & Amberger,
1998), and decrea:;ing particle size (Babare et
aI,
1997). Goenadi et al. (2enO) repofled thatLiquid Culture Supernatant (LCS) instead of
H2S04 in superphosphate (SP) production
and using both with lower H)P04
concen-tration. to bioactivated Morrocan RP as raw materials increased the solubility of P in 2%
citric acid. It seemed that LCS could replace
H2S04 in the production of SP, and believed
to yield a more eco-friendly P fertilizer than conventional SP.
The objectives of this study were: (i) to determine phosphate solubilizing (PS)
acti-vity by A. niger BCC Fl94 on four types of
inorganic
P
compounds, (ii) to ・カセャオ。エ・@ effectof organic acids and phosphatase enzyme on RPs dissolution, and (iii) to conduct
agro-nomic evaluation of bioactivated CRP und
MRP for direct application in greenhouse experiment with cocoa, rubber, and oil palm seedlings as test crops.
Materials and Methods
Isolate
Aspergillus niger was isolated from Alfisols soils of Jeneponto, South Sulawesi,
Indonda (Goenadi et al., 1995). This soil
was highly weathered developing over vol-canic materials. Detailed characteristics of
the soil are, 31% sand, 27% silts, and 42%
clays; pH: 6.6 (H20), 5.3 (KC!); organic matter: 1.1% (C ), 0.1% (total N); 105 ppm P, 35 me/l OOg K; and clay mineral of smectite and kaoiinite. The' isolate was lod-ged in BaIitvet Culture Collection no. BCC F
194. Isolate was grown on Pikovskaya
medium in Petri dishes as a sou!'ce of ino-culum.
Phusphate solubilizing (PS) activity
Phosphate soluhilizing activity was assayed in 50 mL aliquots of standard Pikov-skaya's broth and modified PlkovPikov-skaya's
broth prepared by イ・セャ。」ゥョァ@ CalP04):
(19.9% P) with inorganic phosphate i.e.
AIP04 (50.8% P) and different rock
phos-phates, i.e. Cileung:;i (8.9% P) and Madura
(6.9% P). Cileungsi rock phosphate (CRP) and Madura ·rock phosphate (MRP) were ground (200 mesh) and oven dried. For this
purpose,
P
sourCes were sterilized separatelyand then mixed with the sterile Pikovskaya medium.
Two pieces of inocula (0 3 mm) wcre
inoculateri into Pikovskaya liquid medium containing 0.125 % (w/v) resp:::ctive phos-phorus sources, incubated on a mechan i::al shaker at 100 rpm, 28°C for nine days. The dissolved P was then determined by applying
the molybdenum-b!ue method of Olsen &
Sommers (1982). Absorbance was measured
using
a
Spectronic 21 spectrophotometer at693
urn.
Solubilization ofRPs in Pikovskaya medium
Modified Pikovskaya liquid medium supplemented with CRP or MRP of various concentrations (0,0.125,0.25,0.50, or 1.0 % (w!v)) was used. Inoculation was performed
by trans[.::rring two pieces of irocuia
(0 R mm) on to 100 mL eイャセュョ・ケ・イ@ flask
containing 50 mL culture medium. The cul-tures were ir:cubated on a mechanical shaker
at 100 rpm (28cC) for nine days.
The growth of the fungus was measured in standard procedure by drying the decanted
Santi et al.
veight at the end of incubation. Phosphorus
;olubilizing ability was detennined by using
he molybdenum-blue method of Olsen
&セッュュ・イウ@ (1982).
pH of the filtrates was
mea-;ured with Methrom pH meter and organic
.cid concentrations in the culture w<:re
、・エ・イセ@nined by using HPLC
(0 0 IN
H2S0
4mobile
,hase, 210 nm UV detector, 0.5 mLimin
lowrate,
at
50°C)(Cunningham
&Kuiark,
(992).
