Me
PRO
BIOCON
eloidogyne
OMOTIO
NTROL A
e
incognita
ON IN TOM
BR
BOGOR
AGENTS O
a
(Kofoid a
MATO PL
RUCE OC
GRADUA
AGRICU
B
OF ROOT
and White
LANTS (
L
CHIENG’
ATE SCH
ULTURAL
BOGOR
2010
T-KNOT N
e) Chitwoo
Lycopersico
OBURA
HOOL
L UNIVER
NEMATO
od AND G
on esculen
RSITY
ODES
GROWTH
ntum
Mill)
H
I declare that this thesis titled “Root Endophytic Fungi of Tomato and Their Role as
Biocontrol Agents of Root-knot Nematodes
Meloidogyne incognita
(Kofoid and White)
Chitwood and Growth Promotion in Tomato Plants
Lycopersicon
esculentum
(Mill)” was
entirely completed by myself with resourceful help from the Department of Plant
Protection, Bogor Agricultural University. Information and quotes which were sourced
from journals and books have been acknowledged and mentioned where they appear in this
thesis, all complete references are given at the end of the paper.
Bogor, April 2010
BRUCE OCHIENG’ OBURA. Cendawan Endofit Asal Akar Tanaman Tomat dan
Peranannya Sebagai Agen Biokontrol Terhadap Nematoda Puru Akar
Meloidogyne
incognita
(Kofoid and White) Chitwood serta Pemacu Pertumbuhan pada Tanaman
Tomat (
Lycopersicon
esculentum
Mill). Dibimbing oleh SUPRAMANA dan SURYO
WIYONO
Nematoda puru akar, (
Meloidogyne
incognita
) adalah salah satu OPT utama tomat
(
Lycopersicon esculentum
Mill) di seluruh dunia. Tujuan penelitian ini adalah untuk (1)
mengeksplorasi cendawan endofit (2) melihat pengaruh cendawan endofit terhadap
nematoda puru akar (
Meloidogyne incognita
) serta pemacu pertumbuhan pada tanaman
tomat. (3) menginvestigasi mekanisme cendawan endofit menekan nematoda puru akar
secara
in
-
vitro
.
Pada penelitian ini, pengaruh 12 isolat cendawan endofit asal akar tomat yaitu
Nigrospora
sp, isolate XP9,
Fusarium
oxysporum
,
Fusarium
chlamydosporum
,
Chrysosporium
sp,
Trichoderma
hamatum
,
Trichoderma pseudokoningii,
Sterile black 1,
Torula
sp, Sterile black 2,
Ulocladium
sp dan
Fusarium
sp 3 terhadap preferensi inang oleh
Meloidogyne
incognita
serta pengaruh terhadap pemacu pertumbuhan tanaman tomat
dilaksanakan di Laboratorium Nematoda Departemen Proteksi Tanaman Fakultas
Pertanian, Institut Pertanian Bogor (IPB), dan rumah kaca IPB Cikabayan Bogor.
Percobaan ini dilaksanakan mulai dari bulan Februari sampai Agustus 2009.
Hasil penelitian mengindikasikan bahwa dari 12 isolat cendawan endofit yang diuji, 9
isolat yaitu isolate XP9,
Nigrospora
sp,
Chrysosporium
sp,
Fusarium oxysporum, Fusarium
chlamydosporum,
Trichoderma hamatum
,
Trichoderma pseudokoningii,
Sterile black 1,
Sterile black 2 berpotensi untuk menekan nematoda puru akar, baik pada penelitian secara
in vivo
maupun
in vitro
dan juga berpotensi untuk meningkatkan pertumbuhan tanaman
tomat.
BRUCE OCHIENG’ OBURA. Root Endophytic Fungi of Tomato and Their Role as
Biocontrol Agents of Root-knot Nematodes
Meloidogyne
incognita
(Kofoid and White)
Chitwood
and Growth Promotion in Tomato Plants (
Lycopersicon
esculentum
Mill).
Supervised by SUPRAMANA and SURYO WIYONO
Root knot nematode, (
Meloidogyne
incognita
) is a major constraint to tomato
(
Lycopersicon esculentum
Mill) production in the whole world. The aim of this research
was (1) exploration of endophytic fungi from highland and lowland areas in healthy and
nematode infected tomato plants, (2) to assess the potential of endophytic fungi of tomato
to suppress of root knot nematodes as well as in growth promotion of tomato plants (3) ) to
investigate the mechanisms by which endophytic fungi suppress root knot nematodes
in
vitro
In this research, the effect of 12 tomato root endophytic fungi isolates that is
Nigrospora
sp, isolate XP9,
Fusarium
oxysporum
,
Fusarium
chlamydosporum
,
Chrysosporium
sp,
Trichoderma
hamatum
,
Trichoderma pseudokoningii,
Sterile black 1,
Torula
sp, Sterile black 2,
Ulocladium
sp dan
Fusarium
sp 3 against host preference by root
knot nematode
(
Meloidogyne
incognita
) as well as growth promotion in tomato plants. This
research was conducted in Nematology Laboratory, Department of Plant Protection,
Faculty of Agriculture, Bogor Agricultural University, and in the greenhouse at the
University Farm in Cikabayan Bogor from February to August 2009.
The result of this research indicated that from the 12 isolates of endophytic fungi
tested, 9 isolates that is isolate XP9,
Nigrospora
sp,
Chrysosporium
sp,
Fusarium
oxysporum, Fusarium chlamydosporum,
Trichoderma hamatum
,
Trichoderma
pseudokoningii,
Sterile black 1, Sterile black 2 had the potential to reduce root knot
nematode both in vivo and in vitro, and they also had the potential to increase growth of the
tomato plants
BRUCE OCHIENG’ OBURA. A35208861. Root endophytic fungi of tomato and their
role as biocontrol agents of root-knot nematodes
Meloidogyne
incognita
(Kofoid and
White) Chitwood
and growth promotion in tomato plants (
Lycopersicon
esculentum
Mill). Supervised by SUPRAMANA and SURYO WIYONO
Biological control is an environmentally friendly way of controlling plant pests and
diseases. Biological control of root-knot nematodes using endophytic fungi has been
conducted in many studies, hence arises the need to research on the potential of endophytic
fungi against root-knot nematodes in tomato plants. Endophytic fungi appear to be
ubiquitous in healthy plant tissues and much evidence suggests that endophytes of some
plants help hosts tolerate adverse abiotic and biotic factors including pathogens, this
suggests the use of endophytes as biocontrol agents. This study describe issues regarding
endophytes associated with tomato plants
,
with dual goals of how they suppress pathogens
as well as promoting plant growth performance, understanding the abundance and diversity
of endophytes associated with this host, and of assessing those endophytes for use in
biocontrol.
