SYNTHESIS OF BIPHASIC CALCIUM PHOSPHATE MADE
OF EGGSHELLS THROUGH A HYDROTHERMAL METHOD
NOLDY HUTAGALUNG
DEPARTMENT OF PHYSICS
FACULTY OF MATHEMATICS AND NATURAL SCIENCES BOGOR AGRICULTURAL UNIVERSITY
STATEMENT ON THE THESIS AND SOURCE OF
INFORMATION
I hereby declare that the thesis of “Synthesis of Biphasic Calcium Phosphate Made of Eggshells Through A Hydrothermal Method” is my work with
the direction of supervising comitee and has not been submitted in any form to any college. Source of information derived or quoted from the work or not published by other authors mentioned in the text and listed in the refference at the end of this thesis.
Bogor, September 2013
Noldy Hutagalung
ABSTRACT
NOLDY HUTAGALUNG.Synthesis of Biphasic Calcium Phosphate Made of Eggshells Through A Hydrothermal Method. Supervised by KIAGUS DAHLAN and IRMANSYAH.
Biphasic Calcium Phosphate mixed of hydroxyapatite and tricalcium phosphate. A hydrothermal method of synthesizing by mixing CaO as calcium source and (NH4)2HPO4 as phosphate source with distilled water. CaO got from
calcination process made of eggshells. All samples were synthesized using hydrothermal reactor then followed by sintering process. The molar ratio of Ca and P for all samples was 0.5 M : 0.3 M. Hydrothermal temperature and sintering temperature were variated they are 250 ºC, 300 ºC for hydrothermal and 800 ºC, 900 ºC, 1000 ºC for sintering, higher treatment temperature, tricalcium phosphate appears. Final results of synthesis were characterized by using X-ray Diffraction and Fourier Transform Infrared. X-ray Diffraction patterns was ecompared with database Join Committee on Powder Diffraction Standards and found tricalcium phosphate and hydoxyapatite.
Keywords : biphasic calcium phosphate, hydrothermal, hydroxyapatite, tricalcium phosphate
NOLDY HUTAGALUNG.Sintesis Biphasic Calcium Phosphate Berbahan Cangkang Telur dengan Metode Hidrotermal.Dibimbing oleh KIAGUS DAHLAN dan IRMANSYAH.
Biphasic Calcium Phosphate terdiri dari hidroksiapatit dan trikalsium posfat. Metode hidrotermal merupakan metode untuk mensintesis CaO sebagai sumber kalsium dan (NH4)2HPO4 sebagai sumber posfat dengan aquades sebagai
pelarut. Semua sampel disintesis menggunakan reaktor hidrotermal kemudian dilakukan proses sintering. Perbandingan molar antara Ca dan P untuk semua sampel adalah 0.5 M : 0.3 M. Temperatur hidrotermal dan temperatur sintering dilakukan variasi yakni 250 ºC, 300 ºC untuk temperatur hidrotermal dan 800 ºC, 900 ºC, 1000 ºC untuk proses sintering, semakin tinggi suhu maka fase trikalsium posfat semakin banyak jumlahnya. Hasil akhir dari sintesis kemudian dikarakterisasi menggunakan X-ray Diffraction dan Fourier Transform Infrared. Hasil karakterisasi menggunakan X-ray Diffraction dibandingkan dengan database Join Committee on Powder Diffraction Standards kemudian diperoleh fase trikalsium posfat dan hidroksiapatit.