SfJect oforganic acids on RPs dissolution
This experiment was carried out to
;Iariry the relative strength of different types
0f organic acids in solubilizing P from the
eRP and MRP by using a method of IIImer
et .11. (1995).Citrate, oxalate and gluconate
were added separately in different
concen-trations
(0, 0.05, 0.50, 1.0, 3.0and
6.0mM)
to 50
mL Pikovskaya mt!dium containing
0.125%
(w/v)CRP or MRP, then incubated
for seven days on a mechanical shaker, at
100rpm
(28°C).P-concentration in
super-natant solutions was detennined Ilsing the
method of Olsen
&Sommers
(I982).Effect of phosphatase enzyme on RPs and inorganic P dissolution
The isolate of
A. nigerBCC F
194was
grown in
50mL modified Pikovskaya liquid
medium with vcrious levels of CRP or MRP
(0, 0.125, 0.25, 0.50and
1.0%(w/v)). The P
sources were sterilized separately and then
mixed with the sterile Pikovskaya medium.
The cultures were incubated for nine days on
a mechanical shaker at
100rpm,
(280C) for
the production of extracellular phosphatase.
Correlation between activity of phosphatase
(S0uciet
et al., 1980)and P-solubilization
was detennined. One enzyme unit is the
amount which catalyses the hydrolysis of
1!lmol of pNPP per min. under the
experi-mental condition.
Inactivation of phophatase in
Iiquid
cul-ture supernatant (LCS) of
A. nigerBCC
F194
was studied at temperature of
40, 50, 60, 70.80,
and
90°
C.Further,
50mL LCS
or
A. niger
BeC
F J 49contained inactivated
phosphatase was added with sterile
0.125%(w/v) AIP04
and incubated on mechanical
shaker at
100rpm, 28
°
Cfor 24 h. Activity of
phosphatase enzyme (Souciet
el al., 1980),P-solubilization, and concentration of organic
acid (mainly citric acid) were detennined.
Agronomic evaluation
of
bioaetivated CRP andMRPBioactivation was conducted by
reac-ting
8.5mL LCS with the highest contents of
organic acids (mainly citric acid from
A. niger
BCe F194 isolate) and
28mL
H3P04 52%(v/v) on
55 gRPs
(200mesh)
(Goenadi
et al., 2000).The most efficient
bioactivation of CRP and
MRP were
evaluated on the oasis of soluble P contents
in water and citric acid, as we II
as
perchlorate-extractable P content (SII
0029-73, 1984). Effectiveness of b0th granular and
powder biosuperphosphate prototypes in
substituting conventional P fertilizer,
i.e. SP-36,was determined on the basis of relative
agronomic effectiveness (RAE) (Mackay
elaI.,
1984) in a completely random design
experiment with four levels of P dosages,
i.e.
Solubilization of insoluble phosphaJes by Aspergillus niger ...
Results and Discussion
Phosphate solubilizing (PS) activity
A. niger BCC F 194 solubilized Ca3
(P0
4)2,
CRP, and MRP, but showed poorsolubilization of AIP04 in nine-day
incu-bation period. The amount ofP solubilized by
A. niger BCC F194 was directly related to the
decrease in pH of the medium, except AIP04
treatment. These phenomena suggested that PS activity depends on the types and
cons-tanta solubility product (Ksp) of insoluble
phosphate (Ksp for AIP04 セ@ 10.
30
and Ca3 (P04)2 - 2.0 x 10.2°) - as supposed by many
investigators (Mc Lean, 1976; Narsian et al.
1993; IIImer et al., 1995; Nahas, 1996).
Solubilization ofRPs in Pikovskaya medium
Employing a modified Pikovskaya
me-dium, A. niger BCC Fl94 produced oxalic
acid (3.75 mM), citric acid (2.0 mM), and gluconic acid (0.9 mM) as the main. organic acids from MRP and CRP as P source of
Pikovskaya medium lit 0 1.0% (w/v) level.
