i
ACTIVITY OF BIOACTIVE COMPOUNDS
FROM SPONGE-ASSOCIATED BACTERIA
ON HUMAN LEUKEMIC CELL LINES
AI KARWATI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
i
STATEMENT OF ORIGINALITY
I hereby declare that this thesis entitled “Activity of Bioactive Compounds from Sponge-Associated Bacteria on Human Leukemic Cell Lines” is the result of my own work through the guidance from my academic supervisors and has not been submitted in any form for another degree at any other university. Sources of information derived from published and unpublished works of other authors is mentioned in the text and listed in the list of references at the end of this thesis.
I hereby assign the copyright of my thesis to Bogor Agricultural University.
Bogor, December 2015
RINGKASAN
AI KARWATI. Aktivitas Senyawa Bioaktif dari Bakteri yang Berasosiasi dengan Spons terhadap Sel Leukimia. Dibimbing oleh ARIS TRI WAHYUDI, NAHROWI RAMLI dan JUN NOMURA.
Spons dilaporkan sebagai penghasil utama senyawa alami yang berasal dari laut. Namun, spons belum siap untuk dikembangkan lebih lanjut terkait dengan ketersediaannya yang dibutuhkan dalam jumlah besar dan berkelanjutan. Diketahui hampir 40-60% dari total biomassa spons adalah mikroorganisme, maka isolasi bakteri yang berasosiasi dengan spons dapat menjadi salah satu alternatif untuk menghasilkan berbagai senyawa bioaktif dalam jumlah besar melalui pengkulturan mikroba. Beberapa penelitian menunjukkan bahwa mikroorganisme yang berasosiasi dengan spons merupakan penghasil senyawa bioaktif tersebut. Bioaktif inimemiliki banyak potensi diantaranya sebagai antibakteri, antifungi, antivirus, immunosupressant, antitumor, dan antikanker.
Kanker merupakan sel abnormal yang tumbuh akibat mutasi genetik baik itu yang bersifat turunan maupun yang di induksi oleh lingkungan. Leukemia adalah salah satu sel kanker yang terjadi pada sel darah. Saat ini, kanker berhasil ditanggulangi dengan cara operasi dan kemoterapi. Meskipun pengangkatan sel kanker secara fisik melalui operasi merupakan usaha optimal, namun sangat sulit untuk menghilangkan sel kanker secara menyeluruh dan dapat menyebabkan penyebaran sel kanker ke organ yang lain. Sementara itu, kemoterapi memiliki banyak efek samping. Pengembangan antikanker baru yang tidak memiliki efek samping sangat diperlukan untuk penanggulangan kanker. Beberapa bahan bioaktif yang diperkirakan memiliki aktivitas melawan kanker ditemukan pada beberapa organisme, salah satunya adalah bakteri. Tujuan dari penelitian ini adalahmengisolasi senyawa bioaktif dari bakteri yang berasosiasi dengan spons dan menguji aktivitasnya melawan sel leukimia.
Bakteri yang berasosiasi dengan spons (SAB) dengan kode SAB E-35, E-38 dan SAB E-40 telah diisolasi dari sponge Jaspis sp. yang berasal dari kepulauan Raja Ampat menunjukkan aktivitas antimikroba terhadap Eschericchia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans dan C. tropicalis. Bakteri tersebut juga telah diuji memiliki sitotoksisitas terhadap Artemia salina melalui uji Brine Shrimp Letality Test (BSLT). Perlu dilakukan ujilanjutan untuk menganalisis aktivitasnya melawan sel kanker, salah satunya leukemia.Sel leukemia yang diujikan pada penelitian ini adalah U937, MOLT4, K562, HL60, Daudi dan CEM6.
iii
toksisitas yang beragam pada sel kanker leukemia. Berdasarkan nilai IC50, SAB E-40 (IC50 = 191.512 μg/ml) memiliki kemampuan paling kuat untuk menghambat sel U937. SAB E-40 juga menunjukkan penghambatan terkuat pada K562, Daudi dan CEM6 dengan nilai IC50 berturut-turut 214.467 μg/ml, 222.138 μg/ml dan 233.963 μg/ml. Untuk sel MOLT4, efek toksisitas terkuat ditunjukkan oleh SAB E-35 dengan nilai IC50 263.93 μg/ml. Untuk sel HL60, efek toksisitas terkuat ditunjukkan oleh SAB E-38 dengan nilai IC50 sebesar 294.923 μg/ml. Beberapa fraksi hasil fraksionasi melalui KLT dan KK menunjukkan efektifitas fraksi dengan nilai IC50 yang lebih rendah daripada ekstrak kasarnya.
Uji apoptosis ditentukan dengan menggunakan Annexin V-FITC Apoptosis Detection Kit. Selanjutnya, analisis siklus sel ditentukan dengan menggunakan pewarna DNA Propidium Iodida kemudian dianalisis menggunakan flow cytometer. SAB E-35 menunjukkan efek apoptosis terkuat pada sel MOLT4 berdasarkan kemampuannya menurunkan jumlah sel yang hidup sampai tersisa 21,6% dan sebanyak 37,3% sel berada pada fase akhir apoptosis. Analisis siklus sel pada sel-sel yang diinkubasi dengan ekstrak mengakibatkan penurunan jumlah sel di fase S, G0 / G1 dan G2 fraksi / M, sebaliknya terjadi akumulasi sel dalam fraksi sub-G1. Akumulasi sel dalam fraksi sub-G1 menunjukkan peningkatan fragmentasiDNA pada proses apoptosis.
Hasil penelitian ini menunjukkan bahwa fraksi Amicon dapat memicu pertumbuhan sel kanker sedangkan ekstrak etil asetat memberikan pengaruh sitotoksisitas pada sel leukemia. Hal ini mengindikasikan bahwa terdapat perbedaan jenis senyawa bioaktif yang terisolasi berdasarkan metode yang digunakan. Hasil lainnya menunjukkan bahwa apoptosis terjadi pada MOLT4 yang diberi perlakuan ekstrak, dikonfirmasi oleh hasil analisis siklus sel. Hasil penelitian menunjukkan bahwa efek apoptosis dari SAB terhadap MOLT4 dapat memberikan kontribusi dalam aktivitas antikanker.
SUMMARY
AI KARWATI. Activity of Bioactive Compounds from Sponge-Associated Bacteria Against Leukemic Cell Lines. Supervised by ARIS TRI WAHYUDI, NAHROWI RAMLI and JUN NOMURA.
Sponge was reported as the best producers of new marine natural compounds. However, they are mostly not yet ready to be developedfurtherbecause obtaining continuous and large supplies.Since almost 40-60% total sponge biomass are microorganisms, isolation of sponge-associated bacteria is an alternative that can be used to produce a wide range of bioactive compounds in large quantities through the production of microbial culture. It is hypothesized that symbiotic marine-microorganisms harbored by sponges are the original producers of these bioactive compounds. Those bioactive compounds have potential as antibacterial, antifungal, antiviral, immunosuppressant, antitumor, and anticancer.
Cancer was characterized as abnormal cells that growth from a relatively small number of inherited or environmentally-induced genetic mutations. Leukemia is a cancer of the blood cells. Recently, cancers can be succesfully treated by surgery and chemoteraphy. While physically removing a tumor is optimal, it is difficult for a surgeon to get all of the cancer cell, it made probability that the cells may spread to other organs. The development of a new anticancer that lack of side effect would be important in cancer treatment. Many bioactive compound was expected to have activity against cancer cells have been found in every species including bacteria. The aims of this study were to isolate bioactive compounds from sponge associated bacteria and analyze their activities against human leukemic cell lines.
