I .
INTRODUCTIONI n East Java, Indonesia, t h e Asian c a t f i s h ( C l a r i a s #
b a t r a c h u s L . ) i s p r e s e n t l y on t h e t h r e s h o l d o f becoining an
i m p o r t a n t species f o r aquaculture. F i s h farmers a r e i n t e n s i f y i n g t h e i r e f f o r t s , s i n c e
C l a r i a s
b a t r a c h u s , 1ocally.known as " i k a n l e l e " , i s t h e h i g h e s t p r i z e d f r e s h - water f i s h species i n t h i s r e g i o n . U n f o r t u n a t e l y t h e farmers f a c e a complex o f problems which hamper t h e i r e f f o r t t o expand and i n t e n s i f y t h e c u l t u r e o f i k a n l e l e . One o f t h e i r problems concerns a r e l i a b l e and c o n t r o l l e d method f o r q u a l i t y and q u a n t i t y f i n g e r l i n g p r o d u c t i o n . T h i s problem can \ b e s o l v e dby
i n d u c t i o n o f r e p r o d u c t i o n and g e n e t i c improvement.A r t i f i c i a l induced breeding techniques i n Asian c a t f i s h have been r e c e n t l y developed by Zonneveld e t a l . ,
(1988).
A s i n g l e i n t r a m u s c u l a r i n j e c t i o n o f cPS ( c a r p p i t u i t a r y suspension) ( dosage 6 mg/kg body welght ) i n combination w i t h a l a t e n c y t i m e o f 17 hours ( a t 25OC )r e s u l t e d i n h a t c h i n g r a t e s up t o 82.5 %.
S e l e c t i v e breeding and s u p p o r t i n g g e n e t i c research I n a q u a c u l t u r e use5 v a r i o u s techniques, i n c l u d i n g s e l e c t l o r i f o r disease r e s i s t a n c e and behavior c h a r a c t e r s ,
I
Chromosomal manipulation and artificial mutations have
long been established a s highly advantageous in plant
breeding. It i s very likely
that they will be j u s t
as \efficient in fish. T h e fish breeder, unlike that of tarn1
animals, can handle both male and female gametes outslde
the organisms
and has manipulative control o v e r the
developing zygote (Moav, 1976).
Polyploidy h a s been induced in a number of fish
species using a variety of techniques (Purdom, 1972;
Valenti, 1975; Refstie e t al.,
1977; Allen and Stanley,
1979; Gervai
e t al.,
1980a; Wolters e t al.,
1981).
Induction of triplaidy in
f i s hmight potentially
beuseful
for t h e control of overpopulation, f o r increasing the
growth rate in juveniles, and for extending survival and
improving growth in mature fish.
Most of the interest in induced triploidy has been
from a n aquaculture perspective
w i t h t h e
hope that
triploids might grow faster than diploid a s juvenile o r a s
mature fish. T h i s might result from triploidy
p e rse
o r
a san indirect result of sterility o f triploids (Thorgaard,
Juvenile triploid have generally been found t o grow n o
faster than diploids.
Growth of j u v e n i l e triploids was
similar t o t h a t
of
diploids
in stickleback
( G a s t e r o s t e u saculeatus) (Swarup, 19591, common c a r p
( C y p r z n u s c a r p z u )3
punctatus) (Wolters
et
dl.,1982a). In t h e blue tilapia
,
(Oreochramis aurea)
,
juvenile triploid w e r e found t o
belarger than diploids {Valenti, 1975). However, in Pacific
s a
1
mon
(Oncurhynchus
kisutch)
(Utter
e t
a1
.
,
1983)
triploids may grow slower than diploids.
Several s t u d i e s have found that triploids may grow
faster than diploids a t sexual maturity, presumably because
energy that i s channeled t o gonadal development in d i p l o ~ d s
i s used for growth.
Triploid
channel
catfish were
significantly heavier than diploids a t t h e a g e of 8 months
and older (Wolters e t
dl.,1982a).
In African catfish
(Clarias gariepinus), t h e growth rate w a s not significantly
affected by triploidy. Body composition, however, w a s
strongly affected.
Triploid fish deposited, per gram of
Qrowth, less protein and more fat (Richter e t al., 1987).
Induction o f triploidy of Clarias batrachus
L.
ispossible through t h e manipulation of chromosome. However,
the problems
are
X )how fish conditon (eggs
and sperm
quality) be s e t up,
2 )
what methods will be used,
3 )how to
determine t h e triploid fish,
and
4)
what
is thethe objectives of the experiments w e r e
:+.
t3develop a practical method for inducing triploidy,
2. t o identify triploidy a t juvenile fish,
3.
t o c o m p a r e t h e growth performance of diploid and
11. L I T E R A T U R E REVIEW
1.
Genotype m a n i p u l a t i o nThe m a n i p u l a t i o n o f chromosomes becomes f e a s i b l e d u r i n g t h e n u c l e a r c y c l e s o f c e l l d i v i s i o n and b a s i c a l l y comprises t h e a d d i t i o n o r s u b t r a c t i o n o f a complete h a p l o i d o r d i p l o i d s e t . I n animals, m e i o s i s i n t h e eggs is t t t e
p r i n c i p a l c e l l d i v i s i o n phase where m a n i p u l a t i o n
is
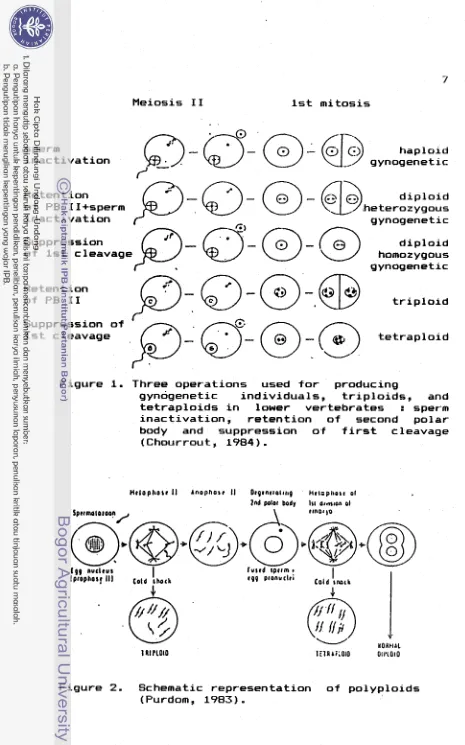
p o s s i b l e , and i n f i s h , and o t h e r animals w i t h e x t e r n a l f e r t i l i z a t i o n , a r t i f i c i a l processes can he a p p l i e d e i t h e r t o t h e gamete b e f o r e f e r t i l i z a t i o n o r t o t h e f e r t i l i z e d egg a t any p e r i o d d u r i n g t h e f o r m a t i o n o f t h e zygote. C o n t r o l o f t h e f i r s t m i t o t i c d i v i s i o n i s a l s o f e a s i b l e i n eggs b u t r e p o r t s o f i t so f a r a r e u n s u b s t a n t i a t e d (Purdom, 1983).I n commercial f i s h , d e v i a t i o n from t h e normal r e p r o d u c t i o n p a t t e r n can be induced e a s i l y ; by r a i s i n g p a r e n t s o f two d i f f e r e n t species ( h y b r i d i z a t i o n ) ; o n l y one p a r e n t (gynogenesis and androgenesis) o r i n c r e a s e o f chromosome numbers ( p o l y p l o i d y ) . These phenomena can be e x p l o i t e d i n p l a n n i n g new schemes o f g e n e t i c improvement f o r animal breeding ( a l r e a d y c l a s s i c a l i n p l a n t ) , such as t h e c r o s s i n g o f i n b r e d l i n e s (produced by gynogenesis) o r t h e use o f p o l y p l o i d s (Purdom, 1983; Chourrout, 1984).