Thesis
Submitted in Partial Fulfillment of the Requirements for the Degree of Sarjana Sains
Department of Physics
SYNTHESIS OF BIPHASIC CALCIUM PHOSPHATE MADE
OF EGGSHELLS THROUGH A HYDROTHERMAL METHOD
DEPARTMENT OF PHYSICS
FACULTY OF MATHEMATICS AND NATURAL SCIENCES BOGOR AGRICULTURAL UNIVERSITY
BOGOR 2013
Research Tittle : Synthesis of Biphasic Calcium Phosphate Made of Eggshells Through A Hydrothermal Method
Name : Noldy Hutagalung Student ID : G74090023
Approved by
Dr Kiagus Dahlan Supervisor
Dr Irmansyah, MSi Co Supervisor
Endorsed by
Dr Akhiruddin Maddu, MSi Head of Physics Department
PREFACE
Praise to my Almighty God for His blessing I could finish this research succesfully. Love and hug to my lovely family for support and pray me all the time specially to my big mommy and daddy, to my sisters Helga, Maria, Yana, and Eve, to my brohers Rommy, David, Elnoa for everything that you gave to make me laugh. My respect and honour to my supervisors : Dr. Kiagus Dahlan, Dr. Irmansyah, M.Si, and Setia Utami, M.Si for all moment to teach me how to do this research well. I thank God to have friends like Jise Hari, Margaretha Septiana, Ratna Sari, Saima Mega, Nasib Pols and PARTARU members for support me all the time. My big thank to 46 Physics student and biomaterialler : Upri, Indri, Budi, Kak Aisyah, Mita for helping me while doing this research, then to Pena stationary : Dede, Vina, Rady and Kania, my dorm mates : Ade, Muga, and Desy. The author hopes much suggestions to make this research will be better and author do hope this research will be usefull to everyone.
Bogor, September 2013
CONTENTS
Sample Characterization by x-ray diffraction (XRD) 7 Sample Characterization by fourier transform infrared (FTIR) 7
RESULT AND DISCUSSION 8
Calcination Process and synthesis of BCP 8
Hydrothermal and Sintering Process 8
Phase, average crystallite size (ACS), and lattice parameter of BCP 9
Phase Groups of BCP 12
Phase of HA with and without Sintering Process 13
LIST OF TABELS
1 Variation of hydrothermal temperature, sintering temperature and
sintering time 6
2 Mass of CaO after sintering process 8
3 Mass of all samples after sintering 9
4 Ratio of HA and -TCP 11
5 Parameter lattice of HA 12
6 Atomic crystall size of all sample 12
LIST OF FIGURES
1 Preparation then followed by calcination process 4 2 Solution and suspension preparation followed by stirring and dropping
process 5
3 Hydrothermal process followed by aging 6
4 Filtration followed by sintering process 7
5 XRD patterns of A1-A3 samples with variations of treatment 10 6 XRD patterns of A4-A6 samples with variations of treatment 11 7 FTIR spectra of A1-A3 samples with variations of treatment 13 8 FTIR spectra of A4-A6 samples with variations of treatment 13
9 XRD patterns of HA1 14
10 XRD patterns of HA2 15
LIST OF APPENDICES
11 Flowchart 19
12 Data JCPDS (Join Committee on Powder Diffraction Standards) 20
INTRODUCION
Background
The bone damage is a serious health problem because bone is one of the most important organs of human. However, bone damage is not only caused by work accidents but diseases can cause bone damage. Some deseases caused bone damage are osteoporoses, bone tumors, certain cancer or a brittle bone desease called osteogenesis.1,2 The bone implantation is the way to treat bone damage and while doing this we need appropriate materials. The composition of material that we will use has to similar with bone composition.
In this era, the development of biomaterial not only talked about synthesis but and characterization but new challanges also. Researchers not only interest focuses primarily on synthesis and characterization, biological behavior of material and porouess are important to know. In order to develop material must have support knowledge of different disciplines and made a colaboration.3 In the
following some interdisiplinaries research that support are biochemistry, biomedical engineering and medical sciences.
An ideal biomaterials that used for implantation should be has biological behaviors such as bioactive, biodegradable, non-toxic and biocompatible with human body.1,2
A biomaterial is a synthetic material used to replace part of living system or to function in intimate contact with living tissue.3 Biomaterial has been acknowledged as a bone graft material in a range of medical and dental applications due to their similar chemical composition with natural bones. Generally, bone substitution materials such as autograft, allograft and xenograft are used to solve problems related to bone trauma and fractures. Material which obtained from the human involved called autograft, from the other human called allograft and from animal called xenograft.2 But, none of these materials provide a perfect replacement of the bone due to mechanical and biological instability and incompatibility. Recently, calcium phosphate bioceramics such as calcium phosphate, tri-calcium phosphate and hydroxyapatite are identified as most suitable bone substitution materials to serve the demand. Unlike other calcium phosphates, HA does not break under physiological conditions. In fact, it is thermodynamically stable at physiological pH and actively takes part in bone bonding.4
A biomaterial is an inert material which implanted into living systems to change living tissue or organ function. The purposes of biomaterials are used to
2
phosphate, hydroxyapatite and biphasic calcium phosphate has difference biological behavior each other.3
Hydroxyapatite is the most commonly used calcium phosphate in the medical fields or dentistry, as it posseses excellent biocompatibility and osteoconductive. It has chemical formula Ca10(PO4)6(OH)2and a Ca/P ratio of 1.67.