Regression analysis indicates that P-solubilization was highly positively corre-lated with organic acid concentration ( rMRP=
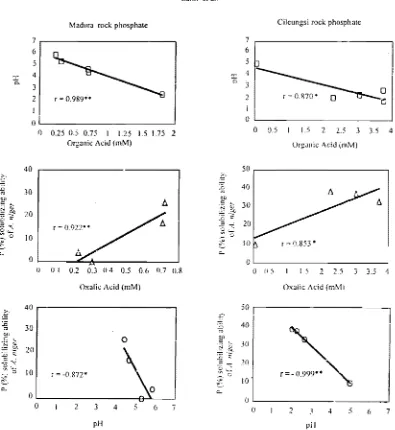
0.92** and fCRP=0.S5**) and ョ・ァ。エゥカセャケ@
correlated with the pH of the medium (rMRP=-0.87* and rCRP=-0.99**) (Figure 1). Organic acid concentrations were also strongly nega-tively correlated with the pH of the medium (rMRP =-0.99** and rCRP=-0.87*).
Effect oforganic acids on RPs dissolution
Production of organic acids is an impor-tant mechanis'11 for solubilizing inorganic ::>hosphate. Gluconic acid concentrations in Pikovskaya medium contained CRP and MRP as P source were comparatively con-;tant, whereqs the increased citrate and oxa-ate concentrations apparently reloxa-ated to the
increase in P-CRP and P-MRP concentrations
in solution (Figure 2). It indicates that citrate
and oxalate have strong influences to the solubilization of P-RPs, rCRP=O.96**, and rMRP=0.99** (citric acid) and rCRP=0.93** and
rMRP= 0.86* (oxalic acid). Oil the other hand,
no correlation between gluconk ;jcid wncen-tration and dissolution of CRP and MRP
(rcR.,,=0.6ns and rMRP= 0.1 ns) were found.
These phenomena lead to the assumption that
citric acid and oxalic acid produced by
A. niger BCC FI94 isolate are responsible [or lowering the pH of medium providing
pro-tons (W) to increase the P-RP solubilization.
In this case, there was relationship between the pKa values of the acids and the amounts of P released. Citric acid has a higher dis-sociation constant (pKa=3.14) than oxalic
acid (pKa = 1.25). Pohlman & McColl (198ii)
reported several factors that are important in determining the degree or rate of dissolution
of RPs,' i.e. : (i) rate of diffusion of organic
acid from bulk solution and diffusion of pro-ducts from the site of reactivity, (ii) contact time between the organic acids and mineral surface, (iii) degree of dissociation of organic acids, (iv) type and position of functional groups, and (v) chemical affinities of chelat-jng agents for the metals.
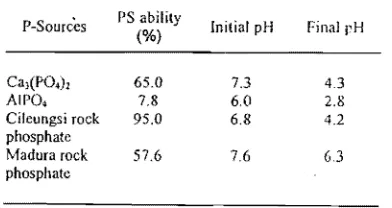
Table I. Solubilization of Ca3(PO.h, AIPO., Cileungsi rock phosphate (CRP), and Madura rock phosphate (MRP) by Aspergillus niger BCC FI94 in nine-day incubation
PS ability
P-Sources (%) Initial pH Final pH
Cal(PO')l 65.0 7.3 4.3
AIPO. 7.8 6.0 2.8
Cileungsi rock 95.0 6.8 4.2 phosphate
[image:6.599.369.565.551.655.2]Santi etal.
Cileungsi mck phosphate Madllra rock phosphate
7 6
7 イMMMMMMMMMMMMMMMMMMMMMセ@
6
5 5
4 :r: -1
:r: c.
3
0.3
2 2
I
oLI____________________
セ@ 00 0.5 1.5 2 2.5 3 3.5 4 (j 0.25 0.5 0.75 1.25 J.S 1.75 2
oイセ。ャャゥ」@ Acid (mM) Organic Acid (mM)
50 40
.2
30
10
O
:.c
m..g 30
ell Nセ@ セ@ . - CI) 20
:E .;:;
セNLN[@
0 ,
-セ@ 0 10
セ@
o
セ____
MFセセ ____________セ@c
o
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8Oxalic Acid (111M)
40r,
.f?
30 '"
'"
:IJ
c::
o
Zセ@
:g
'0 V">0.872*
セ@
c
oセ ______________セセセセ@
o
2 3 4 5 6 7pH
40
....