D i p l o i d gynogenetic i n d i v i d u a l s c o u l d be produced i t
o f sperm, induced r e t e n t i o n o f t h e second p o l a r body, o r induced suppression o f t h e f i r s t cleavage ( F i g . 1 ) . D i p l o i d
1
gynogenesis r e q u i r e s t h e combination o f sperm i n a c t i v a t i o n and d i p l o i d i z a t i o n o f t h e maternal chromosome s e t . I f t h e l a t t e r was achieved by r e t e n t i o n o f second p o l a r body, t h e r e s u l t i n g embryo s t a r t s from two d i f f e r e n t t e r m i n a l p r o d u c t s o f t h e same m e i o s i s and so i s n o t homozygous a t a l l l o c i . I f suppression o f t h e f i r s t cleavage i s used t o d i p l a i d i z e t h e maternal s e t , t h e c o l l e c t e d embryos a r e considered t o be homozygous a t a l l l o c i because they r e s u l t from t h e f u s i o n o f two m i t o t i c p r o d u c t s (Purdom, 1983;
Chourrout, 1984).
A v a r i e t y of r a d i a t i o n t r e a t m e n t s a r e a v a i l a b l e t o i n a c t i v a t e sperm chromosomes. R a d i a t i o n treatments t h a t have been used s u c c e s s f u l l y i n c l u d e i r r a d i a t i o n w i t h gamma r a y s u s u a l l y from 'OCo o r &OCs (Purdom, 1969; Nagy e t
d l . , 1978; Chourrout e t d l . , 1980; R e f s t i e e t d l . , 1982), X
M e i o s i s I 1 1 s t m i t o s i s
I
0 .
sperm
i n a c t i v a t i o n
-
9
h a p l o i dr ' -
gynogeneticR e t e n t i o n
o f i n a c t i v a t i o n PB II+sperm
@ - 0 - @ - @ 3
h e t e r o z d i p l o i d ygous gynogenetic Suppressiono f 1st cleavage
p-
0-
@
-
@
homoz d i p l o i d ygous g y n o g e n e t ~ c Reten t i o nof
PB
I 1
t r i p l o i dI
Suppression o f
1st cleavage
,p
-
Gi
@
-
@
t e t r a p l o i dF i g u r e 1. Three o p e r a t i o n s used f o r producing
gynogenetic i n d i v i d u a l s , t r i p l o i d s , and t e t r a p l o i d s i n lower v e r t e b r a t e s : sperm i n a c t i v a t i o n , r e t e n t i o n o f second p o l a r body and suppression o f f i r s t cleavage
(Chourrout, 1984).
H t l o p h o r c 11 Anophosr 1 1 0t98nrrol1119 H t ~ o p l ~ o t t ol 2nd polof body 1st ormlon ol
rmnd )o
199 n u ~ l r u r fuctd sptrm 8
( P ~ ~ P ~ O S ! 11) Cold 'hock 899 ~ l o ~ v c l t ~ c o l d
I
sMch5.
w
'8'Y O H b l l L
1 R I f'lOIO l r l R A i ; 0 1 0 Oll'LOIO
[image:113.540.46.511.4.749.2]2. P o l y p l o i d p r o d u c t i o n
I
Induced p o l y p l o i d y r e f e r s t o t h e p r o d u c t i o n o f i n d i v i d u a l s w i t h e x t r a s e t s o f chromosomes. T h i s can be done by t r e a t i n g f e r t i l i z e d eggs w i t h e i t h e r temperature shock, h y d r o s t a t i c pressure o r chemical treatment. I f t h e treatments a r e a p p l i e d s h o r t l y a f t e r f e r t i l i z a t i o n , t r i p l o i d s can be produced due t o r e t e n t i o n o f t h e second p o l a r body o f t h e eggs. I f t h e t r e a t m e n t s a r e s h o r t l y b e f o r e t h e f i r s t cleavage d i v i s i o n , t e t r a p l o i d s can be produced.
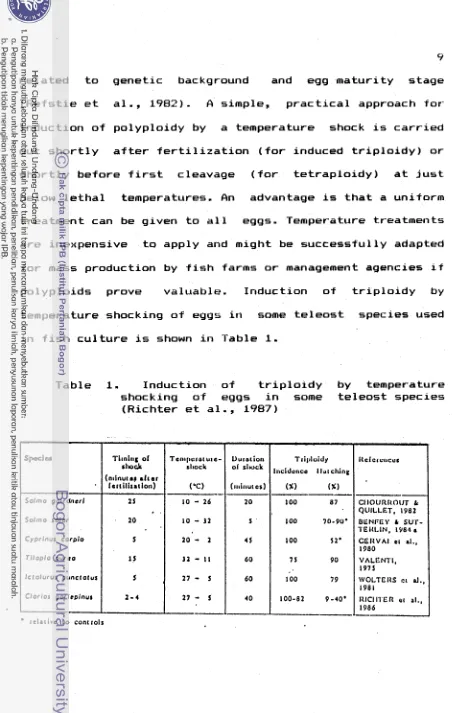
I n t h e method o f temperature shocking, Purdom (1983) presented t h e scheme f o r p o l y p l o i d s p r o d u c t i o n
(Fig.
2).
I n t h a t scheme t r i p l o i d f i s h can be produced by c o l d shocking f e r t i l i z e d eggs a t t h e metaphaseI 1
stage, and t e t r a p l o i d s by c o l d shocking t h e f e r t i l i z e d eggs a t t h e metaphase stage d u r i n g t h e f i r s t cleavage d i v i s i o n i n embryos.Temperature, t r e a t m e n t s o f f e r t i l i z e d eggs have been w i d e l y used t o suppress t h e second m e i o t i c d i v i s i o n
or
second p o l a r body e x t r u s i o n i n f i s h , b o t h c o l d shock (e.g., Purdom, 1969; V a l e n t i , 1975; Nagy e t al.,1978; Chaurrout,-.
1980; R e f s t i e e t d l . , 1982; R i c h t e r e t al.,1987; Lomen e t
a l . , 1988), and heat shock (Chourrout, 1980; Thorgaard
et
al., 1981#
BenPey and S u t e r l i n ,1984).
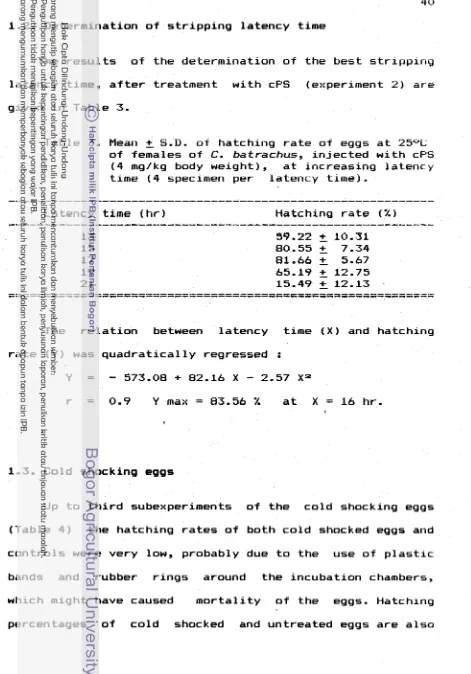
r e l a t e d t o g e n e t i c background and egg m a t u r i t y stage 1 R e f s t i e e t al., 1982). A simple, p r a c t i c a l approach for- i n d u c t i o n o f p o l y p l o i d y by a temperature shock i s c a r r i e d o u t s h o r t l y a f t e r f e r t i l i z a t i o n ( f o r induced t r i p l o i d y ) o r s h o r t l y b e f o r e f i r s t cleavage ( f o r t e t r a p l o i d y ) a t j u s t below l e t h a l temperatures. An advantage i s t h a t a u n i f o r m t r e a t m e n t can be g i v e n t o a1 1 eggs. Temperature treatments a r e inexpensive t o apply and m i g h t be s u c c e s s f u l l y adapted f o r m a s s p r o d u c t i o n by f i s h farms o r management agencies i f p o l y p l o i d s prove v a l u a b l e . I n d u c t i o n o f t r i p l o i d y by temperature shocking o f eggs i n some t e l e o s t species
used
i n f i s h c u l t u r e i s shown i n Table 1.Table 1. I n d u c t i o n o f t r i p l o l d y by temperature shocking o f eggs i n some t e l e o s t species
( R i c h t e r e t d l . , 1987)
Solmo gairdnerl
Solmo salor 1 0
-
3 2Cyprinus corpio
I
5 1 2 0 - 2I I
-
~ e l a t i v c t o controls
IVOLTEItS C I id., 1 9 8 1
I t I C I I I ' E R ut al.,
[image:115.540.48.506.30.743.2]Hydrostatic pressure ha5 been used t o black second
polar body extrusion o r first mitotic division. Streisinger
*e t dl.,
( 1 9 8 1 ) worked wlth zebra fish CBrachydanio reria),
and Yamazaki
(1983) and Chourrout (1984) with rainbow
trout(Salmo gairdneri)
.
