Jurnal Teknologi Proses
Media Publikasi Karya Ilmiah Teknik Kimia
6(1) Januari 2007: 31 – 38 ISSN 1412-7814
Thermal Characteristics of Eutectic Mixture of Capric-Lauric Acids as
Phase Change Material (PCM) in Gypsum Board
Medyan Riza
Department of Chemical Engineering, Faculty of Engineering, Syiah Kuala University [email protected]
Abstract
Thermal characteristics of some eutectic mixtures of fatty acids as phase change materials (PCM) for passive solar building heating and cooling application have been studied previously. This study looked at the effect of using capric – lauric acids eutectic mixture with a composition of 65: 35 w/w % as PCM in gypsum board. Capric – lauric acids eutectic mixture has melting point of 17.48oC and latent heat of 133.08 kJ kg-1. The melting point is considered suitable to maintain a comfortable temperature in a space having a moderate ambient temperature. Gypsum board samples were immersed for 1 hour in the PCM and the thermal characteristics before and after immersion were analysed. The 12.5 mm thick gypsum board was found to have PCM content of 25.6 % of the PCM and the Differential Scanning Calorimetric (DSC) showed that the melting point and latent heat were 17.9oC and 33.1 kJ kg-1, respectively. Meanwhile for 6 mm thickness the PCM content was 29.2 %, the melting point was 18.1oC and the latent heat was 36.8 kJ kg-1. From the result it can be seen that the thermal characteristic of PCM - gypsum board has the same properties as the PCM. Indeed, the immersion process also did not affect the physical properties of gypsum.
Keywords: phase change materials (PCMs), thermal characteristic, fatty acids, eutectic mixtures.
Introduction
Thermal energy storage in domestic solar space heating and cooling application has been given attention since it can utilise this renewable energy to reduce the greenhouse gas emissions. It also provides a reservoar of energy to adjust the mismatch between peak and off peak time and meet the energy demand at all times. Thermal energy storage is basically classified as latent, sensible and chemical energy storage. The concept of using latent heat storage as energy saving vehicle provides the advantages of storing a large amount of energy in a small mass/volume and the phase transition occurring at nearly constant temperature.
The selection of PCM has recently been directed towards the use of low melting organic materials in an effort to avoid some of the problems inherent in inorganic phase change materials, such as supercooling and segregation. Special attention has been given to fatty acids since they can easily be obtained from renewable sources such as oils/fats (Feldman and Banu, 1996).
Fatty acids show solid-liquid transitions within narrow temperature ranges. They possess some superior properties over other PCMs such as melting congruency, good chemical stability, non-toxicity and suitable melting temperature range for solar passive heating and cooling applications. In the liquid phases, these materials have surface tensions in the order of 20-30 dyne cm-1 and are therefore high enough to be retained in the structure of the host material. These materials possess elevated latent heat of transition and high specific heat (in the range 1.9-2.1 J g-1 oC). They also exhibit only small volume changes during melting or solidification (example: melting dilatation is around 0.1-0.2 ml g-1). Because of the protected carboxyl group, fatty acids based PCMs are chemically, heat and colour stable, low corrosion activity and non-toxic (Feldman et al, 1995).
PCM can be utilised as a single component or eutectic mixtures (binary mixtures that exhibit fixed melting/solidification points at a certain composition between two single components and act as single component).
In passive solar application, three methods are proposed for the incorporation of a thermal storage material within the construction element: encapsulation of the storage material in high-density polyethylene pellets mixed with a gypsum board material, direct incorporation and simple immersion of conventional board in molten PCM. The latter method contributes to the economic impact of the passive solar wall (Zelba et al 2003).
Several works have been carried out in order to investigate the thermal properties of the binary mixtures of fatty acid and its
compatibility with the building materials. Study that carried out by Feldman and Banu (1996), investigated thermal characteristics of some PCM and found eutectic mixture of butyl palmitate and butyl stearate (49: 41 % weight) with melting point of 17oC and latent heat of fusion of 140 kJ/kg, to be compatible with
gypsum wallboard. Nikolić et al. (2002)
analysed thermal properties of methyl stearate, methyl palmitate and their binary mixtures use for solar storage in gypsum wallboard. No obvious changes in the thermal performance of materials were recorded after 50 thermal cycles. Their findings indicate that the latent heat of fusion has contributed to the overall heat storage capacity in a wallboard impregnated with the esters and their mixtures.
Besides, Shapiro et al. (1987) has shown several PCMs to be suitable for introduction into gypsum wallboard with possible thermal storage applications for the Florida climate. These materials were mixtures of methyl-esters, methyl palmitate, and methyl stearate and mixtures of short chain acids and capric and lauric acid. Although these materials had relatively high latent heat capacity, the temperature ranges required in achieving the thermal storage did not fall sufficiently within the range of comfort for buildings in hot climates. Rudd (1993) analysed coconut fatty acids as PCM in gypsum and found that for absorption of 25 % weight of acids, the melting point is 24.9oC and latent heat is 22.26 kJ/kg. Room scale test showed that the PCM wallboard has an average thermal storage capacity of 24.2 kJ/kg. This varies by only 8.7 % compared to the latent heat recorded by DSC.