Nセ
'"
"
セ@
'0
() 0.5 1.5 2 2.5 3 3.5 4
Oxalic Acid (mMl
50
I
40
30
"
'"
Gセ@
20
"-'"""
C
イ]MoNYYYBセ
10
O
0 2 4 5 7
[image:7.599.168.563.119.549.2]pH
Figure I. The correlation between pH and organic acid production [top). P solubilizing ability of Aspergilus niger BCC
FI94 lind oxalic acid production (middle), and P SOlubilizing ab;lity and pH (bOUOln) with rock phosphate from
Madura and from Cilcul1gs: as
r
sources in Pikovskaya medillmSolubilization of insoluble phosphates by Aspergillus niger .. _
Madura rock phosph;;te
70
1
Ys 9,9409x -;- 1.5739
rs = 0.9992"
60
1
,
/ /
/
Yo 0.1904x +4,7402ro ojセVQWG@
Y ('
=
0.0672x + 4_25:' rG = 0_1049":/) 20 • Citric Acid
.6. Oxalic Acid
10
II •
Gluconic AcidlP=::::::::i:====!
O'F,r.,.-o
2 4 6Organic Acid Concentration (mM)
140
120
セ@
c
.e
EiuG
セ@
セ@ 8(; ::::
.,
u
c
0
u
A. 60
;,
:3
::l
0
Vi
20
0
Cileungsi rock phosphate
Ys = 17,964x + 9.5586
rs = 0,'.1600"
Yo =8.2758x1.3416 /
ro = 0,9318*'
YG = 0
iセ@
73x + 1;,8696/rc 0.6681 /
,;
1
40 I
•
• Citric Acid
lJ. Oxalic Acid
6. • Gluconic Acid
2
セ@
so
c.
e
セ@
40;:;
o
セ@
:,) 30
:c
'"
:::J
"0
0 4 6
Organic Acid Concentration (mM)
'igure 2. Relationship between concentration of selected org2nic acids (citric, oxalic, or gluconic) and
solubilization of rock phosphate from Madura and Cileungsi in modified Pikovskaya medium
セャヲ・」エ@ ofphosphatase enzyme on RPs and
'1organic P dissolution
Phosphate solubilization mediated by lhosphatase enzyme is believed to be taken Jlace for organic P sources (Bishop et al.,
994). There was no correlation between lctivity of phosphatase enzyme and P
;olubilization (rcRP=0.39"S and rMRP=O.22"'),
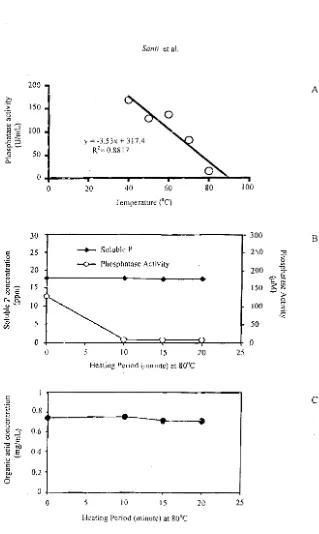
,s well as with P concentration (rMRP=O.39n$ md rCRP=0.23"S). The phosphatase activity 'ikovsakaya medium decreased at 80°C
15 U/mL) and 90°C (5.8 U1mL) (Figure 3A).
'::omplete denaturation of protein by heating Jrocess possibly occurred at 80°C, therefore lhosphatase enzyme was inactivated. The
corr"lation between phosphatase activity and AI P04 solubilization indicates that there was
no significant Psolubilizing value In liquid culture supernatant of A. niger BCC F 194 unheated (UH) and heated (H) at 80° C, although the phosphatase activity was dif-ferent, i.e. 127.3 U/mL (UH) and 7.9 U/mL (H), respectively. (Figure 3B). On the other
hand, the citric acid con<.entration was
relatively stahle at 020 minute of hearing periods (Figure 3C). These results were in agreement with the assumpHon that phos-phate solubilization mediated by phosphatase enzyme is taken place for organic P sources (Traina el oj., 1986; Bishop et 01., 1994; Sigh
Santi et a!.