The density of this material in theoritical is 3.156 g/cm3. The crystalinity of this material has hexagonal rhombic prisms with the space group being P63/m. Its
units cell contains a complete representation of the apatite crystal, consisting of Ca2+, PO43-, and OH- groups.2,3 The biological behaviors of this material are
bio-inert and resorbable. Synthesis of hydroxyapatite can be prepared by using a variety of method and precursor. The variation of method are wet method and dry method. Wet method by precipitation by involving the neutral reaction of acid and alkaline solutions or calcium salts and phosphate salts, dry method by heating, group R3c with unit cell a=1.0439 nm, c=3.7375 nm (hexagonal setting) with 21
formula units per hexagonal unit cell. The dissolution rate of –TCP was three times higher than that of HA. The dissolution rate increased shown like this : HA < –TCP < α-TCP < TTCP.1,3,5 The biological behaviors of this material are bioactive, bioresorbable, and biodegradable.
HA and TCP have been used, not only for bone implant substitute materials, but have also been identified for applicability as drugs delivery, antiseptics, food supplements, and hypothermia treatment agents. The potential of the material as anticancer agents has been investigated and it has been found that nanoscale HA is effective in inhibiting cancer cells growth.6 HA and TCP although they have similar chemical composition but they different in their biological resorbing
capacity. The mixture material of HA and –TCP is called bihasic calcium phosphate (BCP). Development of BCP especially with HA and TCP has drawn considerable attention.7-10 In medical application they have found as bone graft
substitute. The resorbability of BCP ceramics depends on ratio of HA and –TCP, hight ratio will produce high resorbability.
Some kinds of phosphate have been used to react with CaO of some sources such as eggshell, fishbone, seashell, etc. Kinds of phospate which generally used are H3PO4, (NH4)2HPO4, and Na2HPO4.1-12 The eggshell represents the 11% of
3 Objectives of Research
1 What the effects of variation of temperature, hydrothermal-holding time and sintering-temperature to produce biphasic calcium
phosphate with ratio of hyrdoxyapatite and -tricalcium phosphate ?
2 Hows the final result of characterization by using X-ray diffraction (XRD) and fourier transform infrared (FTIR) ?
Purposes of Research
1 To synthesize biphasic calcium phosphate using eggshells
2 To characterize biphasic calcium phosphate by using XRD and FTIR 3 To get BCP with ratio of HA and -TCP are 60 : 40 and 70 : 30
Usefulness of Research
The useful of this research was to developed biomaterial as bone graft and mixed two different phase which is called biphasic and obtained new calcium source from eggshells.
Scope of Research
The aim of this research was to synthesize biphasic calcium phosphate by hydrothermal method. Calcium source got from eggshells by calcination process by freeing CO2 and (NH4)2HPO4 as phosphate source. Sample was characterized
by X-ray Diffraction and Fourier Transform Infrared.
Hypothesis
Variation of hydrothermal-temperature, hydrothermal holding time and sintering temperature can produce BCP with ratio of HA and -TCP are 60 : 40 and 70 : 30. The ratio as mentioned above can produce an ideal material to implant to human or other living system.
EXPERIMENTAL METHOD
Material and Equipment
The materials which used in this research made of eggshells in chemical form CaCO3 was calcinated by freeing CO2 with final result of calcination was
CaO as calcium source, (NH4)2HPO4 as phosphate source, aquades (H2O). These
4
equipment, and hydrothermal reactor. Samples was characterized by using X-ray Difraction and Fourier Transform Infrared.