Sponge associated bacteria (SAB) coded by SAB E-35, SAB E-38 and SAB-E40 have been isolated from Jaspis sp. taken from Raja Ampat islands shown antimicrobe activity against Eschericchia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans and C. tropicalis. Those bacteria also have citotoxicity towards Artemia salinathrough brine shrimp letality test (BSLT). It can be used as samples for analyze their activities against leukemic cell lines. The cell lines that used in this study were U937, MOLT4, K562, HL60, Daudi and CEM6.
iii
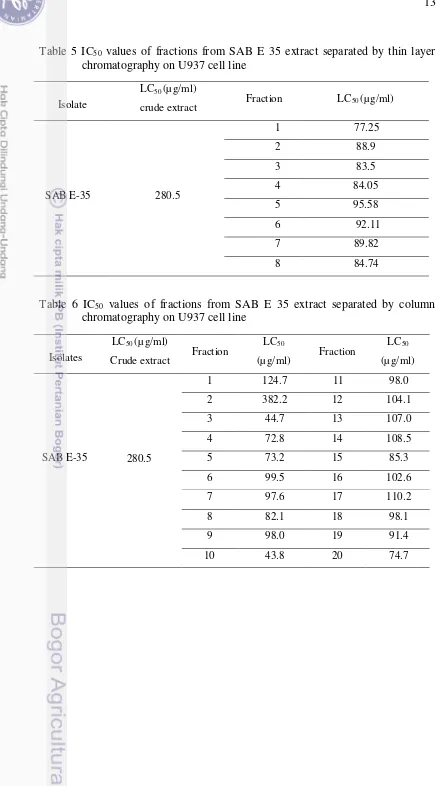
the MOLT4T4 cells, the strongest toxicity effect was showed by SAB E 35 with IC50 value 263.93µg/ml. For the HL60 cells, the strongest toxicity effect was showed by SAB E 38 with IC50 value 294.923µg/ml. Some of extract fraction through TLC and CC resulted the fractions that have IC50 lower than the crude extract.
Apoptosis assay was determined by using Annexin V-FITC Apoptosis Detection Kit. Further, cell cycle analysis was determined by using DNA specific dye Propidium Iodide and analyzed by using flowcytometry.SAB E 35 showed the strongest apoptosis effect on MOLT4 cell line based on its ability to decrease viable cells, that remained only 21.6 % and 37.3 % cells on their late apoptosis phase. Cell cycle analysis on cells incubated with extract resulted decrease in the number of cells in S phase, G0/G1 and G2/M fraction, in addition to the accumulation of cells population in sub-G1 fraction. The proportion of cells in sub-G1 fraction were accumulated indicating that apoptotic DNA cleavage was increased.
The results of this study demonstrated that bioactive compounds from sponge-associated bacteria could be a growth stimulator or inhibitor on leukemic cell lines depend on the isolation method carried. Other results indicated that apoptosis occurred in MOLT4 treated by the ethyl acetate extract, confirmed by the result of cell cycle analysis. The results suggested that the apoptotic effects of sponge-associated bacteria on MOLT4 can provide contribution to their claimed-anticancer activity.
© Copyrightof IPB, 2015
Copyright Reserved
Prohibited for quoting part or all of this thesis without including or citing the sources. Citation is only for educational purposes, research, scientific writing, report writing, criticsm writing, or review of an issue; and citations are not detrimental on behalf to IPB
i
Thesis
As one of the requirements to obtain the degree Master of Science
on
Microbiology Major
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
2015
AI KARWATI
ACTIVITY OF BIOACTIVE COMPOUNDS
FROM SPONGE-ASSOCIATED BACTERIA
(#8)8B)9.#B !9)<)9?B3$*3!:)<#B 3143;2"8B &31B 432'# 883!):#"B !:#5)B ')28:B #;-#1)!B #00B )2#8B
7#B B )B 5=:)B
B
53%B5B 5B !B
3;4#5<)835B
#"B 3%B)!53 )3/3'?B+35B
4453<#"B ?B
;4#5<)8)2'B 311)9:##B
5)B )B
)2B;4#5<)835B
)8!3<#5#"B ?B
53%B;2B31;6B"B("B 3;4#5<)835B
53%B5B B
9#B 3%>1)29)32B B
-:3 #5B
ACKNOWLEDGMENT
Praise and gratitude to Allah SWT for all of His gifts so this thesis has been completed. The tittle of this thesis is ―Activity of Bioactive Compounds from Sponge-Associated Bacteria Against Leukemic Cell Lines‖. I would like to say thanks to Prof. Aris Tri Wahyudi M.Si, Prof Nahrowi Ramli M.Sc and Prof. Jun Nomura MD, PhD as supervisor commission in this thesis research, great thanks to Prof. Anja Meryandini as Head of Microbiology Major and to Dr. Laksmi Ambarsari, MS as examiner beyond commission on the examination. I also thanks to the staff of the Laboratory of Microbiology, Department of Biology Bogor Agricultural University and the Laboratory of Training Division for School Health Nursing (Yogo) Teachers, Faculty of Education Chiba University. This research was partly supported by Insentif SINas 2015 from The Ministry of Research, Technology and Higher Education Indonesia awarded to ATW, and partly supported by academic scholarship from Directorate of Higher Education of Indonesia to AK. Thanks to TWINCLE Program that facilitated the exchange student between IPB-Chiba University.
During the college and research, I gratefully thanks to the family, Microbiology students batch 2013 and Nomura’s lab students who have helped during the research. I wished this research can be beneficial for the knowledge development in the furture.