Although application of hydrostatic
pressure treatments requlres
more
specific equipment
[pressure cell and hydraulic press) than temperature shock
,
treatments, t h e method diserves wide investigation because
it may be 1
s
damaging t o t h e e m b r y o than temperature
shock.
Chemicals may a l s o be used t o block polar body
extrusion o r mitotic division in fertilized eggs. Refstie
e t dl.
(1977) reported producing mosaic polyploid-diploid
Atlantic salmon [ S a l m o salar) after exposing fertilized
e g g s t o cytochalasin B .
Kanka and R a b i n Thorgaard (1983)
reported observing diplaid-triploid
mosaic, triploid and
tetraploid Tinca tinca after treating fertilized e g g s with
cytochalasin
B.
3.
Viability o f polyploids
T h e successful inductions of triploidy in many fish
s p e c i e s [ s e e T a b l e 1) support t h e belief that triploid fish
have good viability.
1980a) apparently survive
aswell a s diploids. Studies
inrainbow trout (Thorgaard et al., 1982) suggest that induced
triploid in t h i s species may be somewhat less viable than
diploids.
Triploidy
may
lead
t o
increased viability in
interspecific hybrids (Sheerer and Thorgaard, 1983). In
frogs, triploid interspecific hybrids a r e sometimes more
viable than diploid hybrids (Bogart
In
Thorgaard, 1983).
Interspecific triploid hybrids could prove useful in
fish culture because hybrid vigor and desirable atributes
of both s p e c i e s might be combined in a relatively healthy
sterile hybrid (Allen and Stanley, 1981).
4.
Gonad development
T r i p l o i d y a p p a r e n t l y i n h i b i t s gonadal developifrent more i n females than i n males. F a i l u r e o f m e i o s i s may p r e v e n t o o c y t e development and t h e a s s o c i a t e d i n c r e a s e i n s i z e o f t h e gonad. T r i p l o i d female rainbow t r o u t a t m a t u r i t y had s m a l l , s t r i n g l i k e gonads w i t h many c e l l s a r r e s t e d a t the pachytene stage o f m e i o s i s (Thorgaard and G a l l , 1979). D i p l o i d s have o v a r i e s about f o u r t i m e s as l a r g e a t m a t u r i t y as t r i p l o i d i n channel c a t f i s h ( W o l t e r s e t al., 1982b3. Gonadal development was a l s o s u b s t a n t i a l l y i n h i b i t e d i n female common c a r p ( G e r v a i e t a l . , 1980b)
I n e x t e r n a l appearence t h e o v a r i e s o f t r l p l o i d C .
g a r i e p i n u s
and s t e r i l e rainbow t r o u t( S a l m o
g a i r d n e r i )
resemble t h e undeveloped gonads (Thorgaard and G a l l , 1979; L i n c o l n and S c o t t , 1983; Yamazaki, 1983; R i c h t e r e t a l . ,1987), b u t t r i p l o i d t e s t e s a r e w e l l developed. Such sexual dimorphism i n gonadal development has p r e v i o u s l y been d e s c r i b e d i n t r i p l o i d f i s h ( Purdom, 1972; Thorgaard and G a l l , 1979; O e r v a i
et
d l . ,1980a;
W o l t e r s e t a l . , 1982a;Johnson
et
d l . , 1986) and amphibians (Fankhauster, 1941 and13
process accur l a t e r i n t h e l i f e o f salmonids (Nakamura, 1982).
1
It
i s apparent t h a t a s m a l l number o f c e l l s succeed i n passing through t h e f i r s t m e i o t i c d i v i s i o n i n b o t h t h e o v a r i e s and t e s t e s o f t r i p l o i d animals; female t r i p l o l d rainbow t r o u t a r e t h e o n l y r e p o r t e d e x c e p t i o n ( L i n c o l n and S c o t t ,1983).
Nothing i s known about t h e m e i o t i c nbechanisrn whereby p o s t m e i o t i c c e l l s a r e produced i n such t r i p l o i d s , b u t i t may be s i m p l y t h r o u g h t h e random s e g r e g a t i o n o f chromosomes. T h i s i s supported by t h e f a c t t h a t t r i p l o i d amphibians g e n e r a l l y produce a n e u p l o i d gametes (Fankhauster and Humprey i n Thorgaard,1983).
However, f u n c t i o n a l m e i o t i c mechanisms have evolved i n gynogenetic t r i p l o i d , i n v o l v i n g e i t h e r t h e f o r m a t i o i l o f t r i p o l a r s p i n d l e s(Cherfas,
1969)
or
t h e e n d o m i t o t i c d u p l i c a t i o n o f chromosomes p r i o r t o normal m e i o t i c d i v i s i o n (Cimino,5. I d e n t i f i c a t i o n o f t r i p l o i d f i s h
T h e assessment a f t h e 'success o f t r e a t m e n t s designed t o produce d i p l o i d s from o t h e r w i s e h a p l o i d eggs i s easy because o f t h e v e r y g r e a t d i f f e r e n c e s i n appearance between d i p l o i d s and h a p l o i d s which become apparent e a r l y i n embryonic development. No such easy o r definitive assay
1 4 methods have consequently been used. Nuclear
site
is
t h e most w i d e l y used c r i t e r i o n t o e s t a b l i s h p l o i d y and f o l l o w s6
t h e e x t e n s i v e work i n amphibians (Fankhausten i n Purdom, 1983). V a s e t s k i i (1967) measured t h e areas o f n u c l e i o f epidermal c e l l s from sturgeon (Acipencer
s t u r i o )
larvae, Purdom (1969) used c a r t i l a g e c e l l n u c l e i o f rainbow t r o u t and v a r i e t y o f authors have used n u c l e i o f r e d blood c e l l s( M o l t e r s e t al., 1982b).
None o f these methods can be r e a d i l y accepted as d e f i n i t i v e because o f v a r i a t i o n i n c e l l o r n u c l e a r s i z e f o r reasons o t h e r than p l o i d y , b u t t h e e r y t h r o c y t e s t u d i e s do employ a r e a d i l y i d e n t i f i a b l e c e l l t y p e and should be r e l i a b l e . I t i s necessary, however, t o r a i s e f i s h t o a reasonable s i z e b e f o r e blood can be c o l l e c t e d .
Measurement o f DNA c o n t e n t o f n u c l e i (Gervai e t al., 1980b) seems t o be more p r e c i s e than s i z e measurement. A n a l y s i s o f chromosome complements i s t h e most d i r e c t assay o f p l o i d y o f any s o r t b u t i t i s d i f f i c u l t i n f i s h and u s u a l l y r e q u i r e s t h a t f i s h be grown t o f r y o r f i n g e r l i n g stage before assay. Chromosome analyses i n blastomere have been used i n salmonids (Purdom and L i n c o l n , 1973; L i n c o l n and S c o t t , 1984) b u t t h e r e s o l v i n g power o f t h e technique i s n o t g r e a t .
(Platichythys f lesus)
and
between
turbot
(Scapthamus
maximus)
and brill
(Scupthamus
rhombus) a r e distinguishable
I
from parental type shortly after hatching by pigment
patterns, and triploids can be recognised a s intermediate
between t h e hybrid and the maternal type (Purdom,
1972).
P r o b l e m s have arisen in t h e measurement of triploidy.
T h e labor intensive chrontosomal preparation has limited
volume of data (Thorqaard e t al.,
1981; 1982),
while
concern about
reliability h a s
arisen regarding data
generated by more rapid procedures (Lemoine and Smith,
1980;
Benfey e t al.,
19843.
Johnson et
dl.(1984) compares t h e u s e of coulters
counter w i t h channelizer and t h e
ICP-22
flow cytometer.