This study was divided into three major sections (1) exploration of endophytic fungi
from healthy and nematode infected tomato plants in highland areas (Puncak) and lowland
areas (Tegallega Central Bogor Indonesia) which resulted to a total of 12 potential isolates
of endophytic fungi
.
(2)
in vivo
trials were conducted to assess the effect of endophytic
fungi treatments on suppression of root knot nematodes as well as growth promotion of
tomato plants and 9 out of the 12 endophytic fungi isolates used that is: isolate XP9,
Nigrospora
sp,
Chrysosporium
sp,
Fusarium oxysporum, Fusarium chlamydosporum,
Trichoderma hamatum
,
Trichoderma pseudokoningii,
Sterile black 1, Sterile black 2
showed significant effect in suppression of root knot nematodes and egg mass formation as
well as growth promotion in tomato plants (3)
in vitro
trials were conducted to assess the
antagonistic mechanisms of endophytic fungi isolates against juveniles of the root-knot
nematodes and 9 out of the 12 endophytic fungi isolates used that is: isolate XP9,
Nigrospora
sp,
Chrysosporium
sp,
Fusarium oxysporum, Fusarium chlamydosporum,
Trichoderma hamatum
,
Trichoderma pseudokoningii,
Sterile black 1, Sterile black 2
showed significant antagonistic effect against root-knot nematode juveniles.
Keywords:
Tomato plants, root-knot nematode,
Meloidogyne incognita,
root
endophytic fungi
© Copyright of Bogor Agricultural University, year 2010
Copy right reserved
1.
No part or whole of this thesis may be excerpted without inclusion or
mentioning the sources.
a.
Excerption only for research and educational use, writing for
scientific papers, reporting, critical writing or reviewing of a
problem.
b.
Excerption does not inflict a financial loss in the proper interest of
Bogor Agricultural University.
2.
No part or all of this thesis may be transmitted or reproduced in any form
BIOCONTROL AGENTS OF ROOT-KNOT NEMATODES
Meloidogyne
incognita
(Kofoid and White) Chitwood AND GROWTH
PROMOTION IN TOMATO PLANTS (
Lycopersicon esculentum
Mill)
BRUCE OCHIENG OBURA
Thesis
Submitted in partial fulfillment of
Master of Science
Major Entomology/Phytopathology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
White) Chitwood and Growth Promotion in Tomato Plants
(
Lycopersicon
esculentum
Mill).
Name : Bruce Ochieng’ Obura
Registration Number: A352088061
Dr. Ir. Supramana. M.Si
Chairman
Coordinator of Major Phytopathology
Dr.Ir. Sri Hendrastuti Hidayat, M.Sc
Examination Date: 7 April 2010
Dr. Ir. Suryo Wiyono. M.Sc. Agr
Member
Dean of Graduate School
Approved
Approved
Advisory Committee
Prof. Dr.Ir. Khairil Anwar Notodiputro, M.S
Thanks be to Almighty God for guiding, strengthening and for His endless blessings
that has seen this research work entitled “Root Endophytic Fungi of Tomato Plants and
Their Role as Biocontrol Agents of Root-knot Nematodes
Meloidogyne incognita
(Kofoid
and White) Chitwood and Growth Promotion in Tomato Plants (
Lycopersicon
esculentum
Mill)” completed.
Sincere thanks goes to my research advisory committee Dr. Ir. Supramana, MSi, and
Dr. Ir. Suryo Wiyono, MSc. Agr, who accorded me invaluable guidance and direction in
conducting this research despite their committed time schedule. Lots of thanks also goes to
my external thesis examiner Dr. Ir. Abdul Munif, MSc. Agr. for the beneficial suggestions
and comments given that shaped the outlook of this thesis.
Special thanks to the Department of Plant Protection for the full support given that
enabled successful completion of this research. Extended thanks goes to Mr. Gatot the
Nematology Laboratory assistant and Ms. Ita of Plant Clinic for all the assistance they
accorded me during the research period, not to forget all the energetic and invaluable
lecturing staff members in the Department of Plant Protection who have in one way or
another imparted knowledge that I acknowledge with gratitude.
Lots of thanks to my sponsor KNB (
Kemitraan Negara Berkembang
) who provided
for my scholarship throughout my masters course. This work would have not reached this
end without the support from KNB.
Further, heartfelt gratitude and thanks goes to my dad, mum, brothers and sisters
Geoffrey, Ismael, Ibrahim, Judith, Afya, Effy and Mercy for all love, care, guidance,
assistance, support, prayers and endless support kindly given during the course of the
research. Special thanks to Uncle John Ong’any Opiyo and his family for all the endless
support, love, prayers kindly given during the course of the research.
I’m very much indebted to appreciate the contributions of Mr. Francis Wanaswa,
Mrs. Sally Wanaswa, Mrs. Christine Kisenga and Mrs. Catherine who contributed
immensely in shaping the outlook of this work as they always provided for academic,
social, moral and psychological support, they always stood by me during the difficult times
of academic work.
Acknowledgement
also
goes
to
my
fellow
colleagues
in
Entomology/Phytopathology major and the KNB family, for the support and assistance
family members, friends and fellow students, may this work help us turn the world into a
better place than we found it. May God bless us all.