200 A
a
150
Zセ@
u
セ
.,
'"
",....l100
セ@ E
.::;;:-
"'-セセ@
<.l 50
c::
0
100
Temperature (He)
• 300 B
250 ..",
:::r 0
"t:J
'"
200 :::r
セGB@
1= g 150 sZZセ@
セ^M
g
100 セZ@
Q
50
0
0 5 10 15 20 25
Heating Period (l11inute) at BOile
0
y -3.53x + 317.4 Rl_ 0.881.7
20 40 60 80
30
'"
0 25
·E
20;::
''
t )
RE
15P- o. 0.
0 " 10
:E
::J
<5 5
C/l
0
+ Soluble P
0 Ph0sphatase Activity
t:
c
.g
O.B
f
t:
g
•
..
0
t )
0.6
c
t: .::J
0 E
"t:J
-. on
gg
0.4 'ct )'"
on 0.2
0
00 10 15 20 25
[image:9.601.210.529.78.612.2]hセ。エゥョァ@ Period (,ninute) at 8U"e
Figure 3. Effect of temperature (A). and heating period (minute) セエ@
so·e
011 acti·.'ity or phosphatase enzyme (8). and effect of heating period M 80· C on organic acid (citric acid) concentration (e)•
Solubilization 0/ insoluble phosphates by Aspergillus niger, ,
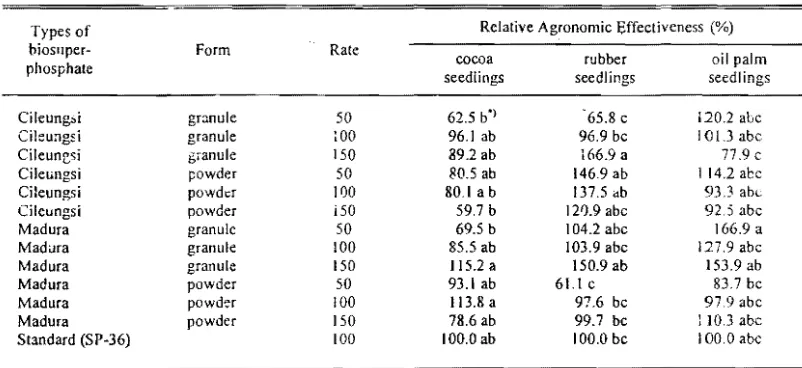
Table 2. Relative agronomic effectiveness of constructed effect ofbiosupcrphosphate (SPab) of cocoa, rubber, and oil
palm seedlings dry weight '
Relative Agronomic Effectiveness (%) Types of
biosllper- Form Rate
cocoa rubber oil palm
phosphate
seediings seedlings seedlings
CHeung:>i granule 50 62.5 b') 65.8 c 120.2 abc
Cileungsi granule :00 96.1 ab 96,9 bc IOLJ abc
cゥャ・オョセウゥ@ granule 150 89.2 ab 166.9 a 77.9 c
CiJeungsi powder 50 SO.5 ab 146,9 ab 1142 abc
Cileungsi pOWder 100 80.1 a b 137,5 db 93J abc
Cileungs! powder i50 59,7 b 121).9 abc 92.5 abc
Madura granule 50 69.5 b 104.2 abc 166.9 a
Mad<Jra granule 100 85.5 ab 103.9 abc 127.9 abc
Madura granule 150 115.2 a 150.9 ab 153.9ab
Madura powder 50 93.1 ab 61.1 c 83.7 be
Madura powder 100 113.8 a 97,6 be 97,9 abc
Madura powder 150 78,6 ab 99.7 be 1103 abc
Standard (SP-36) 100 100.Oab 100,0 be 100,0 abc
Note: *) Figures in each column/ollowed by the same leller (s) are not Significantly different (P<O.05) according to Duncan's multiple range lest
Agronomic evaluation of bioactivated CRP and MRP for direct application in green-house experimem
Application of biuactivated CRP and MRP resulted in significant increase of leaf
number dod height of the three-month old
co-coa and oil pCllm seedlings. For rubber seed-lings, significant responses have been obser-ved staning at four months after treatment (P<0.05).
There were no significant different-ces between conventional SP and 「ゥッウセー・イᆳ
phosphate (SPab) applied on height, leaf number, girth, and dry weight of cocoa, rubber, and oil palm seedlings (unpublished
data). At the end C'f the experiment (four
months after treatment for cocoa and rub!Jer, and six months for oil palm seedling), CRP
and MRPoriginating SPab In granular and
powder form applied at 100% rate equivalent to standard SP application indicated a higher value of relative agronomic effectiveness
(RAE) (Table 2).