Bogor, October 2015
iii
Sponge as Marine Natural Products 2
Marine Sponge-Associated Bacteria 3
Bioactive Compound from Sponge-Associated Bacteria 4
Leukemia 4
3 METHODS 6
Materials 6
Culture of Sponge Associated Bacteria 6
Culture of Leukemic Cell Line 6
Framework of Research 6
Time and Places 6
Fractionation of Bioactive Compound Using Amicon Filter 7 Extraction of Bioactive Compound Using Ethyl Acetate 7 Fractionation of Bacterial Extract Using Thin Layer Chromatography 8 Fractionation of Bacterial Extract Using Column Chromatography 8
Cytotoxicity Assay 8
Apoptosis Assay 8
Cell Cycle Analysis 9
4 RESULTS AND DISCUSSION 9
Fractionation of Bioactive Compound Using Amicon Filter 9
Cytotoxixity Assay Using Amicon Filter Fraction 9
Extraction of Bacterial using Ethyl Acetate 10
Cytotoxicity Assay of Ethyl Acetate Extract 11
Fractionation of SAB E 35 Extract Using Thin Layer Chromatography (TLC)
LIST OF TABLES
1 Clinically important bioactive compounds from sponge-microbe associations as anticancer and antitumor according to review by
Remya et al (2010). 1
2 Yield of fraction from sponge-associated bacteria 9 3 Yield of ethyl acetate extract from sponge-associated bacteria 11 4 IC50 values of crude extract from sponge-associated bacteria on cell
lines 12
5 IC50 values of fractions from SAB E 35 extract separated by thin layer
chromatography on U937 cell line 13
6 IC50 values of fractions from SAB E 35 extract separated by column
chromatography on U937 cell line 13
7 Apoptosis effect on MolT4 cells incubated with samples for 2 hours 15
LIST OF FIGURES
1 Flowchart of the research 7
3 Growth stimulation of fraction 1 SAB E 35 separated by pH
differences on U937 10
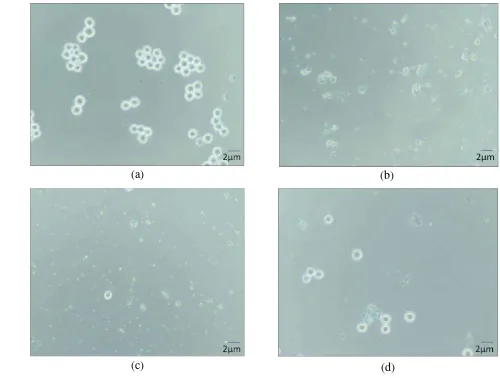
2 Growth stimulation of Amicon fraction of SAB E 35 on U937 10 4 Cytotoxicity effect of SAB E 35 (b), SAB E 38 (c), and SAB E 40 (c)
on MOLT4, using DMSO as control (a) 14
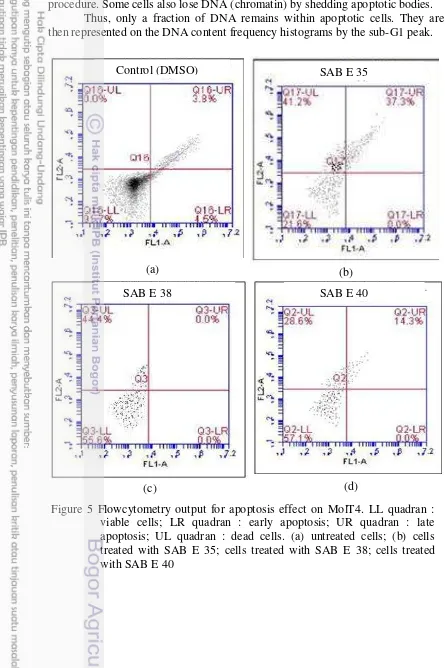
5 Flowcytometry output for apoptosis effect on MolT4. LL quadran : viable cells; LR quadran : early apoptosis; UR quadran : late
apoptosis; UL quadran : dead cells. (a) untreated cells; (b) cells treated with SAB E 35; cells treated with SAB E 38; cells treated with SAB E
40 16
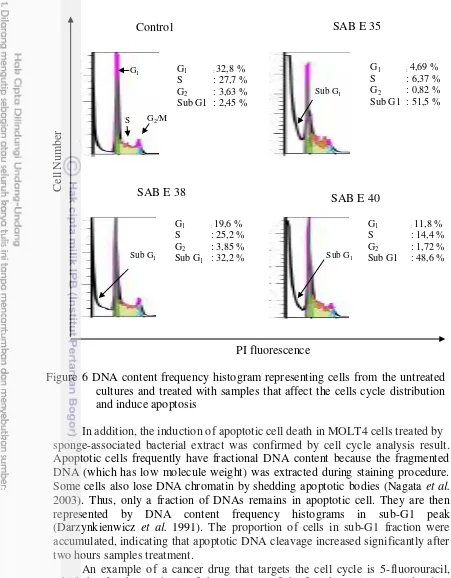
6 DNA content frequency histogram representing cells from the untreated cultures and treated with samples that affect the cells cycle
distribution and induce apoptosis 20
LIST OF APPENDICES
1 Cytotoxicity rate of SAB on leukemic cell lines 23
2 Calculation of % Cytotoxicity Rate and IC50 25
3 Retardation Factor (Rf) values of TLC fraction 26 4 Article published on International Journal of Pharmacy and
1 almost 40-60% total sponge biomass are microorganisms, isolation of sponge-associated bacteria is an alternative that can be used to produce a wide range of bioactive compounds in large quantities through the production of microbial culture (Wang 2006). Previous study proved that sponge associated bacteria are producer of diverse bioactive compound from sponge (Piel 2006). It is hypothesized that symbiotic marine-microorganisms harbored by sponges are the original producers of these bioactive compounds (Proksch 2002; Radjasa 2007; Zhang 2005; Newman & Hill 2006). Those bioactive compounds have potential as antibacterial, antifungal, antiviral, immunosuppressant, antitumor, and anticancer (Mehbub et al. 2014). Piel et al. (2004) reported that uncultured bacterium from sponge Theonella swinhoei taken from Hachijojima Island, Japan produced Onnamide A that potential as antitumor. Liu et al. (2005) reported that Saccharopolyspora sp. nov. (Actinobacteria) isolated from sponge Mycale plumose produced Metacycloprodigiosin and Undecylprodigiosin which can used as anticancer.
Cancer was characterized as abnormal cells that growth from a relatively small number of inherited or environmentally-induced genetic mutations. It can causing over 100 distinct diseases in many different tissues (Renan MJ 1993). Cancer figure among the leading causes of morbidity and mortality worldwide, with aproximately 14 million new cases and 8,2 million cancer related death in 2012 (WHO 2014). In Indonesia, cancer is major public health. Based on Basic Health Research 2007, cancer is the 7th cause of death among all causes of death (5.7%). National prevalence of cancer is 4.3 per 1000 population (Wahidin et al. 2012). Leukemia is a cancer of the blood cells, compared with other cancers such as lung cancer and breast cancer, leukemia was happened rarely. However, leukemia was the most commonly found in children, 32 % of cancer patients was a childrens under 15 years old and 74% of them was leukemia (Belson et al. 2007).
Recently, cancers can be succesfully treated by surgery and chemoteraphy. While physically removing a tumor is optimal, it is difficult for a surgeon to get all of the tumor cells which is generally embedded in normal tissues, it made probability that the cells of tumor may spread to other organs (Sadava et al. 2011). The development of a new anticancer that lack of side effect would be important in cancer treatment. Many bioactive compound was expected to have activity against cancer cells have been found in every species including bacteria.
inhibition against microbes (Putra 2012). Furthermore, Fadhillah (2013) analyzed its citotoxicity through brine shrimp letality test (BSLT) towards Artemia salina, the LC50 value was 117.1 µg/ml-620 µg/ml. There are 3 best isolates coded as SAB E 35, SAB E 38 and SAB E 40. Based on molecular identification SAB E 35, SAB E 38 and SAB E 40 are closely related to Providencia rettgeri YL, Bacillus aerius 24K, and Bacillus subtilis YRLD2, respectively. It can be used as samples for analyze their activities against leukemic cell lines.
Issue Formularization
The treatment of cancer through surgery and chemotheraphy have many side effect. Sponge-associated bacteria was excpected can produce bioactive compound that have potential as anticancer including leukemia. Those bacteria could be important in the development of a new anticancer that lack of side effect.
Aim of Research
The aim of this study was to isolate bioactive compounds from sponge-associated bacteria and analyze their activity on leukemic cell lines through apoptosis.
Benefit of Research
This study is important to give information about type of bioactive compound produced by sponge associated bacteria and its activities against cancer through in vitro assay. Further, this study may be needed to find new alternative for leukemia therapeutic agent.