Bogor , April 2010
TABLE OF CONTENTS
Page
LIST OF TABLES ... xi
LIST OF FIGURES ... xii
LIST OF APPENDICES ... xiii
I. INTRODUCTION ... 1
1.1 Background ... 1
1.2 Research Objectives ... 3
1.3 Hypothesis ... 3
1.4 Research Benefits ... 3
II. LITERATURE REVIEW ... 5
2.1 Root-knot Nematode ... 5
2.2 Mechanisms of Root Infection by Root-knot Nematodes ... 6
2.3 Taxonomy of Root-knot Nematode (Meloidogyne incognita) ... 6
2.4 Morphology of Root-knot Nematode (Meloidogyne incognita) ... 7
2.4.1 Larva ... 7
2.4.2 Male adult ... 7
2.4.3 Female adult ... 7
2.5 Life-cycle of Root-knot Nematode ... 7
2.6 Anatomy of Root-knot Nematodes ... 9
2.7 Factors Influencing Development of Root-knot Nematodes ... 9
2.7.1 Temperature ... 9
2.7.2 Host suitability ... 10
2.7.3 Soil moisture ... 10
2.7.4 Nutrition availability ... 10
2.8 Root-knot Nematodes as Pest of Tomato Plants ... 11
2.9 Symptoms of Root-knot Nematodes in Tomato Plants ... 12
2.9.1 Above ground symptoms ... 12
2.9.2 Underground symptoms ... 12
Page
2.10 Possibility of Biocontrol by Endophytic Fungi ... 15
2.11 Plant Tissue Colonisation Process by Endophytic Fungi ... 18
2.12 Interaction between Endophytic Fungi and Plant Parasitic Nematodes ... 20
2.13 Antagonistic mechanisms of Endophytic Fungi Against RKN... 20
2.13.1 Antibiosis ... 20
2.13.2 Change in host physiology ... 21
2.13.3 Induced resistance ... 22
2.13.4 Competition ... 22
III. MATERIALS AND METHODS ... 23
3.1 Time and Location of Study ... 23
3.2 Exploration of Endophytic Fungi ... 23
3.2.1 Isolation and identification of endophytic fungi ... 23
3.2.2 Selection of endophytic fungi based on pathogenicity test .... 23
3.2.3 Inoculation of seeds with suspension spores ... 24
3.2.4 Re-inoculation of tomato plants with suspension spores ... 25
3.3 Colonisation Test ... 25
3.4 Meloidogyne incognita Egg Mass Inoculation ... 26
3.4.1 Root-knot nematode extraction and inoculation ... 26
3.4.2 Plant management practices ... 27
3.5 Antibiosis In vitro Test ... 27
3.6 Parameter Observation and Data Analysis ... 28
3.6.1 Assessment of damage in tomato plant roots by RKN ... 28
3.6.2 Assessment of plant growth parameters ... 28
3.6.3 Experimental design and data analysis ... 28
IV. RESULTS AND DISCUSSION ... 30
4.1 Results ... 30
4.1.1 Exploration of Endophytic Fungi ... 30
4.1.1.1 Isolation of endophytic fungi ... 30
4.1.1.2 Pathogenicity test ... 31
Page
4.1.3 Antagonistic Effect of Endophytic Fungi against RKN
in planta ... 32
4.1.4 Effect of Endophytic Fungi on Plant Growth ... 33
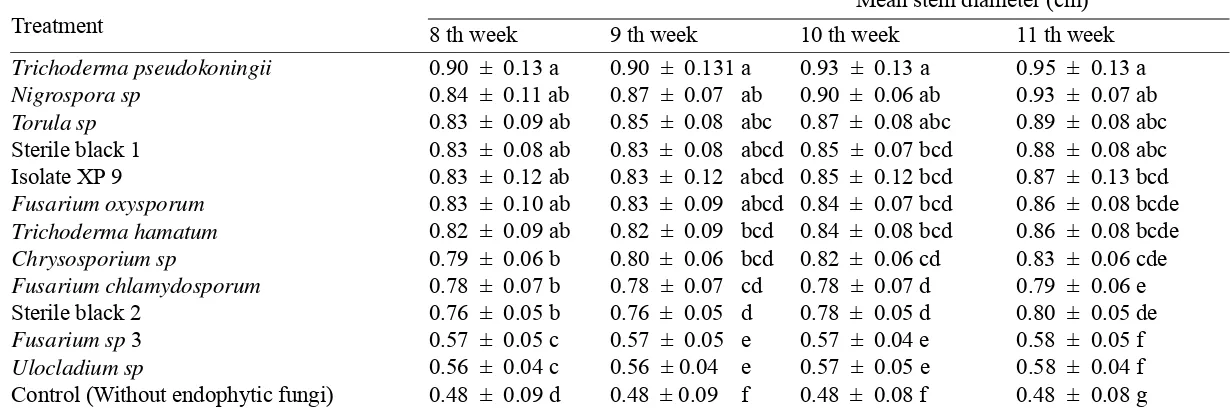
4.1.4.1 Effect on height and stem diameter of RKN inoculated plants ... 33
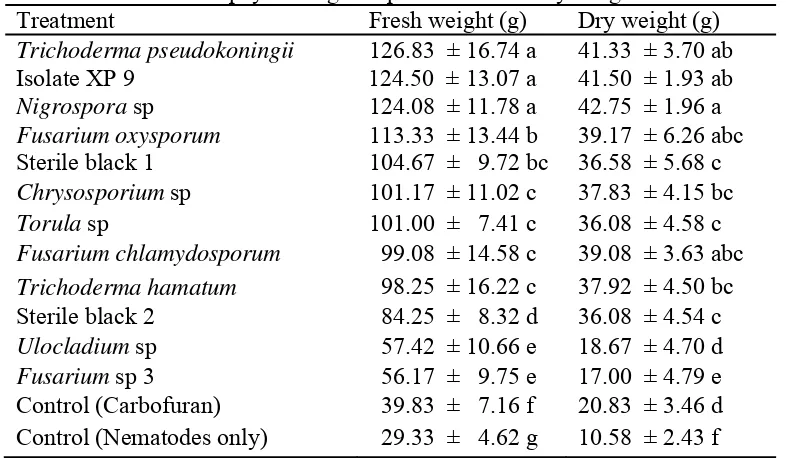
4.1.4.2 Effect on plant fresh and dry weight of RKN inoculated plants ... 36
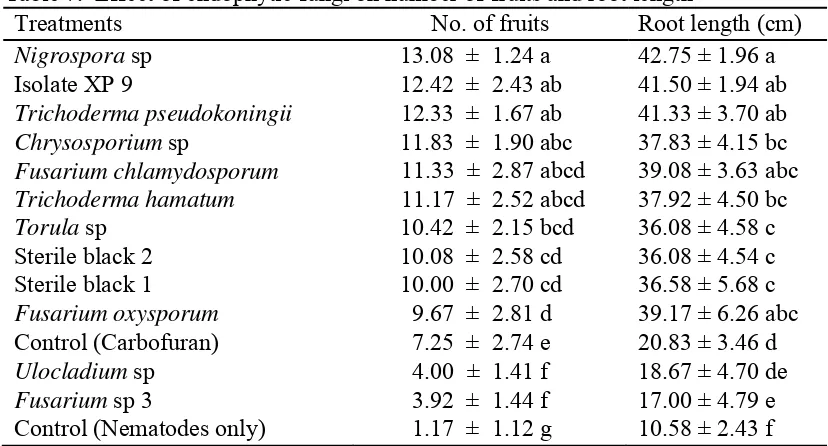
4.1.4.3 Effect on number of fruit and root length of RKN inoculated plants ... 37
4.1.4.4 Effect on height and stem diameter of RKN free plants ... 38
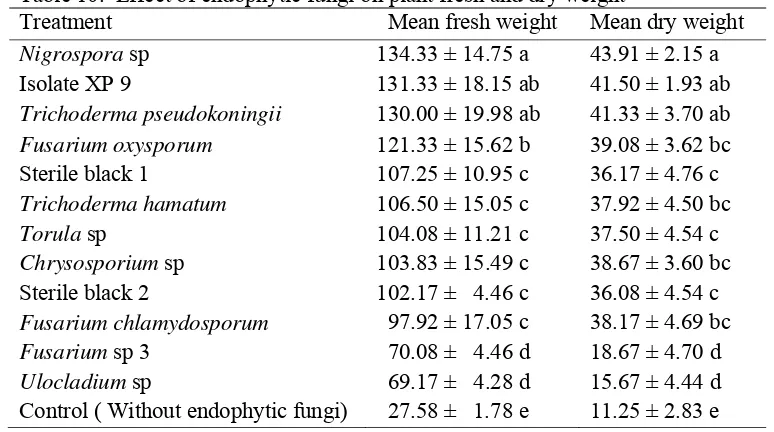
4.1.4.5 Effect on plant fresh and dry weight of RKN free plants ... 41
4.1.4.6 Effect on number of fruit and root length of RKN free plants ... 41
4.1.5 Antibiosis In vitro Test ... 42
4.2 Discussion ... 48