Both equipment allowed rapid identification of diploid and
triploid.
However
differences
in
accuracy
between
instruments w a s revealed in comparison of data from the
s a m e individuals.
Ploidy measurement on c o h o salmon w a s clear for the
t w o instruments in
8 5
o f
100
individuals
( 2 2
triploids,
63diploids). T e n of t h e remaining individuals had skewed
histagram and t h u s indeterminate from
coulter counter
analyses, but were definitely triploids based o n f l o w
cytometery.
T h e five additional individuals produced n o
histogr-ams in t h e coulter counter and were determined
LyBoth the coulter counter with channelizer and
ICP-22
flow cytometer are able to rapidly differentiate triploid
blood samples, however the flow cytometer is more accurate.
The flow cytometer measures ploidy by
fluorescent staining
of nuclear
DNA
and the coulter counter by measuring
erythrocyte cellular volume.
The differences of accuracy
could be attributed to the fragility of cellular shape and
volume a s contrasted to the maintenance of integrity of
nuclei
under conditions
of shear, altered osmotic
environments, and storage conditions.
Analysis by flow
cytometry i s therefore resilient to cellular disruptions
that d o not affect
DNA
fluorescence.
The use of flow cytometry is very accurate, it is
reasonable to assume that the fish with
ahigh
DNA
content
were triploid, since both aneuploids (Gervai et dl.,
198th;
Lincoln, 1981a) and tetraploids (Thorgaard et al., 1981;
Allen, 1983; Chourrout, 1984) are generally non viable.
The
identification of triploid fish can be based
solely on the measurement of the major axis of either the
cell or the nucleus (Thorgaard and Gall, 1979; Chourrout
and Quillet, 1982;
Wolters et al.,
1982a; Beck and
Biggers, 1983; Richter et
dl.,1987).
The calculation of
cell surface area or nuclear volume, which necessitates the
measurement of the second axis, does not increase
t h eprobability of identifying triploids correctly.
1 7
c o u l t e r counter s i z i n g o f e r y t h r o c y t e s appears t o
be
t h e most s u i t e d f o r t h e r o u t i n e screening o f t r i p l o i d f i s h . I n #t h e absence o f a c o u l t e r counter c h a n n e l i z e r o r a f l o w cytometer, t h e measurement o f e r y t h r o c y t e c e l l o r nucleus major a x i s from t h e blood smears can be used as a r e l i a b l e a l t e r n a t i v e method f o r i d e n t i f y i n g t r i p l o i d s . However, t h i s technique i s more time consuming and, hence, l i m i t s t h e number o f f i s h t h a t can be screened i n a p a r t i c u l a r study
6. 4spects and c h a r a c t e r i s t i c s o f t r i p l o i d s
The i n t e r e s t o f producing t r i p l o i d f i s h has been based on t h e assumptian t h a t they would be s t e r i l e and consequently might a v o i d o v e r p o p u l a t i o n problems, and p o s s i b l y grow f a s t e r o r s u r v i v e longer than normal f i s h . T r i p l o i d s a r e expected t o be s t e r i l e because t h e odd number o f thrbm~some $ e t s w i l l l e a d t o d i s r u p t i o n o f meiosis and e i t h e r a f a i l u r e o f gonad development o r p r o d u c t i o n o f aneuploid gametes. The f a i l u r e o f gonad development might, i n t u r n , prevent t h e appearance o f u n d e s i r a b l e s i d e e f f e c t o f sexual maturation, such as poor meat q u a l i t y , slower growth, and h i g h m o r t a l i t y . I t appears t h a t t r i p l o i d f i s h a r e indeed f u n c t i o n a l l y s t e r i l e , secondary sexual c h a r a c t e r s a r e n o t always suppressed.
18 t h e f a c t t h a t t r i p l o i d s have h i g h e r heterozygousity than d i p l o i d s ( A l l e n d o r f and Leary, 1984). T h i s has been shown t o be associated w i t h h i g h e r developmental s t a b i l i t y
as
measured by f l u c t u a t i n g asymmetry (Leary e t al.,1985).
Bingham i n Crosby (1986) proposed t h a t increased h e t e r o z y g o u s i t y was a primary advantage o f u s i n g p o l y p l o i d i e s i n p l a n t breeding programs. Heterozygousity might be maximized i n induced t r i p l o i d s by u s i n g h y b r i d s between two s t r a i n s as t h e female parent and crossxng t o male o f a t h i r d s t r a i n .Purdom (1972) induced t r i p l o i d p l a i c e , flounder, and h y b r i d s by c o l d shocks ((3 t o 5OC f o r 2 t o 4 hours) a p p l i e d t o newly f e r t i l i z e d eggs. T r i p l o i d h y b r i d s s u r v i v e a t a s i g n i f i c a n t l y lower r a t e than
athar
hybrids. I nlarval
pigment p a t t e r n , number o f vertebrae, and metamorphosis c h a r a c t e r i s t i c s , t h e t r i p l o i d s d i s p l a y a d d i t i v e i n h e r i t a n c e i n v o l v i n g a l l t h r e e s e t s o f chromosomes. Concerning t h e growth r a t e , t h e r e was some i n d i c a t i o n t h a t t r i p l o i d
per
semay r e s u l t i n an excessive growth r a t e .
s t e r i l e , probably because abnormal spermatogenesis was t a k i n g place, b u t gonad s i r e appeared t o be unaffected. Female t r i p l o i d h y b r i d s contained o v a r i e s which were normal i n appearance, b u t t h e mean ovary weight was l e s s than 13 %
o f t h e d i p l o i d c o n t r o l . H i s t o l o g i c a l examinations revealed t h a t o v a r i e s o f t r i p l o i d h y b r i d s were more abnormal than those o f d i p l o i d hybrids. The oocytes appeared t o undergo degeneration, and o v u l a t i o n was n o t observed.
T r i p l o i d f i s h do n o t appear t o be s t r i k i n g l y m o r p h o l o g i c a l l y d i f f e r e n t from d i p l o i d s (Thorgaard and G a l l , 1979; Gervai e t dl., 1980b). Swarup (1959) found t h a t t r i p l o i d s t i c k l e b a c k (Gasterosteus
aculeatus)
had s h o r t e r t r u n k s and longer t a i l s than t h e d i p l o i d c o n t r o l s . However t r i p l o i d i n t e r s p e c i f i c h y b r i d s may be r e a d i l y d i s t i n g u i s h e d from d i p l o i d h y b r i d s i n some cases because o f d i f f e r e n c e s i n gene dosage from t h e parent species.I
7. Application
The primary i n t e r e s t i n induced t r i p l o i d f i s h l i e s i n t h e i r s t e r i l i t y and i n t h e p o s s i b i l i t y t h a t t h i s may lead
*
t o extended growth and/or s u r v i v a l i n mature f i s h . Data on t h e performance o f s t e r i l e t r i p l o i d s a r e s t i l l accumulating. The r e s u l t o f t r i p l o i d may m a i n t a i n t h e i r growth much b e t t e r than d i p l o i d s a r e shown by Wolter e t
al.
( 1982b)
.
I Z C b
c o n t r o l o f r e p r o d u c t i o n
is d e s i r a b l e . T r i p l o i d
grassc a r p
( C t e m p h a r y n ~ o d o n 1
i d e l l w )
a r e
b e i n g
a d o p t e d i n a q u a t i c
*
weed c o n t r o l p r o g r a m s ( T h o r g a a r d , 1 9 8 3 1 ,
a n d t r i p l o i d s may
b e
d e s i r a b l e
f o r
s p e c i e s
w h e r e
o v e r p o p u l a t i o n 1 a n d
a s s o c i a t e d s t u n t i n g o c c u r
( T h o r g a a r d ,
1986).A n o t h e r
a p p l i c a t i o n
o f
i n d u c e d
t r i p l o i d y
lies i n t h e f a c t t h a t
t r i p l o i d
h y b r i d s
are t y p i c a l l y much
more
v i a b l e t h a n
d i p l o i d h y b r i d s ( A l l e n a n d S t a n l e y , 1981; C h e v a s s u s
et
a l . ,
1983; S h e e r e r a n d T h o r g a a r d ,
1983).T h i s m a k e
i t
p o s s i b l e
t o combine
d e s i r a b l e c h a r a c t e r s f r o m
t w o
s p e c i e s i n
a
s t e r i l e h y b r i d .