Preparation of Eggshell
Washing eggshell to eliminate inner membrane of eggshells by using aquades, then use furnace to calcinate it in 1000°C at heating rate 5°C/minute for 5 hours. At this process eggshel transform from calcium carbonate into calcium monoxide by freeing carbon dioxide (CO2).14 The material which produce of
calcination process is CaO (calcium monoxide). Sample preparation and calcination process are shown by the Figure 1 and the chemical reaction of this process is shown below.
CaCO3 → CaO + CO2
Figure 1 Preparation then followed by calcination process
Preparation of Sample
5 calcium was stirred by using stirrer equipment at 300 rpm for 20 minutes. At 5 minutes left after stirrer was ran then mix calcium solution and phosphate solution by using dropping equipment until stirrer stop. Calcium solution, phosphate suspensions followed by stirring and dropping process are shown by the Figure 2 and the chemical reaction of mixing process is shown bellow :
10CaO + 14H2O + 6(NH4)2HPO4→ Ca10(PO4)6(OH)2 + 18H2O
Figure 2 Solution and suspension preparation followed by stirring and dropping process
Hydrothermal Method
6
Tabel 1 Variation of hydrothermal temperature, sintering temperature, and sintering time
The holding time for all samples were 10 hours but special for HA2 was not sintered and the holding time was 24 hours followed by drying process in 50 ºC for 8 hours. After all process done then solutions was kept in hydrothermal tube
for overnight or generally this process is called “aging”. Hydrothermal process
and aging are shown by the Figure 3.
Figure 3 Hydrothermal process followed by aging Sintering Process
Sintering process starting from dropping process. Aging process produced final solution then solution was filtrated. The residue of solution then moved into crucible then put in to furnace at 800°C, 900°C, 1000°C at heating rate 5 °C/minute for 5 hours but sample must be dried first at 110 °C with heating rate 5 °C/minute for 5 hours. Filtration and sintering process are shown by the Figure 4.
Suspension Hydrothermal
7
Figure 4 Filtration followed by sintering process
Sample Characterization by X-ray Diffraction
All samples characterized by using X-ray diffraction GBC EMMA. 2 gr of each sample putted on holder then setted sample’s name, initial angel 10º, final angle 80º, CuKα = 1.54056 and analysis speed of computer. The purpose of this process was to identify crystal structure and lattice parameter of each sample.
Sample Characterization by Fourier Transfrorm Infrared
All samples charcterized by using Fourier Transform Infrared ABB MB 3000. The FTIR spectra used KBr pellet technique. 200 mg KBr mixed with 2 mg of each sample. The fourier transform infrared spectroscopy employed to characterize the different functional groups of hydroxyapatite and tricalcium phosphate. The FTIR used vibration energy of functional groups of HA and TCP such as PO43-groups, CO32- groups and OH- groups.
Filtration Sintering process Sample after
sintering process
8
RESULTS AND DISCUSSION
Calcination Process and synthesis of BCP
The variety of method to synthesize powder are wet and dry method. Wet method by precipitation by involving the neutral reaction of acid and alkaline solutions or calcium salts and phosphate salts, dry method by heating, and hydrothermal method.1 Two kinds of raw material which commonly used to synthesize BCP are synthetic material and natural material. In this research as calcium source was waste material it was eggshells.