4.2.1 Exploration of Endophytic Fungi ... 48
4.2.2 Colonisation Test ... 49
4.2.3 Antagonistic Effect of Endophytic Fungi against RKN In planta ... 50
4.2.4 Effect of Endophytic Fungi on Plant Growth ... 51
4.2.5 Antibiosis In vitro Test ... 52
4.2.6 Correlation Pattern between In vitro Test and In planta Tests ... 53
V. CONCLUSION AND RECOMMENDATIONS ... 54
5.1 Conclusion ... 54
5.2 Recommendation ... 54
VI. LIST OF REFERENCES ... 55
LIST OF TABLES
Page
1. Endophytic fungi isolated from healthy and nematode infected
roots ... 30
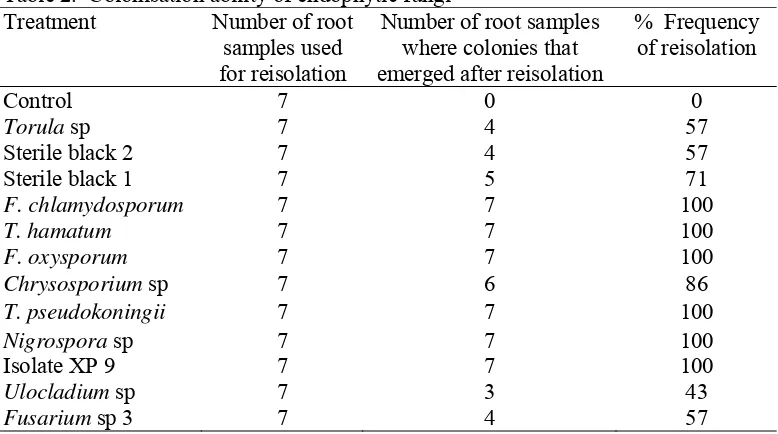
2. Colonisation ability of endophytic fungi... 32
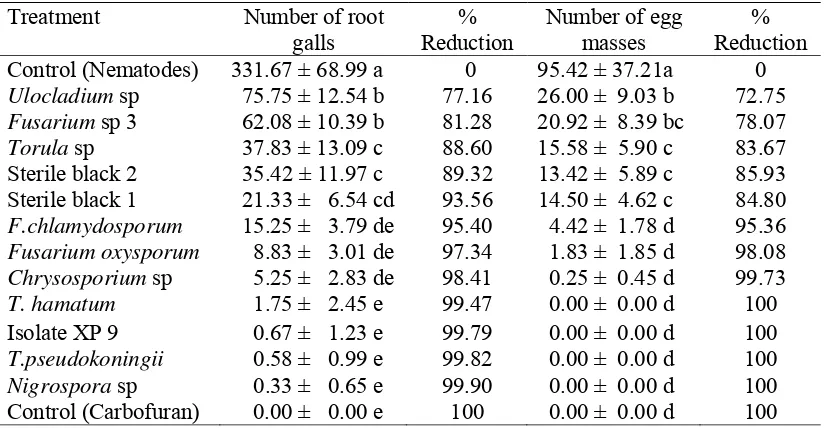
3. Effect of endophytic fungi on number of root galls and egg masses ... 33
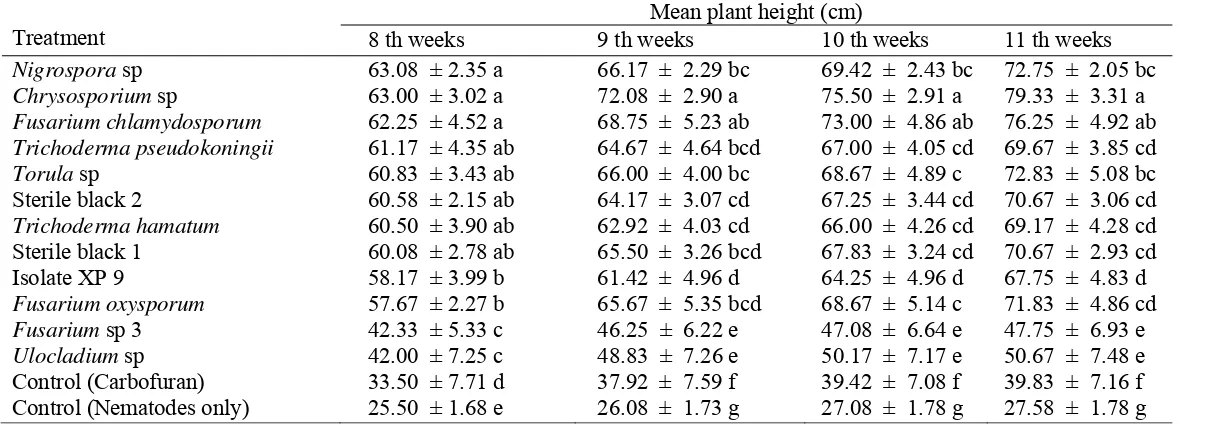
4. Effect of endophytic fungi on plant height of RKN inoculated plants .. 34
5. Effect of endophytic fungi on stem diameter of RKN inoculated plants 35
6. Effect of endophytic fungi on plant fresh and dry weight ... 36
7. Effect of endophytic fungi on number of fruits and root length ... 37
8. Effect of endophytic fungi on plant height of RKN free plants ... 39
9. Effect of endophytic fungi on stem diameter of RKN free plants ... 40
10. Effect of endophytic fungi on fresh and dry weight ... 41
11. Effect of endophytic fungi number of fruits and root length ... 42
LIST OF FIGURES
Page
1. Flow chart diagram on the steps followed in this research ... 4
2. Illustration of lifecycle of root-knot nematodes Meloidogyne incognita in tomato plant roots ... 8
3. Light micrograph of stained endophytic mycelium inside plant tissues showing intercellular colonization of plant tissues by the fungal
endophytes ... 19
4. Pathogenicity test based on germination of tomato seeds on pure
isolates of endophytic fungi and on PDA medium as control ... 31
5. Colonisation test ... 32
6. RKN juvenile percentage mortality rate in different culture
filtrate concentrations ... 45
7. General correlation showing effect of endophytic fungi treatments on reduction of root gall and egg mass formation in comparison to juvenile mortality rate at 30% culture filtrate concentration after
24 hours ... 53
LIST OF APPENDICES
Page
1. Comparison between percentage root colonization rate, percentage
root gall reduction and egg mass reduction by endophytic fungi ... 68
2. Photos showing effect of endophytic fungi on root-knot nematodes in
tomato plants ... 69
3. Photos showing effect of RKN on negative control (endophytic fungi free) and positive control treatments (treated with carbofuran) ... 71
4. Photos shwing effect of endophytic fungi on tomato plants four weeks after re-inoculation ... 72
5. Photos showing effects of endophytic fungi on tomato plant roots four weeks after re-inoculation ... 74
6. Photos showing effect of endophytic fungi on tomato plant roots... 75
7. Photo showing roots of control treatments (without endophytic fungi) .. 77
8. Macroscopic photos of endophytic fungi colony on PDA media
and microscopic photos (Magnifications ×40) ... 78
9. Root-knot nematode attached egg mass perineal pattern of Meloidogyne incognita and pear shaped adult female root-knot
nematode ... 81
I.