T k
m o s t
s u c c e s s f u l a p p l i c a t i o n
o f
p o l y p l o i d
so
f a r
h a s b e e n t h e i n d u c t i o n o f t r i p l o i d s .
Av a r i e t y o f i n d u c t i o n
method h a v e p r o v e n e f f e c t i v e , i n a n i m a l s , s u c h
a s f i s h and
m o l l u s c s , w i t h a r r e s t e d
m e i o s i s
p r i o r
t o f e r t i l i z a t i o n are
c a n d i d a t e s
( A l l e n a n d
S t a n l e y ,
1981). T r i p l o i d h y b r i d s
b e t w e e n p l a i c e a n d f l o u n d e r
are
o b s e r v e d t o b e
m o r e s t e r i l e
v
111. MATERIALS AND METHODS
*
The experiments were conducted a t t h e hatchery o f F i s h C u l t u r e and F i s h e r i e s Department o f Wageningen A g r i c u l t u r e U n i v e r s i t y , t h e Netherlands
fram
October1987
t o September 1988 i n t h e f o l l o w i n g phases.1. I n d u c t i o n o f r e p r o d u c t i o n and t r i p l o i d y o f
C l a r i a s
batrachus
L . c o v e r i n g :1) I n d u c t i o n o f spermatogenesis,
2 )
Determination o f s t r i p p i n g l a t e n c y time, 3 ) c o l d shocking eggs,4)
sperm i r r a d i a t i o n ,5 )
up t o 8 ) gynogenesis.2. I d e n t i f i c a t i o n o f t r i p l o i d f i s h .
3. Growth performance o f t r i p l o i d and d i p l o i d f i s h .
1.
M a t e r i a l s1.1.
P a r e n t a l f i s h , husbandry o f f r y and experimental f i s h#
Larvae o f t h e Asian c a t f i s h
( C l a r i a s b a t r a c h u s )
were c o l l e c t e d from a f i s h pond i n Kabupaten B l i t a r , East Java,f i s h served as p a r e n t a l f i s h . The experiment, s t a r t e d when t h e f i r s t g e n e r a t i o n had reached an age o f
14
months and ew e i g h t o f
200
-
600 g. The techniques used f o r a r t i f i c i a l induced breeding a r e d e s c r i b e d by Zonneveld e t a l .(1988).
F r y produced f o r experiments 2 andJ
were r a i s e d i n 250 1 g l a s s f l o w through tanks, p u t a t i v e d i p l o i d( D
group) and t r i p l o i d ( T group) f i s h were k e p t s e p a r a t e l y . They were f e d n a u p l i i Artemia s a l i n a f o r t h e f i r s t two weeks, f o l l o w e d by a commercial t r o u t d i e t f e d from two weeks onwards a t a r a t i o n o f 16.8 g.kg-O-o.d-z, recommended by Hoogendorn(1981)
as t h e o p t i m a l f e e d i n g r a t i o n f o r commercial p r o d u c t i o n o f C l a r i a s g a r i e p i n w .I n t h e w e i g h t range o f 1 t o 20 g t h e i n c i d e n c e o f " r u p t u r e d i n t e n t i n e syndroms" (RIS) has o f t e n been r e p o r t e d f o r
C l a r i a s
g a r i e p i n u s (Boon e t al.,1987).
J u v e n i l e f i s h seemed t o be l e s s s u s c e p t i b l e t o RIS when f e d a t a low l e v e l . F o r t h i s reason f e e d i n g r a t i o n s were lowered i n t h e mentioned weight range.One s e t o f a q u a r i a w i t h water r e c i r c u l a t i n g system were used f o r eggs i n c u b a t i o n and c o l d shocking treatments. F o r l a r v a e r e a r i n g and growing were used one s e t o f a q u a r i a
-
( 4 aquaria, volume
400
1 ) w i t h f l o w through water system, and one s e t of a q u a r i a( 2 0
aquarla, volume 80 1 ) w i t h water r e c i r c u l a t i n g system were used f o r f e e d i n g experiment.23 2 l / m i n u t e f o r each aquarium (volume 140 1). The dissolved oxygen c o n c e n t r a t i o n o f t h e i n f l o w i n g water was k e p t near
4
s a t u r a t i o n and was always above 40 % s a t u r a t i o n f o r tire
o u t f l o w i n g water. C o n c e n t r a t i o n o f NH4* and
NOn-
never exceeded v a l u e s o f 2 and1
mg/l r e s p e c t i v e l y , w h i l e pH v a l u e s ranged from 7 t o 7.5.1.2. Hormone
The cPE ( c a r p p i t u i t a r y e x t r a c t ) manufactured by Crescent Research Chemicals, V i r g i n i a USA were
used
f o r i n d u c t i o n o f eggs o v u l a t i o n i n t h e a r t i f i c i a l r e p r o d u c t i o n . The cPE powder was suspended i n 0.9 % NaCl (cPS) p r i o r t o i n j e c t i o n .1.3. Equipment
The equipmept f o r analyses o f p r o t e i n ( k j e l t e c ) , f a t ( s o x l e t t ) , and energy (bomb c a l o r i m e t e r ) were used f o r d e t e r m i n i n g body composition. The c e n t r i f u g e , p l a s t i c tubes
(2.5
c c ) , s y r i n g e and needle were used f o r blood sample*
a n a l y s i s . The f l o w cytometer was used f o r r e d blood c e l l s (RBC) o r DNA measurement. The s p e c i f i c a t i o n o f t h e machine
Machine t y p e : Fluorescence Associated C e l l - S o r t e r (FACStar).
Measurements
:Forward scatter
CFSCI;
Side scatter LSSC3;
Fluorescence
1 CFL11;
F l u o r e s ~ e n c e
2
CFt21.
'Set-up
:laser
:488 nm
filter: long pass 585 [default).
The signal from the size measurements
( F S Cand
S S C )were
linearly amplified.
F L Iwas amplified logarithmic,
FL2was
amplified linear.
A 1 1
solutions used for washing, f i x a t ~ a n
and staining of blood cells were filtered with a 0.2 mm
bacterial filter before usage.
Only FSC
and
FL2were used
to measure cells size and amount of DNA respectively.
2.
Methods
2.1.
Induction of reproduction and triploidy
2.1.1.
Artificial reproduction
Three to four days prior to hypophysation the parental
2.1.2.
Cold shocking eggs4
About 200 eggs per sample were f e r t i l i z e d w i t h m i l t and incubated a t 27OC i n p l a s t i c c i r c u l a r chambers
(diameter
10
cm), which were provided w i t h a gauze bottom. Cold shocking was c a r r i e d o u t by t r a n s f e r r i n g t h e eggs from water o f 27*C t o water o f S°C. T h i s was done a t v a r i o u s times a f t e r f e r t i l i z a t i o n . The d u r a t i o n o f t h e shock was c o n s t a n t ( R i c h t e r e t al., 19871 .
A f t e r t h e c o l d shock treatment, t h e eggs were t r a n s f e r r e d again t o water o f 27OC. The e f f e c t s o f t h e treatment were measured by undeveloped eggs(U.D),
hatching r a t e o f deformed l a r v a e(H.D.)
and hatching r a t e o f normal l a r v a e ( H . N . ) . These were expressed as percentages o f number o f eggs incubated.2.1.3.
Assessment o f t r i p l o i d yThe e f f e c t i v e n e s s o f cold-shocking c.q. t h e suppression of t h e second m e i o t i c d i v i s i o n o f t h e egg was determined by f e r t i l i z i n g u n t r e a t e d eggs ( c o n t r o l o f sperm
2.1.4.