Calcination was the process to get CaO from CaCO3 by using furnace in
1000 °C at heating rate 5°C/minute for 5 hours called optimum condition of caltination. At this process eggshells transform from calcium carbonate into calcium monoxide by freeing carbon dioxide (CO2). Chemical reaction of
calcination process is shown below :
CaCO3→ CaO + CO2
CO2 can be eliminated at higher temperature than 750 ºC, Magnesium (Mg)
can be eliminated in 650 ºC, and organic composition can be eliminated in 80 ºC –
100 ºC.1
The synthesis of BCP produced calcium phosphate ceramics which are have an important position among other biomaterials, because they are considered to be almost fully biocompatible with living bodies when replacing the hard bone tissues, they are hydroxyapatite (Ca10(PO4)6(OH)2) and tricalcium phosphate
(Ca3(PO4)2 ) are currently recognized as ceramic materials that significantly
simulate the mineralogical structure of bone. In this research the molarity ratio of Ca and P was 0.5 M : 0.3 M because concentration ratio of calcium and phosphate of HA was 1.67. The reaction of synthesis in hydrothermal reactor as shown below:
10CaO + 14H2O + 6(NH4)2HPO4→ Ca10(PO4)6(OH)2 + 18H2O
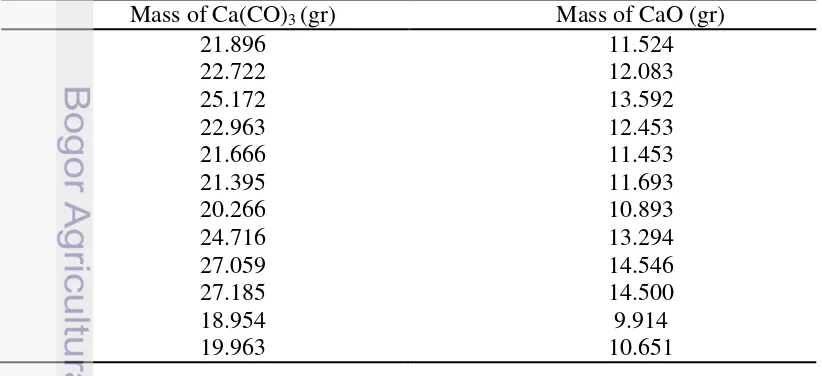
Product of calcination process was CaO and the mass of CaO is shown in Table 2. Table 2 Mass of CaO after calcination process
9 crystal with lattice deffects. The way to obtain large, perfect, single crystal of HA, it is preferable to use the hydrothermal technique.3
Hydrothermal synthesis is characterized by the reaction of aqueous solution in closed recipients under controlled temperature and/or pressure. The temperature can be elevated above the boiling point of water, reaching the pressure of vapor saturation. Hydrothermal method is the best method to get biomaterial high quality, homogenous, high purity, high crystalinity, and high reactivity. There are three main variables in hydrothermal process they are temperature, pressure, and chemist potential. The lattice parameter of HA prepared by the hydrothermal method depend on the reaction temperature and pressure.1
Sintering was the process in high temperature to appear phase of material but not all powder synthesises has to passed this process. The use of natural material such as seashells, eggshells and coral for synthesizing are commonly has to passed sintering process. Although sample had passed hydrothermal process with variety of temperature but it had no effect to appeared phase of material
because hydrothermal process just for creating ion groups of HA and -TCP. Sintering process starting from dropping process. Aging process produced final solution then solution was filtrated. The residue of solution then moved into crucible then put in to furnace at 800 °C, 900 °C, 1000 °C at heating rate 5 °C/minute for 5 hours but sample must be dried first to freeing H2O of
suspensions at 110 °C with heating rate 5 °C/minute for 5 hours.
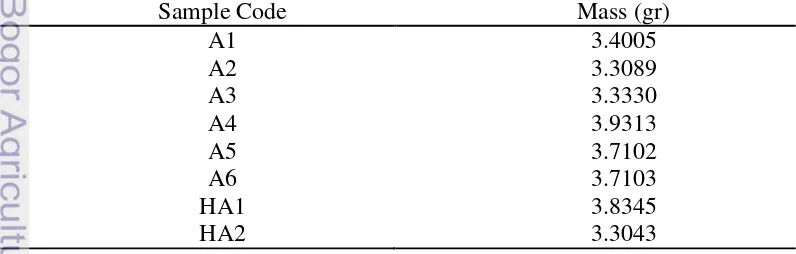
Based on chemical reaction, the result of synthesis was HA but by sintering with high temperature samples might contain TCP because with increasing temperature, the apatite structure changes to a new space group and TCP appears and if in sample contain two different phase it is called biphasic and in this case two different phase were HA and -TCP which is called BCP. The mass of all samples are shown in Table 3.