INTRODUCTION
1.1 Background
Plant parasitic nematodes cause significant damage and losses to most
agricultural crops in the tropics and subtropics (Luc et al. 2005). The need to
control and manage nematode population to acceptable levels remains a big
concern for nematologists. The need to reduce dependent on chemical control
using nematicides and the increased pressure to use pest control measures that do
not pollute or degrade the environment has provided the impetus for more
research geared towards the search and exploitation of potential biological control
agents of plant parasitic nematodes (Cook 1988). Biological control involves the
reduction of inoculum potential of a disease causing pathogen or parasite in its
active or dormant state by one or more organisms accomplished naturally or by
manipulation of environment, host or antagonists or by mass introduction of one
or more antagonists (Baker & Cook 1974). Stirling (1991) defined biological
control of nematodes as “the reduction of nematode population through the action
of living organisms other than the nematode resistant host plant, and which occurs
naturally, or through manipulation of the environment or manipulation of
antagonists.”
Nematodes have long been known to have numerous antagonists
(Kerry 1987). Several organisms have been described and exploited for the
management of plant parasitic nematodes in agricultural crops. A large number of
organisms including fungi, bacteria, viruses, predatory nematodes, insects and
mites have been found to parasitize on the vermiform stages of nematodes or
female eggs of root-knot nematodes or cyst nematodes (Stirling 1991).
More recently the use of endophytic microorganisms resident within plant
tissues for the protection of plants against pests and diseases has been exploited,
the most studied is the grass endophyte association in which endophytic fungi
associated with grasses have been shown to protect grasses against pest and
diseases, most grass endophytes are members of the Ascomycetes family
unpalatable to herbivores and insects (Clay 1988, 1989) detrimental effects of
grass endophytes on fungal pathogens has also been demonstrated. For example
isolates of Acremonium lolii Link ex Fries, and A. coenophialum Morgan-Jones
and W. Gams showed antibiosis against a range of fungal plant pathogens in
culture (White & Cole 1985). Research on grass endophytes has clearly
demonstrated the nature and extent of protection afforded to the host plants by the
interactions, with mutualistic associations between grasses and endophytic fungi
benefiting the host plants in most circumstance (Clay 1990). In mutualistic
association, endophyte-infected plants are protected from attack by some species
of insects, nematodes and fungi while in return, the endophyte is provided with
shelter and nutrition by the host plant (Latch 1993; Saikkonen et al. 1998;
Schardl etal. 2004).
Although most reports on host plant infection by endophytes concern grass
endophytes, symptomless infection of other plants by endophytic fungi belonging
to diverse taxonomic groups have been known for many years (Carroll 1988).
The presence of endophytes has been demonstrated in many plants, including
important crops such as banana (Brown et al. 1998; Pereira et al. 1999;
Cao et al. 2004a; Cao et al. 2004b; Cao et al. 2005), maize Zea mays L.
(Fisher et al. 1992), rice Oryza sativa L. (Fisher & Petrini 1992), and tomato
Lycopersicon esculentum Mill. (Hallmann & Sikora 1994c; Cao et al. 2004a).
Some principle groups of root colonizing plant beneficial fungi, which have
developed symbiotic relationship with the host plants belong to the Fusarium sp
and Trichoderma sp (Haas & Defago 2005).
In this review the role of endophytic fungi in the management of plant
parasitic nematodes as well as plant growth improvement in agricultural crops is
discussed, since limited information is available on the use of endophytic fungi to
control root-knot nematodes Meloidogyne incognita in tomatoes, this review
focused on existing literature between endopyhtes and plant parasitic nematodes
in grasses and other crops, highlighting the implication of plant infection by
endophytic fungi, and discussed the beneficial effects of endophytic fungi in the
1.2Research Objectives
1. Exploration of root endophytic fungi of tomato.
2. To obtain potential endophytic fungi of tomato that can reduce population
of root- knot nematode and improve plant growth.
3. To investigate the mechanisms by which endophytic fungi suppress
root-knot nematodes.
1.3Hypothesis
1. Treatment of tomato plants with endophytic fungi increases induced
resistance of tomato plants against infection by root-knot nematodes.
2. Treatment of tomato plants with endophytic fungi increases growth
performance of tomato plants.
1.4 Research Benefits
Findings in this study are important from the point of view of environmental
pollution likely to be caused while using chemical nematicides to control
root-knot nematodes in tomato plants. The future prospects looks bright for identifying
endophytic fungi to replace the synthetic dangerous and expensive chemicals used
Figure 1. Flow chart diagram on the steps followed in this research EXPERIMENT 1
Exploration of endophytic
-Isolation of endophytic fungi. -Identification
-Diversity index analysis -Similarity analysis -Pathogenicity test
EXPERIMENT 5
In vitro test to evaluate the effect of endophytic fungi culture filtrate
on root-knot nematode juvenile mortality rate.
EXPERIMENT 2
Colonisation test
EXPERIMENT 3 and 4
In planta test to evaluate the effect of endophytic fungi on root-knot nematodes as well as their effect on
plant growth promotion.
II.
LITERATURE REVIEW
2.1 Root-knot Nematode
Root knot nematode had already been reported by 1885 to cause damage to
various plant species, majorly in the tropical and subtropical regions.
According to Chitwood (1949) root-knot nematodes consist of four main species
based on the perineal morphology pattern of the female adult nematodes and other
morphological characteristics, the four species are Meloidogyne javanica,
M. arenaria, M. incognita, M. hapla. By the year 1988 as much as 61 species of
Meloidogyne had been noted (Einsenback & Triantaphyllou 1991). The root-knot
nematode forms the most important plant parasitic nematode with wide host
range, that is around 2000 plant species (Agrios 2005) and most of these crops are
cultivated crops (Jensen 1972). In Indonesia root-knot nematodes of Meloidogyne
incognita has a wide distribution area with 45.4% prevalence and M. arenaria has
38.6% prevalence (Hadisoeganda 1989).