I r r a d i a t i o n o f sperm* The m i l t stock was d i l l u t e d 1 : 1 O w i t h
0.4
%NaCi
s o l u t i o n t o p r e v e n t sperm a c t i v a t i o n . Samples o f
10
m l were spread on a l a r g e watch g l a s s ( i n o r d e r t o o b t a i n a t h i n l a y e r o f spermatozoa) and placed on a p e t r i d i s h f i l l e d w i t h i c e . The m i l t was mechanically s t i r r e d d u r i n g i r r a d i a t i o n ( Komen e t al., 19881 .
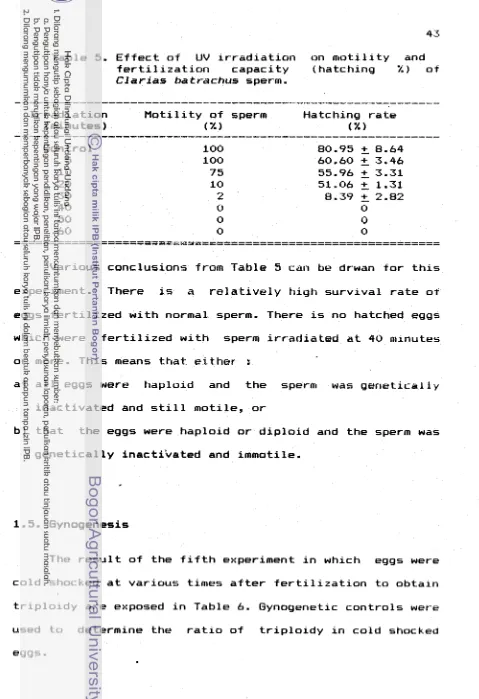
The m o t i l i t y o f sperm(%I
was e s t i m a t e d immediately a f t e r t h e treatment. The d i s t a n c e between t h e lamp and t h e sperm sample was 25 cm. Samples o f200
eggs were mixed w i t h 100 u l o f i r r a d i a t e d sperm ( c o n t r o l on sperm i r r a d i a t i o n ) o r w i t h u n t r e a t e d sperm ( c o n t r o l on eggs q u a l i t yI.
The h a t c h i n g r a t e o f t h e f e r t i l i z e d eggs( % I
was c a l c u l a t e d .2.1.5.
Experimental designs and s t a t i s t i c a l methodsExperiment
1
(23-10-1987)
I
t h e s e m i n a l i s v e s i c u l a somatic index ( S V S I ) were determined
and
t h e sperm q u a l i t y b o t h o f s t r i p p e d m i l t and o f m i l t #o b t a i n e d from t h e t e s t i s was determined by
e g g
f e r t i l i z a t i o n .
Experiment 2 ( 11-11-1987
T h i s experiment was c a r r i e d o u t t o determine t h e b e s t s t r i p p i n g l a t e n c y t i m e f o r
C l a r i a s
batrachus k e p t under "Wageningen hatchery c o n d i t i o n s " . Twenty females were hypophysized and d i v i d e d i n 5 subgroups o f 4 females, whichwere
s t r i p p e d a t l a t e n c ytimes
o f 13, 15, 17, 19, and 21 hours, r e s p e c t i v e l y . I n c u b a t i o n o f f e r t i l i z e d eggs wasI
c a r r i e d o u t a t 29OC and s u r v i v a l r a t e s were c a l c u l a t e d a f t e r w a r d s .
*
Experiment 3 ( 19-11-1987, 24-11-1987, 7-12-1987, A1-12- 1987 )
The t h i r d experiment was designed t o determine t h e
/
28 eggs were estimated. The p l o i d y was n o t determined i n t h i s experiment. T h i s experiment was repeated t h r e e times, because o f unexpected t e c h n i c a l problems, which c o u l d have a f f e c t e d t h e r e s u l t s .
Experiment 4 ( 30-11-1987)
T h i s experiment was c a r r i e d o u t t o determine t h e e f f e c t o f i r r a d i a t i o n d u r a t i o n on g e n e t i c a l i n a c t i v a t i o n and m o r t a l i t y o f sperm. Four males o f
C l a r i a s
batrachus were s a c r i f i c e d and t h e t e s t e s were grinded. The i r r a d i a t i o n d u r a t i o n s o f sperm were 5, 10, 20,30,
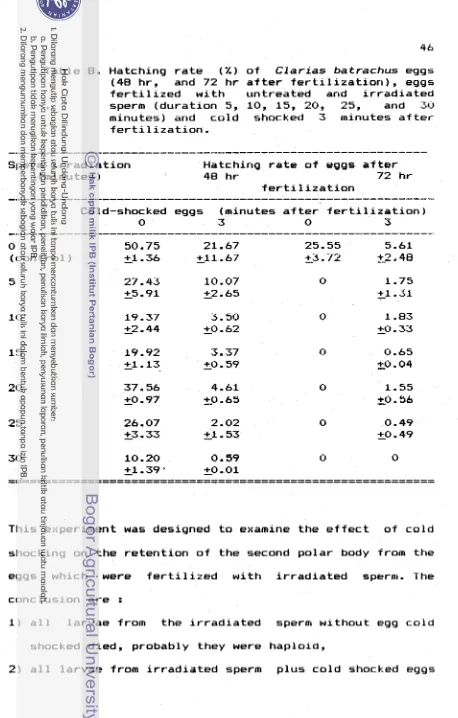
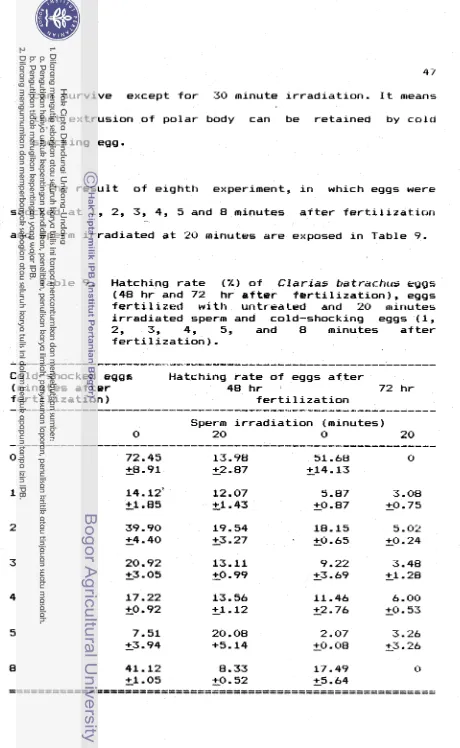
40, 5 0 , and 60 minutes. Sperm m a t i l i t y and t h e hatching r a t e o f f e r t i l i z e d eggs were used as parameters i n t h i s experiment.Experiment 5, 6, 7 and 8 (22-12-1987,7-1-1988, 20-4-1988, 4-5-1988)
.
embryonic development. The e f f e c t o f t h e l a t t e r was compared w i t h hatching percentages o f non t r e a t e d eggs and
I
sperm. Untreated eggs were a l s o f e r t i l i z e d w i t h i r r a d i a t e d sperm t o
check
t h e sperm c a p a c i t y t o f e r t i l i z e deggs.
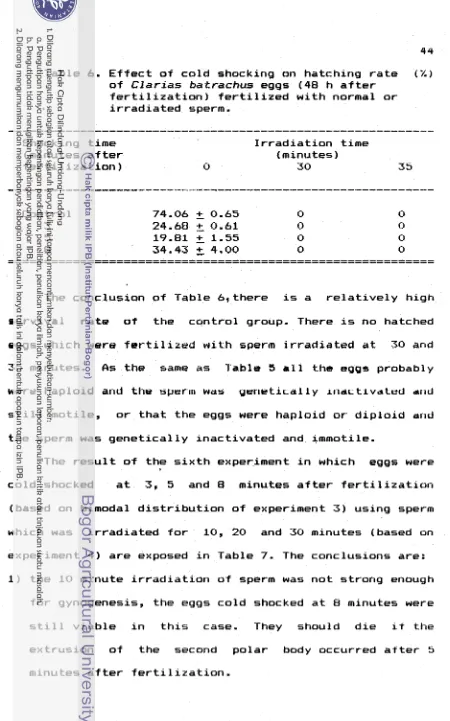
I n t h e f i f t h experiment, eggs were cold-shocked a t 3, 5, and 8 minutes a f t e r f e r t i l i z a t i o n . They were f e r t i l i z e d e i t h e r w i t h u n t r e a t e d sperm o r w i t h i r r a d i a t e d sperm
( i r r a d i a t i o n d u r a t i o n 30 and 35 minutes).