Scope of Research
The scope of this research included the isolation of bioactive compounds from SAB and analyze their activities on leukemic cell lines. The mechanisms of cytotoxicity analysis included the relation between apoptosis assay and cell cycle analysis.
2
LITERATURES
Sponge as Marine Natural Products
3
organisms icluding microorganisms and phytoplankton, invertebrates mainly sponges, tunicates, bryozoans or mollusks, and algae. Among all the marine organisms investigated, marine sponges (Porifera) are recognized as the richest sources of new marine natural products contributing up to 29% of all marine natural products discovered (Blunt et al. 2014). This makes sponges the most marine producers of compounds with more than 200 new compounds reported each year for the last decade (Laport et al. 2009).
Sponges are filter feeders, having numerous tiny pores on their surface, which allow water to enter and circulate through a series of canals where microorganisms and organic particles are filtered out and eaten (Lee 2001). Since sponges are simple and sessile organisms; during evolution they have developed potent chemical defensive mechanism to protect themselves from competitors and predators as well as infectious microorganisms. Studies show that secondary metabolites in sponges play a crucial role in their survival in the marine ecosystem (Thakur & Muller 2004). Numerous studies of biological activities for these compounds, including antibacterial, antifungal, antiprotozoal, anticancer, antiviral, anti-inflammatory, immunosuppressive, antifouling and a range of other bioactivities have revealed (Blunt et al. 2005).
Marine Sponge-Associated Bacteria
Sponges showed as the best producers of new marine natural compound. However they are generally not ready for further development due to the challenge of obtaining continuous and larger supplies of the compounds. Considerable quantities of a drug candidate are vital for clinical trials, but only a few milligrams of most natural products can be isolated from marine samples (Kinghorn et al. 2009). One solution is to scaled up producing sponge compounds that are produced by sponge-associated microorganisms independently without growing the sponges. Sponges are well known to be hosts for a large community of microorganisms, which comprise a significant percentage (up to40–60%) of the biomass of the sponge host (Wang 2006). The role of these diverse microbes in sponge are various from source of nutrition to mutualistic symbiosis with the sponge (Kenedy et al. 2009).
symbiosis, however, is the involvement of bacteria in the production of bioactive metabolites (Thoms & Schupp 2005) that have a role in defense (Proksch 1994).
Bioactive Compound from Sponge-Associated Bacteria
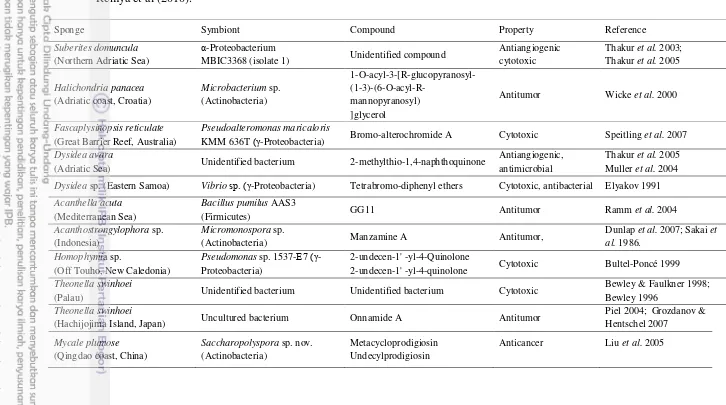
Metabolites produced by sponges and their associated microorganisms can be classified chemically as alkaloids, terpenoids, glycosides, phenols, phenazines, polyketides, fatty acid products and peptides, amino acid analogues, nucleosides, porphyrins, aliphatic cyclic peroxides and sterols (Thakur & Muller 2004; Tilvi et al. 2004). The chemical diversity of bioactive compounds reportedly produced by sponge-microbe associations showed that certain chemical classes such as quinones, steroids, fatty acids, diketopiperazines, alkaloids, terpenes, terpenoids, trichoverroids and prodigiosin derivatives, diglucosyl-glycerol, polyketides, cyclopeptides, glycoglycerolipid, benzoic acid derivatives are responsible for anticancer or antitumor activity (Thomas et al. 2010). Some of clinically important bioactive compounds from sponge-microbe associations as anticancer and antitumor according to review as shown in Table 1.
Leukemia
Cancer was characterized as abnormal cell that growth from a relatively small number of inherited or environmentally-induced genetic mutations (Renan MJ 1993). One of cancer cells is blood cell cancer i.e. leukimia. Leukemia arises in the bone marrow from cell lineage that form the immune cells that circulate in the blood (Harris 2015).
1
Table 1 Clinically important bioactive compounds from sponge-microbe associations as anticancer and antitumor according to review by Remya et al (2010).
Sponge Symbiont Compound Property Reference
Suberites domuncula (Northern Adriatic Sea)
α-Proteobacterium
MBIC3368 (isolate 1) Unidentified compound
Antiangiogenic
Antitumor Wicke et al. 2000
Fascaplysinopsis reticulate (Great Barrier Reef, Australia)
Pseudoalteromonas maricaloris
KMM 636T (γ-Proteobacteria) Bromo-alterochromide A Cytotoxic Speitling et al. 2007
Dysidea avara
(Adriatic Sea) Unidentified bacterium 2-methylthio-1,4-naphthoquinone
Antiangiogenic, antimicrobial
Thakur et al. 2005 Muller et al. 2004
Dysidea sp. (Eastern Samoa) Vibrio sp. (γ-Proteobacteria) Tetrabromo-diphenyl ethers Cytotoxic, antibacterial Elyakov 1991
Acanthella acuta (Mediterranean Sea)
Bacillus pumilus AAS3
(Firmicutes) GG11 Antitumor Ramm et al. 2004
Acanthostrongylophora sp. (Indonesia)
Micromonospora sp.
(Actinobacteria) Manzamine A Antitumor,
Dunlap et al. 2007; Sakai et al. 1986.
2-undecen-1' -yl-4-quinolone Cytotoxic Bultel-Poncé 1999
Theonella swinhoei
(Palau) Unidentified bacterium Unidentified bacterium Cytotoxic
Bewley & Faulkner 1998; Bewley 1996
Theonella swinhoei
(Hachijojima Island, Japan) Uncultured bacterium Onnamide A Antitumor
Piel 2004; Grozdanov &
Anticancer Liu et al. 2005
3
METHODS
Materials
Culture of Sponge Associated Bacteria
Bacterial isolates used in this study were SAB E 35, SAB E 38 and SAB E 40 isolated from Jaspis sp. taken from Raja Ampat Islands, Indonesia. Those bacteria have ability to produce antimicrobial substances against Staphylococcus aureus, Vibrio harveyii, Escherichia coli, Pseudomonas aeruginosa, EPEC K-11, Candida albicans and C. tropicalis (Abubakar et al 2011). The bacteria were cultured in Sea Water Complete (SWC) media (Pepton 5 g/L; Yeast Extract 1 g/L; Gliserol 3 ml/L; Sterilized Sea Water 750 ml/L; Aquades 250 ml/L; Agar Bacto 15 g/L).
Culture of Leukemic Cell Line
The U937, MOLT4, K562, CEM6, Daudi and HL60 cell line was obtained from RIKEN Cell Bank, Japan and was established by Laboratory of Training Division for School Health Nursing (Yogo) Teachers, Chiba University. Cell line were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and 1% penicilin and streptomycin. Cell line were maintained at 37oC in a 5% CO2 atmosphere with 95% humidity.