Root knot nematodes has been known as a disease of vegetable crops since
1855, when (Berkeley 1855) in England first described the disease on cucumber
Cucumis sativus L. roots, (Eisenback & Triantaphyllou 1991). The causal
organism was described as Heterodera radicicola. From 1884 to 1949, root knot
nematodes were considered a single species in combination with cyst nematodes
and referred to by a number of designations (Johnson & Fassuliotis 1984).
Chitwood (1949) described morphological differences among populations, and
re-assigned the root knot nematode to the genus Meloidogyne. At this time
Meloidogyne incognita, M. arenaria, M. javanica, M. hapla and M. exiqua were
recognized primarily on the basis off perineal pattern and other morphological
characteristics.
Initially all root knot nematodes were considered to one extremely
phylophagous species, Heterodera marioni until (Chitwood 1949) re-established
the genus Meloidogyne, although 51 species of Meloidogyne have been described
to date (Jepson 1987), four species are of particular economic importance to
Meloidogyne arenaria, and Meloidogyne hapla. Out of the 1000 root knot
population collected from 75 countries 52% were identified as
Meloidogyne incognita, 30% as Meloidogyne javanica, 8% as Meloidogyne
arenaria, 8% as Meloidogyne hapla and 2% as Meloidogyne exigua or other
species (Taylor & Sasser 1978). M. incognita consists of four races; M. arenaria
has two races, M. javanica and M. hapla show no clearly defined races
M. incognita, M. javanica M. arenaria and M. hapla have the widest host ranges.
M. incognita and M. javanica are commonly found in the tropics, while M. hapla
is a species commonly found in the temperate regions and occasionally in the
cooler upland tropics.
2.2 Mechanism of Infection by Root-knot Nematodes Meloidogyne incognita
The direct mechanical injury inflicted by nematodes while feeding causes
only slight damage to plants. Most of the damage is caused by secretion of saliva
injected into the plant while the nematodes are feeding. The nematodes puncture
cell walls using their stylet, inject saliva into the cells, withdraw cell contents,
they remain sedentary at their feeding site for the whole of their life while feeding
at the site.
The feeding process causes the affected plant cells to react, resulting to dead
or devitalized root tips, lesion forming and tissue break down, swelling and gall
formation, these are caused by the dissolution of the infected tissues by nematode
enzymes which causes tissue disintegration and death of the cells, others are
caused by abnormal cell enlargement (hypertrophy) by suppression of cell
division, or by stimulation of cell division proceeding in a controlled manner and
resulting in the formation of galls, or large number of lateral roots at or near the
point of infection.
2.3 Taxonomy of Root-knot Nematode (Meloidogyne incognita)
Kingdom animalia, Phylum Nematoda, Class: Secernentea, Order
Tylenchida, Family Heteroderidae, Genus Meloidogyne, Species: M. incognita
2.4 Morphology of Root-knot Nematode (Meloidogyne incognita)
Meloidogyne incognita like other a plant parasitic nematode has a colourless
body that is cylindrical in shape (Wallace 1963). Adult female, adult male and the
larva can be differentiated based on their body form.
2.4.1 Larva. First instar (L1) has a blunt tail and molts within the egg, the second instar larva (L2) is hatched and live freely in the soil and look for a host,
according to (Walker 1975) the length of the (L2) is between 375-500 µm with a
diameter of 12-15 µm. The third and fourth larval instas develop within the host
plant tissues.
2.4.2 Male adult. Meloidogyne incognita adult males are stretched cylindrical and are threadlike with the length 1.2-1.5 mm (Agrios 2005). The male
head is composed of head cap and head region provides many good diagnostic
features. The head cap includes labial disk surrounded by lateral and medial lips,
a centrally located prestoma leads to a slit like stoma, four sensory organs
terminate on medial lips (cephalic sensilia), and the head region may or may not
be set off from the remainder of the body.
2.4.3 Female adults. Name of the genus Meloidogyne originated from greek language with the meaning that literally means apple and female because
the body form of the female nematode is apple or pear shaped, with the length of
0.40-1.30 mm and diameter of 0.27-0.75mm (Walker 1975; Agrios 2005) with the
neck of 0.15-0.24 mm wide (Walker 1975), the name Meloidogyne was given for
the first time by Goeldi in the year 1887 (Franklin 1982).
2.5 Lifecycle of the Root-knot Nematode
Root-knot nematodes display marked sexual dimorphism i.e. the females are
pyriform or saccate, the males vermiform. These general differences in the body
form between male and female become established during the post embryonic
development of Meloidogyne incognita. The embryonic development results in
juvenile. This motile vermiform, infective stage migrates through the soil and
enters roots of the suitable host plant, it moves through the plant tissue to a
preferred feeding site and establishes a complex host parasite relationship with the
plant. The second stage juvenile becomes sedentary and as it feeds on special
nurse cells (giant cells), it undergoes more morphological changes, and become
flask-shaped, without further feeding it molts three times into third and fourth
stage juveniles and finally becomes an adult. Shortly after last molt the saccate
adult female resumes feeding and continues to feed for the remainder of her life,
during this post embryonic development, the reproductive system develops and
grows into functional gonads, the sexes can be differentiated based on the number
of gonads (females have two gonads; males only one gonad). The change in shape
from saccate male juvenile to vermiform adult male takes place during the fourth
juvenile stage. The adult male does not feed it will leave the root and move freely
through the soil. Depending on type and mode of reproduction, of a particular
species, amphimixis or parthenogenesis, males may search for females and mate
or remain in the soil and finally die. Length of life cycle of root-knot nematodes is
greatly influenced by temperature, for Meloidogyne incognita is about 29°C, the
first adult females appear 13-15 days after root penetration. The lifespan of
egg-producing females may extend from 2-3 months and they lay upto 2000 eggs, but
that of males maybe shorter.
Figure 2. Lifecycle of the root-knot nematodes Meloidogyne incognita (Source; The American Phytopathological Society 2003)
2.6 Anatomy of Root-knot Nematodes
The nematode body is more or less transparent; it is covered by a
colourless cuticle that molts when the nematode goes through successive juvenile
stages. The cuticle is produced by the hypodermis which consists of living cells
and extends into the body cavity as four chords separating four bands of
longitudinal muscles, the muscles enable the nematode to move.
The body cavity contains fluid through which circulation and respiration
takes place, the digestive system is a hollow tube extending from the mouth
through the esophagus, rectum and anus. Lips usually six in number, surrounds
the mouth. Most plant parasitic nematodes have a hollow stylet or spear that they
use to puncture holes in plant cells and through which to withdraw nutrients from
the cell.