Experimental Method
Materials
Fatty acids used in this work are capric and lauric acid with the purity of 0.99, supplied by Palm-Oleo Sdn.Bhd, Malaysia. The chemicals were used without any purification .Capric acid has melting point of 31oC, whereas for lauric acid is 44oC. The eutectic mixture of capric-lauric acids in the weight ratio of 65.5:35 was prepared by melting the acids together at temperature 80oC (Sari et al., 2004).
Gypsum boards with thickness of 6 or 12.5 mm are used in this study. Gypsum board was prepared by mixing gypsum with water in the ratio of 1.25:1 and poured into 20 x 20 x 1.25/0.6 cm size mould. After stiffening, the gypsum samples were cut into 6 x 15 x 1.25/0.6 cm in size. Physical properties of gypsum samples before and after immersion in PCM were tested.
Method
The thermal properties of eutectic mixture of lauric-stearic acids such as melting point, solidification point and latent heat were first characterised. Gypsum board samples (6 x 15 x 1.25/0.6 cm size) were immersed for 1 hour in
eutectic mixture of acids at 60oC. After
immersion, the samples were taken out, dried and the thermal characteristics of PCM-gypsum board were analysed.
DSC Analysis
Differential scanning calorimetric (DSC) Perkin-Elmer Thermal Analysis Seri 7 was used to measure the thermal characteristics of eutectic mixture and PCM-gypsum board. Samples were weighted in a sealed aluminium pan with a mass of 2.5 - 10 mg. The DSC thermal analysis was performed in the temperature range of -10 - +80oC with a heating rate of 5oC min-1 and under constant stream of nitrogen at atmospheric pressure. The melting temperature of the PCM,
Tm, corresponds to the onset temperature
obtained by drawing a line at the point of maximum slope of the leading edge of the peak. The latent heat, ∆Hfus, was calculated as the area under the peak by numerical integration. In
addition, melting or solidification peak temperatures are defined as the temperature of the points, which are located furthest from the base line.
Result and Discussion
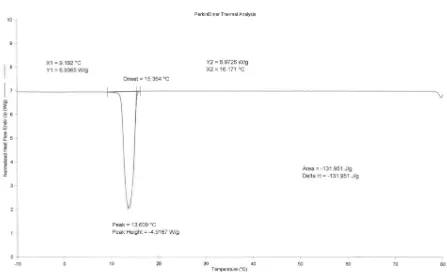
The DSC analysis for eutectic mixtures of capric-lauric acids (65 : 35 w/w %) shows a sharp peak with no secondary peak or hump in the temperature range of -10 – +80oC. It can be seen from Figure 1 that melting temperature, latent heat and melting temperatures range of the eutectic mixture are 17.482oC, 133.081 J g-1 and 14.863 – 22.714oC, respectively. The melting point of eutectic mixtures is lower than those of the single acids. However, the latent heat is high enough that they can be comparable to other PCMs, such as salt hydrates and polyalcohols, which are between 100 – 250 J g-1. Thermal characteristics of solidification process as determined by DSC are shown in Figure 2, which show that the solidification point of 15.364oC, latent heat of 131.951 J g-1 and solidification temperatures range 16.171 –
9.192oC, respectively. The temperature
transitions between melting and solidification processes are 17.482 and 15.364oC and these are considered close. The solid-liquid phase transition was reversible, as it can be seen that heat absorbed as latent heat of fusion was released as heat of solidification (with a small heat loss).
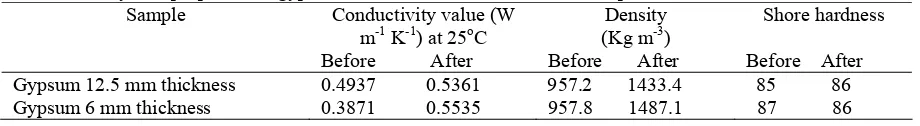
Some physical properties of gypsum board were also tested to show the immersion process do not influence the physical characteristics of gypsum. The conductivity value, which is higher after immersion for better absorption of heat, was caused by the replacement of air in gypsum by acid. The increasing in the thermal conductivity was higher in gypsum 6 mm thickness and this could be due to the higher amount of acid absorbed. The impregnated PCM does not reduce gypsum hardness. Table 4 shows some physical properties of gypsum board sample before and after immersion.