Table 3 Mass of all samples after sintering process
10
Phase, Lattice Parameter, and Average Crystallite Size of BCP
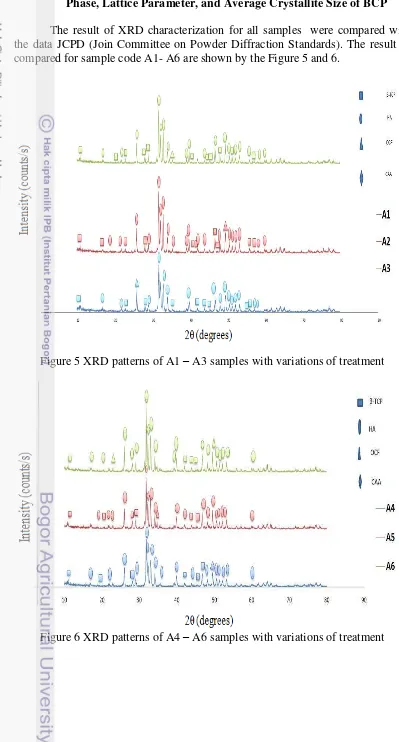
The result of XRD characterization for all samples were compared with the data JCPD (Join Committee on Powder Diffraction Standards). The result of compared for sample code A1- A6 are shown by the Figure 5 and 6.
Figure 5 XRD patterns of A1 – A3 samples with variations of treatment
11 All figures above show that the result of XRD were same generally. Figure 5 shows the result of sample A1, A2, A3, they were same in hydrothermal
temperature β50 ºC but they are different in sintering temperature. That’s why
their characteristic were different each other. According to thermal stability of
HA and –TCP, with increasing temperature, the apatite structure changes to a new space group and TCP apprears.3As mentioned before –TCP is stable up to
11β5°C which is means higher temperature, –TCP appears . Figure 6 shows the result of sample A4, A5, A6, they were same in hydrothermal temperature at 300 ºC but they were different in sintering temperature.
Calcium phosphate bio ceramics such as calcium phosphate, tricalcium phosphate and hydroxyapatite has been acknowledged as a bone graft material in a range of medical and dental applications due to their similar chemical composition with natural bones. Generally, bone substitution materials such as autograft, allograft and xenograft are used to solve problems related to bone trauma and fractures. They are identified as most suitable bone substitution materials to serve the demand. According to result of evaluated based on data JCPDS researcher got nothing sample which contained pure HA and –TCP , researcher got apatite calcium type A (CAA) and octacal cium phosphate (OCP) but majority pahses in each sample were HA and –TCP. The existence of OCP did not matter for ceramics used for implantation because synthetic OCP showed its osteoconductive characteristics in the bone marrow space such as enhance bone regeneration at the initial bone apposition stage and stimulate resorption of the
newly formed bone. In addition, biodegrade rate of synthetic OCP is faster than -TCP ceramics and OCP enchance bone formation more than HA and -TCP ceramics when implanted in living system.13
XRD patterns were used to find phase of material, parameter lattice and atomic crystal size. XRD patterns were compared with data JCDPS and based on result of XRD patterns above we knew that they are different each other specially
for ratio of HA and –TCP. The strongest peak of all samples is appears at 31.7º and it is belong to HA. The result of calculated are shown in Table 4.
12
MDI Jade 6 program. The lattice parameter and average crystallite size are shown in Table 5 and 6.
Table 5 Lattice parameter of HA
Sample code a (Å) c (Å)
Table 6 Average crystallite size of all samples
Sample code ACS (nm)
The lattice constant of HA prepared by the hydrothermal method depend on the reaction temperature and pressure.1 According to the data that we got while
synthesizing BCP higher temperature hydrothermal, higher pressure. The lattice parameters of the synthesized BCP samples were obtained from the diffraction pattern by fitting the peaks of identified reflections. Generally crystallite size of all samples were similar except HA2 because optimum phase of hydroxyapatite would be appeared in high temperature because CaO that we used in this synthesis was wastes material.