The reproductive system of the nematodes is well developed, females have
one or two ovaries followed by an oviduct terminating in a vulva. The male
reproductive structure is similar to that of the females, but there is a testis, seminal
vesicle, and a terminus in a common opening with the intestine. A pair of
protrusible and, copulatory spicules is also present in males. Reproduction in plant
parasitic nematodes is through eggs and may be sexual or parthenogetic to the
species that lack males.
2.7 Factors Influencing Development of Root knot Nematodes
Many factors limits the growth development of root-knot nematodes,
however there are two major important factors that is temperature and host
suitability (Chrystie 1959).
2.7.1 Temperature. Meloidogyne incognita is sedentary endoparasite and completes its lifecycle in 20-25 days within the root cortex at a temperature of
27°C (Agrios 2005). Between 27°C-30°C the development of female root-knot
nematodes begins from infective larva up to egg hatching going on for 17 days, at
temperature of 24°C, egg hatching goes for 31 days, the longest development
with temperatures below 15.4°C and above 33.5°C the development in root-knot
nematodes will fail to take place up to adult stage.
2.7.2 Host suitability. In suitable host plants, eggs produced by the Meloidogyne incognita are many, the more suitable the host plant the more eggs
produced (Chrystie 1959). Occurrence of continuous infection is influenced by
the host suitability. When the larva has already entered the non-suitable host
tissues, in about 4-6 days this infective larva will leave that plant tissue and
invade another plant, or stay in the latter plant tissues with its life development
experiencing disturbance (Dropkin 1980).
2.7.3 Soil moisture. This will influence the development of the Meloidogyne incognita by determining the time taken for the start of egg hatching.
Egg hatching will be impeded in dry conditions with low moisture levels
(Chrystie 1959). Sufficient soil moisture content (in the field capacity), forms the
best condition for the development of root-knot nematodes, but flooded soils also
will have bad consequences, or even cause death. According to Dropkin (1980)
best conditions for the development of root-knot nematodes is in the soils with
little sand, and not good in clay soils.
Water availability will really determine the life process and at the same time
it is an important media for movement of root-knot nematodes in the soil
(Norton 1978). Low moisture conditions will influence mobility acceleration of
Meloidogyne incognita but has not resulted to death, it only changes physiological
mechanisms of root-knot nematodes. Soil moisture content best for the existence
of the root-knot nematodes ranges between 40-60% from field capacity
(Wallace 1963).
2.7.4 Nutrition availability: Total nutrient availability shows much influence in the population ratio between males and females. According to
Norton (1978) host plants tissue that gives abundant nutrition leads to increased
development of the larva to female while host plant tissue that gives less plant
Based on the experimental results it is known that giving of mineral
nutrients to plants is influential to nematode development. Giving solutions of
N, P and K to tomato and potato host plants increases the production of root-knot
nematode eggs (Dropkin 1980). In plants with much nitrogen, nematode
development also increases, however on the contrary in plants with less nitrogen
availability development of Meloidogyne incognita is impeded (Dropkin 1980).
Existence of excess potassium like in cucumber shows increase in the
development of Meloidogyne incognita although this case does not occur with
M. hapla and M. javanica. In pea plant with excess potassium hatching of the first
egg takes place on the 16th day after inoculation, very different from plants with less potassium, hatching of eggs takes place 40 days after inoculation
(Chrystie 1959).
2.8 Root Knot Nematodes as Pests of Tomato Plants
The species of root knot nematodes found to be most detrimental to tomato
plants are those involved in the destruction of primary roots, disrupting the
anchorage system and divitalization of the root tips and eventually death of the
plant in severe cases. The most wide spread and important are
Meloidogyne incognita, it is found worldwide in tropical and sub-tropical regions
and occurs wherever tomatoes are grown (Bridge & Gowen 1993). Areas where
the nematode is known to occur on tomatoes includeAfrica, parts of Asia, Central
and South America, Cuba, Australia and several countries in Southern Europe.
The root-knot nematode second stage juveniles are short (400-600µm) the
cephalic framework is weakly sclerotized, and has indistinct knobs.
The esophageal gland lobe overlaps the intestine ventrally, and tail tapers to a
pointed tip with a clear terminus. The males of root-knot nematodes are 1.0 to 2.0
mm long, the stylet is about 18-24µm and has distinct knobs. The esophageal
gland lobe overlaps the intestine ventrally. The tail is short and rounded and lacks
bursa. The spicules of root-knot male nematodes open a short distance from the
tail tip, unlike those of the cyst nematodes which opens near the terminus. The
females of the Meloidogyne incognita are swollen and pear shaped, pearly white,
protrude from the galled root tissues, unlike cyst nematodes the female root-knot
nematodes usually remain completely endoparasitic.
The species has a pronounced sexual dimorphism in which males are warm
like vermiform and about 1.0 to 2.0 mm long by 30 to 36 micrometer in diameter
(Agrios 2005). Each female lays approximately 2000 eggs in a gelatinous
substance the first stage juveniles develop inside each egg, the second stage
juvenile emerges from the egg into the soil and this is the only infective stage of
the nematode, if it reaches a susceptible host the juvenile enters the roots become
sedentary and grows thick like a sausage (Agrios 2005). Meloidogyne incognita is
a sedentary endoparasite and completes its life cycle in 20-25 days within the root
cortex at a temperature of 27°C (Agrios 2005). Females lay 20-30 eggs per day
for a period of two weeks (Niere 2001). The eggs hatch in 8-10 days and the
juvenile stages are completed in 10-13 days, the nematode cannot survive more
than six months in soil deficient of the host (Ssango & Speijer 1997).
2.9 Symptoms of Root-knot Nematodes in Tomato Plant
Root-knot nematode infection of plants results in appearance of symptoms,
typical symptoms of nematode injury can involve both above ground and below
ground plant parts.
2.9.1 Above ground symptoms: Infected plants will shows inhibited growth (stunting), yellowing (chlorosis) of leaf, reduced yield, poor quality and
quantity of crop products like the tomato fruits, premature leaf fall, erratic stands,
wilting during the day.
2.9.2 Underground symptoms: Infected plants will show excessive branching of secondary roots, overall development of root galls, injured root tips
and egg masses on the root surface, rough root surfaces with club appearance,
infected roots are small and show necrosis.
Interactions involving fungal plant pathogens and plant parasitic nematodes
have been reviewed previously (Powell 1971a; Webster 1985;
Wheeler 1993). Interaction between Meloidogyne incognita and Fusarium wilt
fungi have received special attention and were documented in 20 crop species.
Interactions of these pathogens were especially obvious when the root knot
nematode infection preceded those of the Fusarium wilt pathogens by 3 to 4
weeks. Majority of studies have established that the presence of root-knot
nematodes increases the incidence and rate of development and severity of wilt or
the mortality of the Fusarium-susceptible and tolerant crops. However the role of
root-knot nematodes in the breakdown or alteration of the monogenic type of
resistance to Fusarium wilt fungi (such as tomato cultivars with the dormant
I-genes against F. oxysporum f.sp. lycopersici) remains controversial and requires
further investigation (Mai & Abawi 1987).