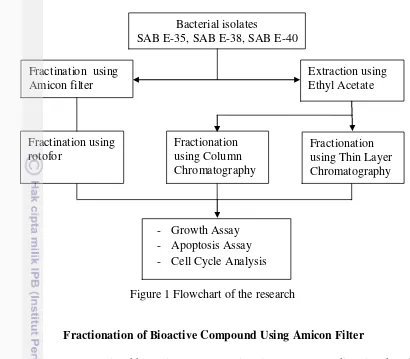
Framework of Research
The flowchart of methods in this study is shown in Figure 1. Isolation of bioactive compound from bacterial isolates SAB E-35, SAB E-38 and SAB E-40 including fractionation using amicon filter and extraction using ethyl acetate. The filter of Amicon filter then fractinated using rotofor based on differences of pH, the ethyl acetate extract then fractionated through thin layer chromatography and column chromatography. The fractions obtained then used as samples for cytotoxicity assay against leukemic cell line, apoptosis assay and cell cycle analysis.
Time and Places
The experiment was conducted from August 2014 to February 2015 in Laboratory of Microbiology Bogor Agricultural University and Laboratory of
7
Fractionation of Bioactive Compound Using Amicon Filter
Sponge-associated bacteria were grown in 250 ml SWC medium incubated for 72 hours on shaker (100 rpm, 30 0C), then sentrifuged at 12000 rpm for 10 minutes. Furthermore, supernatant were fractionate through a 50 kDa then 10 kDa nominal cut-off Amicon filter (Millipore, Billerica, MA, USA) by centrifugation. The resulting fractions were referred to as the fraction 1 (high mass; >50 kDa) fraction 2 (medium mass ;>10 kDa and <50 kDa), fraction 3 (low mass; <10 kDa), they were used for further fractionation according to differences of pH using rotofor.
Extraction of Bioactive Compound Using Ethyl Acetate
Sponge-associated bacteria were grown in 1000 ml SWC medium incubated for 72 hours on shaker (100 rpm, 30 0C). After incubated, culture was added by 1000 ml ethyl acetate and stirred for 12 hours. Ethyl acetate phase uper the culture were separated then concentrated with rotary evaporator to gain crude extract. Crude extract produced stored at 5oC for further purification through Thin Layer Chromatography and Column Chromatography using butanol as solvent.
Figure 1 Flowchart of the research Fractination using
Amicon filter
Extraction using Ethyl Acetate
Fractionation using Thin Layer Chromatography Fractionation
using Column Chromatography Fractination using
rotofor
Bacterial isolates
SAB E-35, SAB E-38, SAB E-40
- Growth Assay - Apoptosis Assay - Cell Cycle Analysis
Fractionation of Bacterial Extract Using Thin Layer Chromatography
As much 10 μl of crude extracts were spotted on TLC plates (MERCK Silica Gel 60 F
254) and eluted with vertical chromatography using n-butanol. The spots on
TLC plate were detected under UV light at 254 nm wave-length. After that, the retardation factor (Rf) values were calculated. The spots on TLC plate were cut off and dried up in room temperature. The developed TLC plates were dissolved on DMSO and used for further assay.
Fractionation of Bacterial Extract Using Column Chromatography
As much 0.5 g of crude extract was dissolved with 0.5 ml DMSO and injected into silica gel-column chromatography (column dimension 0.40 x 150 mm, particle size of silica gel 40 x 63 μm). Eighteen of fractions were collected (1 ml/each fraction).These fractions were dried up and dissolved with DMSO and used for further assay.
Cytotoxicity Assay
Cytotoxicity assay was determined by Cell Counting Kit CCK-8. Approximately 1x104 cells/well of cell line were plated in 100 μl of RPMI 1640 with 2% FBS and seeded into 96 well plates. The cells were loaded with 50 μl of the extract at concentration of 50 μg/ml, 100 μg/ml, 200 μg/ml and 400 μg/ml. The plates were then incubated in CO2 incubator at 37ºC for 48 hours prior to
addition of 10 μl CCK-8 counting reagent each well. The plate were then re-incubated at 37ºC in CO2 incubator for four hours and the absorbance of which was read using microplate reader at 450 nm. This assay was carried out in duplicate.
The percentage of inhibition rate was calculated by formula:
% cytotoxicity rate =Mean OD of control cells−Mean OD of tested cells
Mean OD of control cells x 100
The IC50 (concentration reducing cell viability by 50%) was determined using relation curve between concentration of extract (x) and percentage of inhibition (y). The IC50 is provided in μg/ml.
Apoptosis Assay
9
incubated for 15 minutes in room temperature. The samples were analyzed by flow cytometry. This assay was carried out in duplicate.
Cell Cycle Analysis (0.588/50 ml DDW), 0.45 ml Rnase (10 mg/ml), 0.1 ml 10% Triton X-100, 3.95 ml DDW.
4
RESULTS AND DISCUSSION
Fractionation of Bioactive Compound Using Amicon Filter
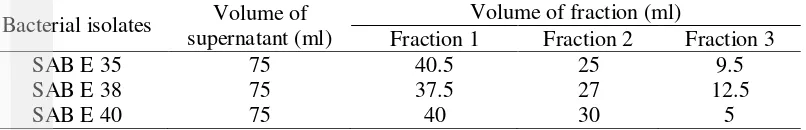
Fractionation of bioactive compound using Amicon Filter showed various result. Commonly, yield of fraction 1 (high mass: >50 kDa) was higher compared with fraction 2 (medium mass: <50 kDa and >10 kDa) and fraction 3 (low mass: <10 kDa). Fraction 3 has the lowest yield (Table 2). Amicon filter can possibly applicate for purification of macromolecular components found in tissue culture extracts and cell lysates. It also can be used for recovery of oligonucleotides and peptides and filtration of substances with molecular weight below the nominal molecular weight limit.
Table 2 Yield of fraction from sponge-associated bacteria Bacterial isolates Volume of
supernatant (ml)
Volume of fraction (ml)
Fraction 1 Fraction 2 Fraction 3
SAB E 35 75 40.5 25 9.5
SAB E 38 75 37.5 27 12.5
SAB E 40 75 40 30 5
Cytotoxixity Assay Using Amicon Filter Fraction
0
1000 500 250 125 62,5 31,25 15,625
OD fractions that had been separated. This results indicated that the fractions 1 consist of more than one bioactive compound that will optimally work in combination. In addition, the ability of growth stimulation of each pH fractions became lower as they separated based on pH differences (Figure 3).
Based on the results, the differences of the size of substances causing the differences of effect on cell lines. It was indicated that the molecular size of substances affect the activation of bioactive compounds on fractions. As mentioned before, the type of compounds that might be contained in the fractions are proteins, oligonucleotide and others substances with molecular weight below the nominal molecular weight limit.