Conclusions
A. niger DCC F 194 performed high abilities in solubilizing inorganic phosphate
(>50%), i.e. Ca3 (P04)2 , CRP, and MRP, but
not for AIP04• Citric and oxalic acids were
important components responsible for
phos-phate solubilization by A. niger BCC FJ 94.
Oxalic acid was the main organic acid
pro-duced by A. niger BCC F194 in modified
Pikovskaya medium', for nine days incubation at 100 rpm, 2S°C. Citrate and oxalate had
strong influences to the solubilization of CRP
and MRP. There was no correlation between phosphatase activity and solubilization of inorganic phosphate. Results of cocoa, rub-ber, and oil palm seedlings experiments in greenhouse showed that the prototype pro-ducts from bioactivation (biosuperphosphate) showed a comparable relative agronomic effecttiveness value to that of the conven-tional SP (SP36).
[image:10.601.163.564.168.352.2]Santi ct al.
Acknowledgment rocks with a P soluLilizing fungus. Soil. Sci.
The authors wish to thank the Riset Unggulan Terpadu VII Project Management
of the Ministry..ofResearcll セョ、@ Technolo.gy
for financial support (Contract No.: 17/SPK!
RUT/BPPT/IV/2000).
References
Babare, /\.M., P.W.G. Sale, N. Fleming, D.L. Garden & D. Johnson (1997). The agro-nomic effectiveness of reactive phosphate rocks. 5, The effect of particle size of a moderately reactive phosphate rock, Aus. 1.
Exp. Agric., 37,969·984,
Bishop, M.L, AC. Chang & R.W.K. Lee (1994), Enzymatic mineralization of organic phos-phorus in a volcanic soil in Chile. Soil BioI. Biochem., 157,238-243.
Cunningham, J.E. & C. Kuiack (1992). Produc-tions of citric and oxalic acids and solubili-zations of calcium phosphate by Penicillium bilaji. App. Env, Microbial., 58(5),
1452-1458,
Gerke, L. (1992), Phosphate, aluminium, and iron in solution of three different セッゥZウ@ in relation to varying ('uncentration of citric acid,
1. Plant Nutr, Soil Sc .. 155, 339-343, Goenadi, D.I-I. (1990). Effect of acidulation on the
mineralogical characteristics of a comercial phosphate rock. lndon. 1. Trop. Agric., 2,
1-5. '
Goenadi, D.H., R. Saraswati, N.N. Nganro &
.lAS, Adiningsih {I 995), Mikroba pelamt
hara dan pemantap 'agregat dari beberapa lanah tropika basah. Menaru Perkebunan, 63(2),60-66.
Goenadi, D.H., R.A. Pasaribu, Isroi, H, Hartono, & R. Misman (1999). Phosphate-solubil-izing fungi isolated from tropical forest soils. Menara Perkebunan, 67( 1).40-51.
Goenadi, D.I-I., Siswimto & Y. Sugiarto (2000). Bioactivation of poorly soluble phosphate
Soc. Am. J., 64, 927-932
Hammond, L. L., S.H Chien & A.U. Mokwunye (1989). Agronomic value of unacidulated and partially acidulated phosphate rocks indigenous to the tropics. Adv. Agr., 40, 89-140,
JIlmer, P., A. Barbato & F. Schinncr (1995), Solubilization of hardly-soluble AIPOd w!th P-solubilizing micloorganisms. Soil Bhl. Biochem, 27 (3), 265-270.
Mackay, A.D.,.I.K. Syers & P.E.H. Gregg (1984). Ability of chemical. extraction procedures to assess the agronomic effectiveness of phos-phate rock materials. New Zealand .f. Agric. Res., 27, 219-230.
McLean, E,O. (I 976}. Chemistry of soil alumi-nium. Communications in Soil Sci. & Plant Anal., 7, 619-636.
Moersidi (1999). Fo;fat Alam sebagai Bahan Baku dan Fupllk Fosfat. Bogor, Pusat Penelitian Tanah dan Agroklimat, pp 82.