Phase Groups of BCP
13
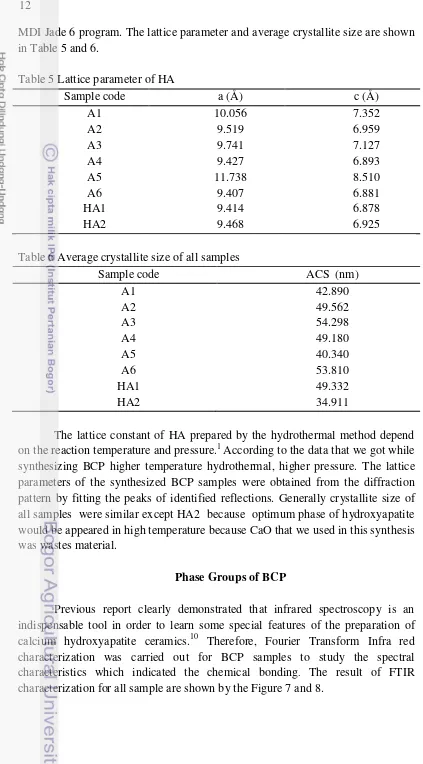
Figure 7 FTIR spectra of A1 – A3 samples with variation of treatment
14
The spectra indicates the formation of a typical HA structure containing PO43- band and OH- band, TCP structure containing PO43- band, and OCP
structure containing HPO42- band. Phosphate characteristic absorption bands
appear at wavenumber 650 - 550 cm-1 is called v4, 1200 – 900 cm-1 is called v3,
Phase of HA with and without Sintering Process
In this research two different samples were addded to synthesize as shown in Table 1 which are different in treatment of variation. For HA1 was hydroxyapatite with temperature of hydrothermal is 150 ºC, hydrothermal holding time is 3 hours, and sintered in 800 ºC for 5 hours. Another one was hydroxyapatite with temperature of hydrothermal 200 ºC, hydrothermal holding time 24 hours without sintering process (HA2). XRD patterns were compared with the data JCPDS. The result of these synthesises were different each other specially for peak strong. HA1 had a stronger peak of all peak in this sample at 31.7° with intensity at 385 counts/s. HA2 had stronger peak at 31.7º with intensity at 267 counts/s. The result of compared for two samples hydroxyapatite are shown by the Figure 9 and 10.
15
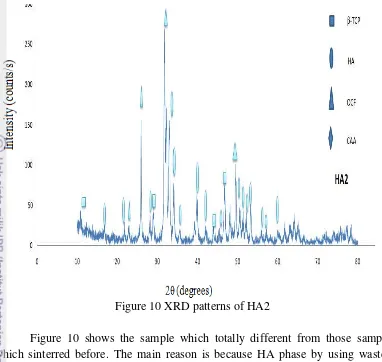
Figure 10 XRD patterns of HA2
Figure 10 shows the sample which totally different from those sample which sinterred before. The main reason is because HA phase by using wastes material such as eggshells, seashells and others wastes material will be created if we sintered in high temperature. When hydrothermal process was run sample synthesized in 250 ºC for 24 hours and analyzed result by using database JCPDS
we got OCP, HA, and -TCP and majority phase is HA. But if we compared result analys of HA2 and sample with sintering process, sample with sintering better than HA2 because the crysstalline structure of this sample was amorphous as we see in Table 6. Otherwise if we use calcium nitrate tetrahydrate (Ca(NO3)2.4H2O)
16
CONCLUSION AND SUGGESTIONS
Conclusion
This research was to synthesize BCP made of eggshells through a
hydrothermal method. Biphasic calcium phosphate was mixed of HA and -TCP.
Calcium source was from waste materials it was hen’s eggshells. The chemical form of eggshells is CaCO3. Calcination was the process to get CaO by freeing
CO2 from CaCO3 using furnace at 1000 °C with heating rate 5 °C/minute for 5
hours. Each sample was mixed of calcium suspension and phosphate solution. Ratio molar of Ca and P was 0.5 M : 0.3 M to synthesize BCP. Hydrothermal was the method to synthesize calcium monoxide be a biomaterial high quality, high crystalinity and high reactivity. Hydrothermal temperature and sintering temperature were variated. For sintering process higher temperature, tricalcium phosphate appears and for hydrothermal process higher treatment of temperature, higher pressure in hydrothermal tube. Three main variables in hydrothermal process were temperature, pressure, and chemist potential.
Product of sintering process was characterized by using XRD and FTIR. XRD patterns were compared with the data JCPDS to know what the phase of
product. The result of comparing process was majority phases were HA and -TCP. The lattice parameter and average cryztallite size were calculated by comparing XRD pattern with database JCPDS. Higher treatment sintering temperature had effect on atomic crystal size of BCP. To support the calculated result of characterized by using XRD we got functional groups of HA and -TCPas PO43-groups, CO32- groups and OH- groups of FTIR characterized.