Figure 3 Growth stimulation of fraction 1 of SAB E 35 separated by differences of pH on U937
11
Extraction of Bacterial Extract Using Ethyl Acetate
Extraction is a technique that use to separate compounds based on the differences of solubilities in two solvents that do not mix. Most commonly, one of the solvents will be water and the other will be an immiscible organic solvent. In general, very non-polar compounds will partion to the organic solvent and very polar compounds and salts will partion to the aqueous phase. Since the two solvents do not mix, they can be separated in a separatory funnel providing a very quick and easy way to separate compounds (WFU 2015). To extract organic compounds from the mixture, it is needed an extracting solvent that will solubilize the compounds of interest, not itself dissolve in the water, also does not react with the compound to be extracted. The selection of a solvent in the extraction process is largely determined by the solubility properties of the compounds, type of substrate, the partition coefficient and the distribution ratio of solvent system (Jeffery et al.
1989). Ethyl acetate is semipolar solvent that was chosen to extract the compounds from the mixture. Based on differences of solubility, the separation of ethyl acetate layer from liquid culture of bacteria was more easily done. The compounds that extracted from microbial culture was espected as extracellular secondary metabolites produced by bacteria. Metabolites produced by sponges and their associated microorganisms can be classified chemically into alkaloids, terpenoids, glycosides, phenols, phenazines, polyketides, fatty acid products and peptides, amino acid analogues, nucleosides, porphyrins, aliphatic cyclic peroxides and sterols (Thakur & Muller 2004; Tilvi et al. 2004).
The three isolates of SAB produced crude extracts as yield, ranging from 0.01% to 0.02% weight per volume with SAB E 38 as the highest yield producer (Table 3). Bioactive extraction from microorganisms was more efficient comparing with extraction from sponge directly because it need shorter time in production process and harmless towards preservation of sponge. stated that
Cultivation of sponges take 2-3 years to yield (MacMillan 1999). Extraction from sponge Haliclona sp. obtained 24.43 gram from 1.2 kg sponge (Lee et al. 2012), 150 gram from 2 kg sponge Jaspis sp. (Tang et al. 2012), and 80 gram from 500 gram sponnge Jaspis splendes (Ebada et al. 2009).
Table 3 Yield of ethyl acetate extract from sponge-associated bacteria Bacterial
Cytotoxicity Assay of Ethyl Acetate Extract
by SAB E 35 with IC50 value 294.923 µg/ml. One appereance of the cytotoxicity effect of SAB on MOLT4 cell line displayed on Figure 4. The cell lines undergoing lysis as they treated by the extracts compared with the untreated cell lines. This result indicated that the extracts have ability as bioactive compound that effective on the cell lines. The chemical diversity of bioactive compounds produced by sponge-associated microbes revealed that certain chemical classes such as quinones, steroids, fatty acids, diketopiperazines, alkaloids, terpenes, terpenoids, trichoverroids and prodigiosin derivatives, diglucosyl-glycerols, polyketides, cyclopeptides, glycoglycerolipid, benzoic acid derivatives, are responsible for anticancer or antitumor activities (Thomas et al 2010).
Table 4 IC50 values of crude extract from sponge-associated bacteria on cell lines
Samples IC50 Values on Cell Lines (µg/ml)
U937 MOLT4 K562 HL60 Daudi CEM6
SAB E 35 280.529 263.930 299.126 320.080 274.193 244.238
SAB E 38 354.474 331.075 426.024 294.923 388.628 317.351
SAB E 40 191.512 291.700 214.467 393.479 222.138 233.963
There are different results between bioactive compound isolation using Amicon filter and ethyl acetate extraction. The amicon fractions resulted bioactive that can stimulate the growth of cell line, the ethyl acetate extract gave the cytotoxicity effect instead. Those results indicated that the type of methods can affecting the type of bioactive compound isolated.
Fractionation of SAB E 35 Extract Using Thin Layer Chromatography (TLC) and Column Chromatography (CC)
TLC and CC aims to separate the component of bacterial crude extracts. The principles of TLC were based on the difference of absorption, partition and solubility of the chemical components that will be moved with the polarity of eluent. Meanwhile, on the CC technique, the solvent was allowed to flow through the column due to the gravity or the pressure. The compounds will move through the column at the different rates, separated and collected as the fractions out of the column base (Hurtubise 2010).
13
Table 5 IC50 values of fractions from SAB E 35 extract separated by thin layer chromatography on U937 cell line
Isolate
LC50 (µ g/ml)
crude extract Fraction LC50 (µ g/ml)
SAB E-35 280.5
1 77.25
2 88.9
3 83.5
4 84.05
5 95.58
6 92.11
7 89.82
8 84.74
Table 6 IC50 values of fractions from SAB E 35 extract separated by column chromatography on U937 cell line
Isolates
LC50 (µ g/ml)
Crude extract Fraction
LC50
(µg/ml) Fraction
LC50
(µg/ml)
280.5
1 124.7 11 98.0
2 382.2 12 104.1
3 44.7 13 107.0
4 72.8 14 108.5
SAB E-35 5 73.2 15 85.3
6 99.5 16 102.6
7 97.6 17 110.2
8 82.1 18 98.1
9 98.0 19 91.4
(a) (b)
(c) (d)
15
Apoptosis Assay
Cytotoxicity mechanisms can occur through apoptosis which is a genetically-regulated form of cell death. Apoptosis is controlled by signal, which may come from inside or outside the cell. Internal signals may be linked to the absence of mitosis or the recognition of damage DNA. External signals can cause a receptor protein in the plasma membrane to change its shape, and in turn activate a signal transduction pathway. Both internal and external signal can lead to the activation of a class of enzymes caled caspases. These enzymes are proteases that hydrolyze target molecules in a cascade of events. As a result, the cell dies as the caspases hydrolyze protein of the nuclear envelope, nucleosomes and plasma membrane (Sadava et al. 2011). Extract from sponge associated bacteria could be one of the external signal that activate the signal transduction pathway. In the early stages of apoptosis, several changes occur on cell surface. One of the plasma membrane alterations is the translocation of phosphatidylserine (PS) from the inner side of plasma membrane to the outer side, by which PS becomes exposed on cell external surface. PS changes can be detected using Annexin-V anticoagulant which has affinity binding to PS. Cell membrane integrity is lost during apoptotic process. Using DNA-specific viability dyes such as Propidium Iodide (PI), it is possible to distinguish early apoptotic, late apoptotic, and dead cells (Vermes et al. 1995).
The result of apoptosis assay revealed that SAB extract can induce apoptotic activity. The viability of cells treated with SAB extract decreased and they showed their apoptosis effect as late phase apoptosis. SAB E 35 showed the strongest effect based on its ability to decrease the number of viable cells, that remained only 21.6% and 37.3% cells on their late apoptosis phase. However,dead cells cannot be detected whether they death or not by apoptosis effect or any other mechanisms (Table 7, Figure 5). The number of cells treated for this analysis has dramatically decreased, compared with untreated cells. This was expected because cells were dead early while being treated and wasted away during assay process. Table 7 Apoptosis effect on MolT4 cells incubated with samples for 2 hours
(b) (a)
(d) (c)
(b) (a)
Apoptotic cells often have fractional DNA content due to the fact that the fragmented (low molecul weight) DNA undergoes extraction during the staining procedure. Some cells also lose DNA (chromatin) by shedding apoptotic bodies.
Thus, only a fraction of DNA remains within apoptotic cells. They are then represented on the DNA content frequency histograms by the sub-G1 peak.