I n t h e s i x t h experiment, eggs were c o l d shocked a t 3, 5,
and
8 minutes a f t e r f e r t i l i z a t i o n . D u r a t i o n o f sperm i r r a d i a t i o n was 5, 10, 20, o r 30 minutes.In
t h e seventh experiment, eggs were c o l d shocked a t 3 minutes a f t e r f e r t i l i z a t i o n , and sperm i r r a d i a t e d f o r 5, 10, 15, 20, 25, and 30 minutes.I n t h e e i g h t experiment, eggs were c o l d shocked a t 1, 2, 3, 4, 5, and 8 minutes a f t e r f e r t i l i z a t i o n , and sperm i r r a d i a t e d f o r 20 minutes.
Hatching r a t e o f eggs, 48 h r and 72 h r a f t e r f e r t i l i z a t i o n ware used
as
v a r i a b l e i n these experiments. Hatching r a t e o f 48 h r a f t e r f e r t i l i z a t i o n i s t h e hatching r a t e o f eggs, i n c l u d i n g deformed and h a p l o i d larvae. *Hatching rate
=((nl)/(nl+ud+dl))SlOO
nl
=normal larvae
0
ud
=
undeveloped eggs
dl
=
deformed larvae
(Richter et dl.,
1985)
Statistical analyses
The data were tested for normality using W-values and
homogeneity of variance using Bartlett's test (Sokal and
Rohlf, 1981).
The data were normalized by arcsine square
root transformation and subsequently, difference between
groups were tested with students t-test (Sokal and Rohlf,
1981). Calculation of
W
values, t-student test, and Anova
was performed using An Interactive Statistical Analysis
Program for Microcomputers by NH Analytical Software (Nimis
and Heisey, 1982).
2.2.
Identification of triploid
and
diploid fish
2.2.1.
Mass production of diploid and triploid fish
.
The cold-shocked
fish, putative triploid
( T )and
untreated fish or diploid
(D)
were mass produced using the
best procedure from previous experiment.
Five Clarias
suspended w i t h p h y s i o l o g i c a l NaCl 0.9 %
.
A l l eggs were mixed and placed i n 5 t r a y s .One p a r t o f them were f e r t i l i z e d w i t h 1 p a r t o f t h e mixed sperm and incubated i n water o f 27OC t o produce t h e normal ( d i p l o i d ) f i s h . T r i p l o i d f i s h were produced by f e r t i l i z i n g t h e remaining eggs w i t h sperm and c o l d shocking them from 27% t o 5% a t 3 minutes a f t e r f e r t i l i z a t i o n f o r 20 minutes (based on t h e experiment 3 and 8 ) .
Eggs hatched a t 4/3/88. F i s h were r a i s e d i n 250 1 g l a s s f l o w t h r o u g h tanks a t (25
t
1 ) O C and were fed n a u p l i i A r t e m i asalina
f o r t h e f i r s t two weeks, and a f t e r t h i s p e r i o d a commercial t r o u t d i e t a t a r a t i o n o f16.8 g.kg-o-6.d-a (Henken e t a l , 1987; Hoogendorn,
1981).
2.2.2. I d e n t i f i c a t i o n o f t r i p l o i d f i s h
Blood samples o f 1.5 m l were drawn from t h e caudal v a s c u l a t u r e o f , 4 0 randomly s e l e c t e d f i s h o f b o t h group
D
washing procedure was repeated t h r e e times. A f t e r t h e t h i r d d e c a n t a t i o n c e l l s were suspended i n TBS c o n t a i n i n g 1 % #
f o r m a l i n and s t o r e d o v e r n i g h t a t 4%. The n e x t morning samples were again washed t h r e e times w i t h TBS-Na. A f t e r washing packed c e l l s were suspended i n 1 m l o f a s o l u t i o n c o n t a i n i n g 5 % propidium i o d i d e ( a DNA s p e c i f i c f l u o r e s c e n t dye) and 1 % N a - c i t r a t e and vortesed f o r 1 minute. The sample was s y r i n g e d through a 26 gauge needle t o a v o i d clumping. Sample were s t o r e d f o r two hours a t room temperature. A f t e r s t a i n i n g samples were again washed t h r e e t i m e s and resuspended i n a volume o f 3 m l TBS. Flow c y t o m e t r i c a n a l y s i s was performed w i t h i n two hours a f t e r t h e l a s t washing.
DNA f l u o r e s c e n c e (FL) and c e l l s s i z e
(FSC),
which i s p r o p o r t i o n a l t o t h e amount o f DNA present, was measured u s i n ga
FACStar f l o w cytometer. Fluorescence o f 10,000 t o 20,000 c e l l s were measured from every f i s h , and t h e modal v a l u e ( W ) was kecorded. The corresponding channel number [mean peak l o c a t i o n[MPLI)
was chosen as assay u n i t , s i n c e i t i s s u f f i c i e n t l y l i n e a r ( t r i p l o i d M P L=
1.51
d i p l o i dMPL
).
-
2.2.3. S t a t i s t i c s
c a l c u l a t e d , u s i n g t h e f o l l o w i n g formula o f A.
where XI
=
c r i t i c a l l e v e lX
=
a r i t h m a t i c mean peak l o c a t i o n t,-a=
t - v a l u e a t n-1 degrees o f freedom s=
standard d e v i a t i o n .OC = c r i t i c a l l e v e l a t 0 - 0 5
2.3. Growth performance o f t r i p l o i d and d i p l o i d f i s h
The feeding experiment was done i n two steps. The f i r s t experiment was done when t h e f i s h a t 109 days o f
age
( t h e gonad o f normal f i s h s t a r t e d t o develop). T h i s experiment was conducted from J u l y t o August 1988. The second was done when t h e f i s h a t 178 days of age ( t h e normal f i s h was mature), T h i s experiment was conducted from August t o October 1988. Each experiment r e q u i r e d two weeks o f a d a p t a t i o n and 6 weeks o f feeding. Both experiments used t h e same p o p u l a t i o n o f f i s h , which were produced i n March 1988 (mass p r o d u c t i o n ) .
2.3.1. Experimental desiqns
-
Both experiments contained groups o f u n t r e a t e d ( d i p l o i d , C D l l and o f c o l d - t r e a t e d ( t r i p l o i d , L T I ) f i s h . W i t h i n group D and T, f o u r feeding r a t i o n s were employed, r e s u l t i n g i n 8 treatment combinations
( 2
p l o i d y and 4resulting in t h e best feed conversion ratio for
C l a r l a s gariepinus(Hoogendorn,
1 9 8 1 ) .Each treatment combination
#
w a s carried o u t in duplicate. D ~ s t r i b u t i o n
o ft h e various
treatment combinations over t h e experimental aquaria
1 5given in Fig. 3. Treatments w e r e not placed a t random s i n c e
t h e laboratory condition
was
sufficiently homogeneous.
.... ... I';'-"-"..." .. r-.----.-- r-.---.----. .
-
r-- --.-- ,--.--
...-...- .-....-
.
---.- p-..-.--..-. . "-.,
r."---"--l1
I i
I I
,Ii
i
I
I I
I
I
I I
I
I
I IIi
111
211
311
411
511
611
711
8
1 1I
II
I
I
I
I I
I
I
I I
I
I
i I
I
I l l C D , l lII
CT,1111 CD,2111 CT,2111 CD,3111 CT,3111 CD,4111 CT,4111 [image:140.540.44.504.12.763.2]II
<I I
I I
II
I I
I I
II
I I
II
...
...
I
.L--r ....-..-.
4 L .--..-...-...-,. 4 L .--....-.-..- 1 L ...-.-.. 1 I...-.--- ..-.. -2 L ..-...-.-...-.. --.-J L. ..--- ..-...-.... I L I. . . . . . . . . .
.
.