Suggestions
1. As mentioned before with increasing temperature, the apatite structure
changes to a new space group and TCP apprears that’s why we suggest touse sintering temperature higher than 1000 ºC to get more various of ratio of HA
and -TCP.
2. In this research all samples were characterized by using XRD and FTIR, the author recomend to use SEM to know about the morphologh structure of BCP. 3. To get sample without impurities the residue of filtration must be washed by
using water destilled.
REFERENCES
1. Fajriah HA.Hydrothermal Synthesis of Biphasic Calcium Phosphate.[Thesis]. Bogor. Faculty of Mathematics and Natural Sciences, Bogor agricultural University.2008;1-15.
17 3. Shi D.Biomaterials and Tissue Engineering.New York:Springer.2003;4-15 4. Sudip M, Biswanath M, Apurba D, Sudit SM.Studies on Processing and
Characterization of Hydroxyapatite Biomaterialsfrom Different Bio Wastes. Journal of Minerals & Materials Characterization & Engineering.2012;11(1):55-67.
5. Mohd AAMN, Maryam MR, Zainal AA.Synthesis and Characterization of -Tricalcium Phosphate Ceramics Via Sol-Gel Method.Journal of Nuclear And Related Technologies.2009;6(1):199-205.
6. Asep SFA, Iis S.Low temperature hydrothermal synthesis of calcium phophate ceramics: Effect of excess Ca precursor on phase behaviour.Indian Journal of Chemistry.2009;48A:1492-1500.
7. Victoria EC, Gnanam FD.Synthesis and Characterisation of Biphasic Calcium Phosphate.Trends Biomater.Artif.Organs.2002;16(1):12-14.
8. Nezahat K, Cuneyt TA.Synthesis of Calcium Hydroxyapatite-Tricalcium Phophate (HA-TCP) Composite Bioceramic Powders and Their Sintering Behaviour.J.Am.Cetam.Soc.1998;81(9):2245-2252.
9. Zyman ZZ, Tkachenko MV, Polevodin DV.Preparation and Characterization of Bihasic Calcium Phosphate Ceramics of Desired Composition.J Mater Sci: Mater Med.2008;19:2819-2825.
10. Nuzulia NA.Study of Biphasic Calcium Phosphate Ceramics and
HA-Chitosan Composite Implanted Into Sheep’s bone.[Thesis].Bogor.Faculty of
Mathematics and Natural Sciences, Bogor agricultural University;2009. 11. Earl JS, Wood DJ, Milne SJ.Hydothermal Synthesis of Hydroxyapatite.
Journal of Physics: Confrence series.2006;26:268-271.
12. El BH, Ginebra MP, Vert M, Boudeville P.Wet or Dry Mechanochemical Syhthesis of Calcium Phosphates? Influence of The Water content on DCPD-CaO Reaction Kinetics.Acta Biomaterialia.2008;4:378-386.
13. Sasikumar S, Vijayaraghavan R.Low Temperature Synthesis of Nanocrystaline Hydoxyapatite from Egg Shells by Combustion Method.Trends Bioamater. Artif. Organs.2006;19(2):70-73.
14. Eric MR, Miguel A, Witold B, Victor MC, Diaz EJR, Hernandez R, Rogelio RJ.Synthesis of Hydroxyapatite fro Eggshell.Material Letters .1999;41.128-134.
15. Liga BC, Natalija B.Research of Calcium Phosphates Using Fourier Transform Infra Red Spectroscopy.[www.intechnopen]. Riga Technical University.
18
19
Appendix 1 Flowchart
No
Figure A7 Steps which author did as basic principle to research Materials and equipment preparation
Ready
Sample preparation
Hydrothermal synthesis CaO, (NH4)2HPO4, H2O
Sintering
FTIR and XRD characterization
Data analysis
20
Appendix 2 Data JCPDS of (a) -TCP, (b) HA, (c) OCP and (d) CAA
(a)
21
(c)
22
Appendix 3 XRD pattern of sample A1
XRD pattern of sample A2
23 XRD pattern of sample A3
24
XRD pattern of sample A5
25 XRD pattern of sample HA1
XRD pattern of sample HA2
26
FTIR spectra of sample
27 FTIR spectra of sample A3
FTIR spectra of sample A4
28
FTIR spectra of sample A5
29