SAB E 38
Figure 5 Flowcytometry output for apoptosis effect on MolT4. LL quadran : viable cells; LR quadran : early apoptosis; UR quadran : late apoptosis; UL quadran : dead cells. (a) untreated cells; (b) cells treated with SAB E 35; cells treated with SAB E 38; cells treated with SAB E 40
Control (DMSO) SAB E 35
20
In addition, the induction of apoptotic cell death in MOLT4 cells treated by sponge-associated bacterial extract was confirmed by cell cycle analysis result. Apoptotic cells frequently have fractional DNA content because the fragmented DNA (which has low molecule weight) was extracted during staining procedure. Some cells also lose DNA chromatin by shedding apoptotic bodies (Nagata et al. 2003). Thus, only a fraction of DNAs remains in apoptotic cell. They are then represented by DNA content frequency histograms in sub-G1 peak (Darzynkienwicz et al. 1991). The proportion of cells in sub-G1 fraction were accumulated, indicating that apoptotic DNA cleavage increased significantly after two hours samples treatment.
An example of a cancer drug that targets the cell cycle is 5-fluorouracil, which blocks the synthesis of thymine, one of the four bases in DNA. The drug taxol prevents the functioning of microtubules in the mitotic spindle. Both drugs inhibit the cell cycle, and apoptosis causes tumor shrinkage (Sadava et al. 2011). More such research on going is Pterostilbene, a polyphenolic compound present in grapes and other fruits. Pterostilbene at the IC90 concentration of 44 μM inhibited
with pterostilbene resulted in a transient accumulation of cells in the G0/G1-cell cycle phase followed by the S-phase arrest (Siedlecka-Kroplewska et al. 2012).
In summary, combining the cytotoxicity assay and the detection of apoptosis confirmed with cell cycle analysis could be applied to efficient screening of the potent bacterial isolates and also predicting the type of the compound. These compounds could be developed and applied in pharmaceutical industry in order to treat the leukemia.
5 CONCLUSION AND SUGGESTION
Conclusion
The results of this study demonstrated that sponge-associated bacterial extracts gave the cytotoxicity effect on leukemic cell lines. Other results indicated that apoptosis occurred in MOLT4 treated by the extract, confirmed by the result of cell cycle analysis. Crude extract of SAB E 35 is highly potential as anticancer. Further study may needed to find new alternative cancer therapeutic agent.The results suggested that the apoptotic effects of sponge-associated bacteria on MOLT4 can provide contribution to their claimed-anticancer activity.
Suggestion
22
REFERENCES
Abubakar H, Wahyudi AT, Yuhana M. 2009.Skrining bakteri yang berasosiasi dengan spons Jaspis sp. sebagai penghasil senyawa antimikroba. Ilmu Kelautan : 16:35-40.
Bewley CA, Faulkner DJ. 2004. Lithistid sponges: Star performers or hosts to the stars. 1998. AngewChem Int Ed37 : 2162–2178.
Bewley CA, Holland ND, Faulkner DJ. 1996. Two classes of metabolites from Theonellaswinhoei are localized in distinct populations of bacterial symbionts. Experientia 52 : 716–722.
Belson M, Kingsley B, Holmes A. 2007. Risk factors for acute leukemia in children: a review. Environ Health Perspect 115 : 138-145.
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. 2014. Marine
Bultel-Poncé V, Berge J, Debitus C, Nicolas J, Guyot M. 1999. Metabolites from the spongeassociated bacterium Pseudomonas species. Mar Biotechnol 1 : 384–390.
Collins SJ, Bodner A, Ting R, Gallo RC. 1980. Induction of morphological and functional differentiation of human promyelocytic leukemic cells (HL60) by compounds which induce differentiation of murine leukemic cells. Int J Can 25 : 213.
Darzynkienwicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. 1991. Features of apoptotic cells measured by flowcytometry. Cytometri 13:795-808.
Dunlap WC, Battershill CN, Liptrot CH, Cobb RE, Bourne DG, Jaspars M, LongPF, Newman DJ. 2007. Biomedicinals from the phytosymbionts of marine invertebrates: Amolecular approach. Methods 42 : 358–376.
Ebada SS, Lin WH, Proksch P. 2010. Bioactive sesterterpenes and triterpenes from marine sponges:occurence and pharmacological significance. Marine Drugs. 8:313-346.
Elyakov GB, Kuznetsova T, Mikhailov VV, Maltsev II, Voinov VG, Fedoreyev SA. 1991. Brominated diphenyl ethers from a marine bacterium associated with the sponge Dysideasp. CellMolLife Sci 47 : 632–633
Fadhillah M. 2014. Toxicity of Sponge-Associated Bacteria Bioactive Compounds Extract and Analysis of DNA that play role in its Biosynthesis. [thesis]. Bogor : Departement of Biology, Mathematic and Sains Faculty Bogor Agricultural University
Faulkner DJ. 2002. Marine natural products. Nat Prod Rep 19 : 1–48.
Grozdanov L, Hentschel U. 2007. An environmental genomics perspective on the diversity andfunction of marine sponge-associated microbiota. Curr Opinion Microbiol 10 : 215–220.
Harris RE. 2015. Global Epidemiology of Cancer. USA: Jones & Bartlett Publishers.
Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J, Moore BS. 2002. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol 68 : 4431–4440.
Hurtubise RJ. 2010. Adsorption Chromatography. In : Cazes J, editor. Encyclopedia of Chromatography Third Edition. New York : John Wiley & Sons, Inc.
Jeffery GH, Bassett J, Mendham J, Denney RC. 1989. Textbook of Quantitative Chemical Analysis. Fifth Edition. New York: John Wiley & Sons, Inc.
Kennedy J, Baker P, Piper C, Cotter PD, Walsh M, Mooij MJ, Bourke MB, Rea
MC, O’Connor PM, Ross RP, Hill C, O’Gara F, Marchesi JR, Dobson ADW.
2009. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar Biotechnol11 : 384–396.
Kinghorn AD, Chin YW, Swanson SM. 2009. Discovery of natural product anticancer agents from biodiverse organisms. Curr Opin Drug Discov Dev 12 : 189–196.
Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, Vanky F. 1976. Properties of the K562 cell line, derived from a patient wtih chronic myeloid leukemia. Int J Can 4 : 421-431.
Laport M, Santos O, Muricy G. 2009. Marine sponges: Potential sources of new antimicrobial drugs. Curr Pharma Biotechnol 10 : 86–05.
Lee YK, Lee JH, Lee HK. 2001. Microbial symbiosis in marine sponges. J Microbiol 39 :254–264.
Lee Y, Jang KH, Jeon J, Yang WY, Sim CJ, Oh KB, Shin J. 2012. Cyclic Bis-1,3-dialkylpyridiniums from the Sponge Haliclona sp. Mar Drugs 10: 2126-2137.
Liu R, Cui C, Duan L, Gu Q, Zhu W. 2005. Potent in Vitro anticancer activity ofmetacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomyceteSaccharopolyspora sp. nov. Arch Pharm Res 28 : 1341–1344.
MacMillan SM. 1999. Starting A Succesful Commercial Sponge Aquaculture Farm. Hawaii (US): CTSA Publication.
McClintock JB, Baker BJ. 2010. Marine Chemical Ecology; USA : CRC Press Boca Raton.
Mehbub MF, Lei J, Franco C, Zhang W. 2014. Marine sponge derived natural products between 2001 and 2010 : trends and opportunities for discovery of bioactive. Mar Drugs 12 4539-4577.