Conclusion
Eutectic mixture of capric-lauric acids (65: 35 w/w %) has melting point of 15.364oC, latent heat of 131.951 J g-1 and close temperature transition. When impregnated in gypsum board, the thermal characteristics of the mixtures are practically unchanged, with sharp peak and no additional peak or hump occurs. Indeed, the immersion process does not affect the physical characteristics of gypsum board. For further study it is recommended an accelerated thermal cycle test to be conducted in order to detect thermal behaviour change (if any) during long-term period of used.
Acknowledgement
The author would like to thank Advanced Oleochemical Technology Centre (AOTC), Malaysian Palm Oil Board (MPOB)
and Palm-Oleo Sdn.Bhd, for their supports of this work.
References
Beghi G. 1982. Thermal energy storage, Dordrecht, Hollaand: D. Reidel Publising Company. Feldman D. and Banu D. 1996. DSC analysis for the
evaluation of an energy storing wallboard,
Thermochimica Acta, 272, 243- 251.
Feldman D., Banu D. and Hawes D. 1995. Low chain esters of stearic acids as phase change materials for thermal energy storage in building, Solar Energy Materials and Solar Cells, 36, 311-322.
Nikolić R., Marinović-Cincović M., Gadzurić S.and Zsigrai I. J. 2003. New materials for solar thermal storage-solid/liquid transitions in fatty acid ester, Solar Energy Materials and Solar Cells, 79, 285–292.
Rudd A.F. 1993. Phase change material wallboard for distributed thermal storage in buildings,
ASHRAE Transaction, 99, Part 2, 3724. Sari A., Sari H., Onal A. 2004. Thermal properties
and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials, Energy Conversion and Management, 45, 365–376.
Shapiro M.M., Feldman D., Hawes D. and Banu D. PCM. 1987. Thermal storage in drywall using organic phase-change material,” Passive Solar Journal, 44, 419-438.
FIGURE 1: DSC thermogram of eutectic mixture of capric-lauric acids (65:35 w/w %) scanned at 5oC min-1 (Heating).
TABLE 1: Percentage of eutectic mixture (PCM) in gypsum board after immersion
Sample Replicate 1
(%)
Replicate 2 (%)
Replicate 3 (%)
Average (%)
Gypsum 12.5 mm thickness 25.5 25.6 25.4 25.5
Gypsum 6 mm thickness 29.7 29.2 30.0 29.6
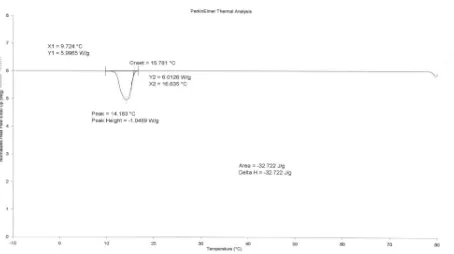
FIGURE 3: DSC thermogram of PCM-gypsum board 12.5 mm thickness scanned at 5oC min-1, loading 25.6 % weight (Heating).
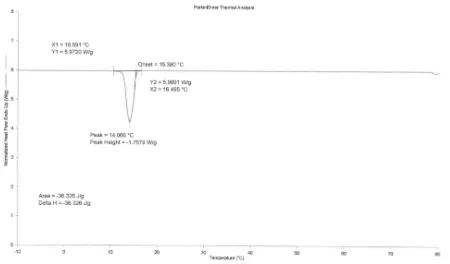
FIGURE 5: DSC thermogram of PCM-gypsum board 6 mm thickness scanned at 5oC min-1, loading 29.2 % weight (Heating).
TABLE 2: Thermal characteristics of PCM-gypsum board (heating) Sample Replicates Melting Point, Tm,
(oC)
Latent Heat
∆Hfus (J/g)
Melting Temperature Ranges (oC)
1 17.7 31.4 15.5-23.8 2 17.9 33.1 15.9-24.0 Gypsum 12.5 mm
thickness
3 16.5 34.0 13.8-23.1
1 18.1 37.3 11.3-23.8 2 18.1 36.8 15.9-23.3 Gypsum 6 mm
thickness
3 18.2 35.1 15.9-23.4
TABLE 3: Thermal characteristics of PCM-gypsum board (cooling) Sample Replicates Solidification
Point, Tm,(oC)
Latent Heat
∆Hfus (J/g)
Solidification Temperature Ranges (oC)
1 15.9 31.3 16.6-9.4 2 15.8 32.7 16.6-9.7 Gypsum 12.5 mm
thickness
3 15.2 33.4 15.9-8.3
1 15.3 35.8 16.1-10.9 2 15.4 36.3 16.5-10.6 Gypsum 6 mm
thickness
3 15.4 34.8 16.2-10.4
TABLE 4: Physical properties of gypsum board before and after immersion process Sample Conductivity value (W
m-1 K-1) at 25oC Before After
Density (Kg m-3) Before After
Shore hardness