Narsian, V" 1. Thakkar & H.B. Patel (1993). Solubilization of natural rock phosphates and pure i'1soluble inorganic phosphates by Aspergillus awamori. Ind. J Exp. BioI., 31, 747-749.
Olsen, S.R. & LE. Sommers (1982). Phosphorus. In Page, A.L.. R.H, Miller & D.R. Keeney (Eds.) Methods of Soil Analysis. Agronomv series. No.9. Madison, American Society of Agronomy, p. 403-430.
Pohlman, A.A. & .I.G. McColl (1986). Kinetics of metal dissolution from forest soils by soluble organic acids. J. Environ. Qua!', 15,86-92.
Solubilization of insoluble phosphates by Aspergi!lus ョゥゥ[セイ@ .
Sagoc, Ct, T. Ando, K. Kouno & T Nagaoka. (1996). Response of italian ryegrass to phosphorus in organic - acid treated phos-phate rocks. 1. Fac. Appl. Bioi. Sci., 35, 199-209.
Singh, CPo & A. Affiberger (i 998). Organic acids and phosphorus solubilization in straw
COIll-posted with rock ph0sphate. Bioresollrce Technol., 63( I), 13-16.
Souciet, G., 1. Attias & 1. d' Auzac (1980). A neutral cytoplasmic phosphatase from the latex of Hevea brasiliensis. Phytochem., 19, 20Q9-2102.
Thomas, G.V., M.V. Shantaram & N. Saraswathy (1985). Occurrence and activity of phos-phate-solubilizing fungi from coconut plan-tation soils. Plant & Soil, 87, 357·364.
Traina, SJ, G. SJ:osito, D. Hesterberg &
u.
Ka!kafi (1986). Effects of pH and organic acids on orthophosphate solubility in acidic, montmorillor.ltic soil. Soil Sci. Soc. Am. J.,50,45-51.
Ulrich, B. & M.E. Sumner (1991). Suil Acidity. Berlin, Springa- Verlag.
Vussiiev, N., M. Vasslleva & R. Azcon (1997), Solubilization of rock phosphate hy immo-bilized Aspergillus niger. Bioresource i'echnol., 59, 1-4.
Vassileva, M., R. Azcon, J. Barea & N, Vassilev (1998). App!ication of an encapsulated filamentuus fungi in solubilization of inorganic phosphate. 1. Biotechnoi.. 63 (I), 67·72.
•
..
Menara Perkebunan, 2000 68(2)
CONTENTS
Research Reports
Page
Transfonnation of Cojlea arabica using chitinase gene and regeneration of plantlets from transformed-zygotic embryos (Transformasi cッュセ。@ arabic a menggunakarl gen kinitase dan regenerllsi planlet dad embrio zigotik haL<£! transfarmasi) - A. Budiani,
T. Chaidamsari, Priyono, S. Mawardi & Siswanto ... .
I 11
Overexpression of chitinase gene with a GCrich synthetic enhancer in tobacco plant
(Nicotia't/a tabacum) (Overeskpresi gen kinitase dengan enhancer sintesis kaya GC pada tanaman tembakau (Nicotiana tabacum) D. SantoslJ, T. Chaidamsari, A.
Budiani, H. Minarsh, S. Dwi Utomo & Siswanto ... .. 12 20 Development of tobacco plant cells
in
the presence of kanamycin at various levels fortransgenezis (Perkembangan sel tanaman tembakau pado berbagai konsentrasi
kanamisin untuk transgenesis) - D. Santoso, Ferry I Cugito & H. Minarsih ... .. 21 28
Extraction and characterization of humic acid from plantation's solid organic waste composts (Ekstraksi dan karakterisasi asam humat dari kompos limbah padat organic
Perkebunan) Laksmita P. Santi, D.H. Goenadi, H. Widiastuti, N. Mardiana & Isroi 29 36
Solubilization of insoluble phosphates by Aspergillus niger (Pelarutan fosfat sukar larut oleh Aspergillus niger) Laksmita P. Santi, D.H. Goenadi, Siswanto, I. Sailah &