Müller WEG, Thakur NL, Ushijima H, Thakur AN, Krasko A, Pennec G, IndapMM, Perovic-Ottstadt S, Schröder HC, Lang G, Bringmann G. 2004. Matrix-mediated canalformation in primmorphs from the sponge Suberites domuncula involves the expression of aCD36 receptor-ligand system. Cell Sci117 : 2579–2590.
Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. 2003. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 10:108-116.
Newman DJ, Hill RT. 2006. New drugs from marine microbes: the tide is turning. J Ind MicrobiolBiotechnol 33 : 539–544.
24
Piel J. 2006. Bacterial symbionts: prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem 13: 39–50.
Proksch P, Edrada RA, Ebel R. 2002 Drugs from the seas: current status and microbiologicalimblications. Appl Microbiol Biotechnol 59 : 125–134.
Proksch P. 1994. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon 32 : 639–655.
Putra I. 2012. Extraction and Bioactivity of Antimicrobial Compounds from Bacteria Associated with Sponges. [thesis]. Bogor : Departement of Biology, Mathematic and Sains Faculty Bogor Agricultural University
Radjasa OK, Martens T, Grossart H, BrinkhoffT, Sabdono A, Simmon M. 2007. Antagonistic activity of a marine bacterium Pseudoalteromonas luteoviolacea TAB4.2 associated with coralAcropora sp. J Biol Sci 7 : 239–246.
RammW, Schatton W, Wagner-Dobler I, Wray V, Nimtz M, Tokuda H, Enjyo F, Nishino H, Beil W, Heckmann R, Lurtz V, Lang S. 20014. Diglucosyl-glycerolipids from themarine sponge-associated Bacillus pumilus strain AAS3: their production, enzymaticmodification and properties. Appl Microbiol Biotechnol 64 : 497–504.
Remya AT, Kavlekar DP, LokaBharathi PA. 2010. Marine drugs from sponge-microbe association-a review. Mar Drugs 8:1417-1468.
Renan MJ. 1993. How many mutations are required for tumorigenesis? Implications from human cancer cells. Mol Carcinog 7:139–146.
Sadava D, Hillis D, Heller C, Berenbaum M. 2011. Life, The Science of Biology Ninth Edition. USA : The Courier Companies, Inc.
Sakai R, Higa T,; Jefford CW, Bernardinelli G. 1986. Manzamine A, a novel antitumor alkaloidfrom a sponge. J Am Chem Soc108 : 6404–6405.
Selvin J, Shanmugha Priya S, Seghal Kiran G, Thangavelu T, Sapna Bai N. 2009. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol Res 164 : 352–363.
Siedlecka-Kroplewska K, Jozwik A, Kaszubowska L, Kowalczyk A, Boguslawski W. 2012. Pterostilbene induces cell cycle arrest and apoptosis in MOLT4 human leukemia cells. Fol Histochem Et Cytobio 50:574–580
Speitling M, Smetanina OF, Kuznetsova TA, Laatsch H. 2007. Bromoalterochromides A and A',unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM 636T.Antibiot. 60 : 36– 42.
Sundstorm C, Nilsson K. 1976. Establishment and characterization of a human histtiocytic lymphoma cell line (U937). Int J Canc 17 : 565.
Tang S, Xu R, Lin W, Duan H. 2012. Jaspiferin A and B: two new secondary metabolites from the south China sea sponge Jaspis stellifera. Rec Nat Prod. 6:398-401.
Thakur AN, Thakur NL, Indap MM, Pandit RA, Datar, VV, Müller WEG. 2005. Antiangiogenic, antimicrobial and cytotoxic potential of sponge-associated bacteria. MarBiotechnol 7 : 245–252.
Thakur NL, Hentschel U, Krasko A, Pabel CT, Anil AC, Müller, WEG. 2003. Antibacterialactivity of the sponge Suberites domuncula and its primmorphs: potential basis forepibacterialchemical defense. Aquat Microb Ecol 31 : 77– 83.
Thakur NL, Müller WEG. 2004. Biotechnological potential of marine sponges. Curr Sci86 :1506–1512.
Thomas TRA, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association—A review. Mar Drugs 8 : 1417–1468.
Thoms C, Schupp P. 2005. Biotechnological potential of marine sponges and their associated bacteria as producers of new pharmaceuticals (Part II). J Int Biotechnol Law 2 : 257–264.
Tilvi S, Rodrigues C, Naik C, Parameswaran P, Wahidhulla S. 2004. New bromotyrosine alkaloids from the marine sponge Psammaplysilla purpurea. Tetrahedron 60 : 10207–10215.
Vermes, I. Haanen, C. Steffens-Nakken, H. Reutellingsperger, C. 1995. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunolog Meth 184:39-51
Wang G. 2006. Diversity and biotechnological potential of the spons-associated microbial consortia. J Ind Microbiol Biotechnol 33: 545-551.
Wahidin M, Noviani R, Hermawan S, Andriani V, Ardian A, Djarir H. Population-based cancer registration in Indonesia. Asian Pac Jour of Canc Prevent 13:1709-1710.
[WHO] World Health Organization. 2014. World Cancer Report 2014. Stewart BW, Wild CP, editor. Geneva : IARC Nonserial Publication.
[WFU] Wake Forest University. 2015. Extraction.
http://www.wfu.edu/chemistry/courses/organic/extract/extraction.html (11 November 2015).
Wicke C, Hners M, WrayV, Nimtz M, Bilitewski U, Lang S. 2000. Production and structureelucidation of glycoglycerolipids from a marine sponge associated Microbacterium species. Nat Prod 63 : 621–626.
Wilkinson C, Fay P. 1979. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature 279 : 527–529.
25
Appendix 2 Calculation of % Cytotoxicity Rate and IC50 Example :
Cytotoxicity rate of SAB E 35 on U937 at concentration 400 μg/ml
Appendix 3 Retardation Factor (Rf) values of TLC fraction Fraction Retardation Factor
1 0.10
2 0.40
3 0.43
4 0.50
5 0.55
6 0.77
7 0.91
27
BIOGRAPHY
The author was born in Subang on 17th May 1991 as the 3rd daughter from Bapak Narman and Ibu Anah. The author was graduated from MAN 1 Subang in 2008. In the same year, the author was acceptrd as undergraduated student of Biology Major, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University through Invitation of IPB Entrance Selection (USMI) then graduated in 2012. In 2013, the author was accepted as a graduate student of Microbiology Major, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University and grantee academic scholarsip from Directorate of Higher Education of Indonesia.
During became a graduate student of Microbiology Major, the author has participated on student exchange program between IPB and Chiba University, Japan on August-November 2014 and January-February 2015. As one of requirements to obtain the degree of Master of Science, the author conducted a research entittled ―Cytotoxicity of Crude Extract from Sponge-Associated Bacteria Against MOLT4 Leukemic Cell Lines Through Apoptosis‖. The research was supervised by Prof. Aris Tri Wahyudi M.Si, Prof. Nahrowi M.Si and Prof. Jun Nomura Md, phD. This research article has been published in the International Journal of Pharmacy and Pharmaceutical Sciences 7(12): 246-249 on December 2015, also has been presented on the Mini Symposium of International Conference of Biosciences on 4 August 2014 in Bogor, West Java and on Annual Scientific Meetings of Indonesian Society of Microbiology on 8-9 October 2015 in Semarang, Central Java.