-
"-( ,"."...*l.--""-"..l P.."""'." .-.--....-....,
r.-"".-.." ,.".- -.-.--
... ..-..--.--, C' I 1I
I
I I
I
II
I11
I
I
I I
I
I I I11
911
1011
1111
1211
1311
1411
19
II 16 II1 I
I I
I I
11 II
I I
II
I I11
I I C T r l l II CD,4111CT,41 l l C D , l I llCT,21 llCD,23 llCT,31 llCD,31
ii
I
I
I
II
II
I11
I I
I
I I I I I L.&rL-czL-;-z :----.."-...--- l L :2::zzz::-;- f,C= .L:;z;;: :z;;2:yI:: 2.;=;;
I' J-L. IL'Tzz;zz;:z ,JJ;::; L:z;-LTI';:L !,k ;;:,;,: I::yL.-Ploidy
: D=
diploid ,(normal)
T=
triploid (putative)
1 2 1=
numbers of aquaria.
CD.11, CD,2I,.CD,41 =
diploid fish with feeding ration
1,2,.4
o f optimum feeding ration for
C l a r i a s gariepinus.CT,13, ~ ~ ~ 2 1 . .
L T .
,41=
putative triploid fish with feeding
ration
1,2. . 4optimum
feeding
ration
for Clarias
gariepinus.F i g u r e 3.
Schematic representation of t h e
2.3.2.
E x p e r i m e n t a l procedure*
A t f e e d i n g experiment 1, 360 f i s h were s e l e c t e d from b o t h D and T group. Both samples were d i s t r i b u t e o s e p a r a t e l y o v e r 8 a q u a r i a , r e s u l t i n g i n
16
groups o f 45 f i s h each. F i s h were a l l o w e d t o adapt t o i n e wenvironment f o r two weeks. I n t h ~ s p e r i o d they were f e d 12.6 g.kg-O-Q.d-L. A t day Q , when t h e experiment s t a r t e d , t h e number o f f i s h i n aquarium was reduced from 45 t o 35. The f i s h had reached an age o f 123 days and a weight o f 50.1 g a t t h a t time. Ten randomly sampled f i s h o f b o t h group D and T were used f o r assessment o f body c o m p o s i t i o n a t t h e s t a r t o f t h e experiment. Ten f i s h o f each sex and p l o i d y were sampled f o r d i s s e c t i o n .
fit f e e d i n g experiment
2
each group c o n t a i n e d 40 f i s h a t t h e s t a r t o f t h e a d a p t a t i o n p e r i o d , which was lowered t o 25 a t day 0. F i s h had reached an age o f 192 days and a w e i g h t o f 114.1,g a t t h a t time. Other procedures used a r e t h e same as i n experiment I.I n t h e evening o f day I t h e e x p e r i m e n t a l f e e d i n g r a t i o n s were i n t r o d u c e d f o r t h e f i r s t t i m e u s i n g conveyar- b e l t t y p e feeders. The f i s h were f e d c o n t i n o u s l y o v e r n i g h t . . Mean f r e s h body weight and m o r t a l i t y o f e v e r y aquarium was determined b i w e e k l y and r a t i o n s were a d j u s t e d a c c o r d i n g l y . Feeding stopped a t l e a s t 12 hours b e f o r e weighing.
A t t h e end o f t h e experiment [day 43) f i s h were weighed and counted and sex r a t i o s were determined. A t
l e a s t s i x f i s h from every aquarium were sampled f o r body c o m p o s i t i o n a n a l y s i s .
*
A t d i s s e c t i o n body weight, weight o f ovary o r t e s t e s and seminal v e s i c l e , m e s e n t e r i a l f a t weight and g u t t e d weight (body weight minus weight o f a l l i n t e s t i n e s ) were determined. Together w i t h t h e samples taken a t t h e s t a r t and a feed samples t h e
16
f i n a l samples were analyzed f o r d r y m a t t e r , crude p r o t e i n ( N K j e l d a h l Y 6 - 2 5 ] , crude f a t( h e x a n e - e x t r a c t i o n ) and g r a s s energy.
i
2.3.3.
S t a t i s t i c sThe f o l l o w i n g v a r i a b l e s were c a l c u l a t e d f o r each o f t h e 16 f i s h groups :
V a r i a b l e s on g r o w t h and body c o m p o s i t i o n :
-
M e t a b o l i c g r o w t h r a t e = MGR=
Cg-kg-o-=,d-a) MGR=
GR / pWgO-eGR
=
(We-
Wo)/dBWg
=
exp ( t l n W e + I n Wo)/2) GR = growthrate
=
CglBWg
=
geometric mean body w e i g h t=
L k g l Wo and Wt = w e i g h t a t day0
and day t=
Cg] d = t i m e (day)-
Feed c o n v e r s i o n r a t i o CFCRI:FCR = F /
G
F = amount o f feed g i v e n
=
CglG = f r e s h body weight g a i n ( W e
-
W O ) = Cgl-
P r o t e i n g a i n = PG=
[g-kg-o-e-d-llPG = RP /
1000
/BWgO-*
Po and Pt = p r o t e i n i n t h e f i s h a t day O and day t C X I
.
- F a t g a i n
= F G = Cg.kg-o-e.d-*3I
F G = R F / 1000 / bWgO-P
RF
=
r e t a i n e d f a t = (Wt*Ft)-
( k * F o )=
C Q ~
-
Energy g a i n=
E G=
CkJ.g-13E G
=
E t-
E OE t and EO
=
energy a t day t and day 0=
C i ~ J . g - ~ l .- P r o t e i n e f f i c i e n c y r a t i o (PER)
= Cg.g-ilPER
=
G / GPG
=
weight g a i n = ( W t-
WO) = CglGP
=
gross p r o t e i n=
p r o t e i n provided = Cg3-
Apparent n e t p r o t e i n u t i l i z a t i o n (app.NPU) =C % l
NPUa= (RP / GP)
t
100 %= C % l
RP
=
r e t a i n e d p r o t e i n = CglGP = gross p r o t e i n = p r o t e i n provided
=
Cg1- E f f i c i e n c y o f energy g a i n
= EEGE E G
=
E G / GE = C % lEG = amount o f energy g a i n = CkJ.9-=I
GE
=
gross energy i n food=
u n i t o f energy provided Ck3.g-.+I-
F a t - p r o t e i n r a t i o=
FPHFPR
=
percentage o f f a t d i v i d e d by percentage o f p r o t e i n ( f r e s h w,eight b a s i s ) .-
F a t percentage, p r o t e i n percentage and energy i n d r y m a t t e r and/or f r e s h m a t e r i a l s .D i s s e c t i o n v a r i a b l e s
-
Ovarian weight-
M e s e n t e r i a l f a t weight-
W e i g h t
a f t e r g u t t i n g as t h e f i s h weight a f t e r - r e m o v a l n t t h e i n t e s t i n e-
Gonado somatic index(GSI
C % l )
as gonad weight S 10938
-
Fat index (FSI) a 5 mesenterial fat weight
$1 0 0 divided
by fresh body weight.
-
Coddition factor a s (Weight/Lengthf)*lOO
-
Yield after gutting
=
((Weight after gutting)S100)
divided by fresh body weight.
*
Dissection data
were tested for normality using
Kolmogorov-Snirnov
( Wvalues) and for homogeneity using
Bartlett's test. When necessary data were normalized
using
an arcsin square root transformation, N o apriory tests were
performed on data concerning growth and body composition
since there were only two replicates for every treatment
combination. Further, data were analyzed by analysis
o fvariance. Both body composition and dissection data were
initially analyzed with the following
6NOVAmodel:
where
:=
value of 'the
jSeeding level group and i
ploidy
=
overall mean
=
effect of ploidy ( i
=
2,
3
for diploids and
triploids respectively)
=
effect of feeding ration
I j
=1,2.,3,4)
8
Fj1
=
two-way interaction between ploidy
and feeding ration
I V . RESULTS
1,
I n d u c t i o n o f r e p r o d u c t i o n and t r i p l o i d y *1.1.
I n d u c t i o n o f spermatogenesisThe r e s u l t s o f t h e i n d u c t i o n o f spermatogenesis experiment a r e presented i n Table 2.
Table 2. Means
2
SD o f t e s t i s somatic index ( T S I ) , s e m i n a l i s v e s i