Many example of disease complexes are known (Pitcher 1963;
Powell 1971a; Powell 1971b; Taylor 1979; Webster 1985). Tomato plants wilt
more quickly and can be killed when Fusarium oxysporium is simultaneously
present along side with nematodes, resistance of tomato cultivars to fungal wilt
caused by Fusarium oxysporum f.sp. lycopersici was reduced in the presence of
Meloidogyne incognita (Jenkins & Coursen 1957; Sidhu & Webster 1977).
Damage to the root system caused by root knot nematode attack has been
considered responsible for the increase in the intensity of bacterial wilt caused by
Pseudomonus solanacearum (Valdez 1978) and bacterial canker caused by
Corynebacterium michiganense (Moura et al. 1975). Several of the viruses that
are transmitted by the nematodes cause significant economic losses on major food
crops such as tomato and tobacco ring spot virus. Meloidogyne incognita race 1
was shown to increase wilt caused by both R. solanacearum and
F. oxysporum f.sp. lycopersici on resistant tomato cultivars when inoculated
simultaneously (Chindo et al. 1991). Van Gundy et al. (1977) demonstrated that
leaching of nematode infected plants applied to tomato inoculated with
Rhizoctonia sp resulted in the appearance of severe rots. The presence of root-knot
nematodes play a major role in increasing the incidence and severity of bacterial
wilt diseases caused by Pseudomonas solanacearum on various crops including
Yield loss in tomatoes due to root-knot nematodes in the world has been
estimated to be approximately $ 100 billion world wide annually
(Sasser & Freckman 1987). The root knot nematodes have a worldwide
distribution but are more abundant in warm temperate and tropical soils. Losses
due to Meloidogyne incognita in tomatoes can be as high as 50% (Niere 2001).
In addition to the direct crop damage caused by the nematodes, many nematode
species have also been shown to predispose plants to various infections by fungal
or bacterial pathogens, or to transmit virus diseases.
Several control methods are available for the control of the tomato root-knot
nematodes. The most important cultural control method is use of clean planting
materials, only seedlings with roots free of galls should be selected for
transplanting, the nurseries should also be free from root knot nematodes and seed
beds should be selected on sites where previously there were no host plants. Crop
rotation reduces the impact of root knot nematodes in tropical region and it’s the
main management strategy to regulate the population of nematodes but its success
is often limited because of the wide host range of most root knot nematodes
species and the frequent occurrence of infestation composed of more than one
species (Sikora et al. 1988), galled roots remaining in the field after harvest
should be eliminated by uprooting and destruction, other control practices include
bare fallowing and flooding.
Nematicides are widely used by growers producing fruits for export trade, a
number of organophosphates and carbamates are used. However, their use is often
prohibitive for many resources poor small scale farmers, registered products are
highly toxic, expertise is required for application and most of them have been
phased out of the market. The pesticide usually inactivates the nematode within
the plant tissue or in the soil, which after microbial degradation the nematode
recovers and damage continues (Sikora & Pocasangre 2004).
Possible agents for biological control of root knot nematodes are fungal
antagonists that include nematode trapping or predacious fungi, endoparasitic
fungi, parasites of nematode eggs, and fungi that produce enzymes and
metabolites toxic to the nematode (Coosemans 1993). Research on way of
root knot nematode resistant cultivars would substitute the toxic nematicides
currently in use and permit cultivation where farmers could not previously afford
nematode control (Sikora et al. 2003). Research on the control of root knot
nematodes suggests that no single control strategy will provide complete control
(Sikora et al. 2003). A broad integrated pest management (IPM) approach
including new components of pest control is necessary to safe guard sustainable
tomato production. The biological enhancement of tomato plants with mutualistic
fungal endophytes is a new approach that seems as a strong option for sustainable
and ecologically sound nematode control. Inoculation of tomato plants with
endophytes has resulted in reduced nematode reproduction, numbers and damage
in pot experiments by more than 30% over controls (Sikora et al. 2003).
Conducted research on ability of the fungal endophytes to persist in the tissues of
inoculated plants and the interactions between the fungal endophytes and tomato
plants has yielded conclusive results (Paparu et al. 2004; Niere et al. 1999).
2.10 Possibility of Biocontrol by Endophytic Fungi
Several definitions of endophytism have been proposed
(Carroll 1988; Clay 1990), for the purpose of this research, the term endophyte
refers to fungi or bacteria, which for all or part of their life cycle invade and live
inside tissues of living plants without causing any disease symptom or any
apparent injury to the host (Petrini 1991; Wilson 1995), while epiphytes are
bacteria or fungi that colonize plant surface tissues, in contrast to the epiphytes,
endophytes are contained entirely within plant tissues, are asymptomatic and may
be described as mutualistic (Clay 1990), fungi associated with root rhizospheres
of the plants are called plant growth promoting fungi (PGPF). Some of the
important PGPF belong to the genus Trichoderma and Gliocladium and the
arbuscular mycorrhizal fungi (AMF), which form symbiotic association with plant
roots and are also capable of colonizing the roots of their hosts
(Gera Hol & Cook 2005).
Estimated yield loss due to plant parasitic nematodes range from 20-80% in
tomato production systems (Swennen & Vulysteke 2001). The control methods
system (Niere 2001). The strategic use of naturally occurring antagonistic
organisms to control pest population and increase crop production represents a
viable option (Marshall et al. 1999). Endophytic fungi are potentially effective
biological control agents for plant parasitic nematodes management
(Niere et al. 1999; Sikora et al. 2003). A wide diversity of endophytic fungi has
been isolated from healthy tomato tissues with majority of isolates being from the
genus non pathogenic Fusarium sp (Niere2001;Pocasangre et al. 2000).
Culture filtrates for a number of isolates of non-pathogenic
Fusarium oxysporum screened for in vitro activity against the root knot
nematodes have shown high nematicidal activity causing mortality rates of
82-100%. Production of secondary metabolites by endophytic fungi is thought to
be one of the mechanisms leading to plant pest control, these has been shown for
grass endophytes but has not been elucidated for endophytes of crop plants
(Niere et al. 2002).
The use of endophytes for control of plant parasitic nematodes is relatively
a new approach. Since endophytes spend most of their life cycle inside plant
tissues they are less exposed to the environment factors, hence they don’t entirely
depend on the environment for multiplication and survival
(Siddiqui & Shaukat 2003a). Endophytes occupy a similar niche as pests and thus
are in close contact with the pest which make them an edge over other biological
control agents (Hallmann et al. 1996b; Hallmann et al. 1997b). Inside the plant
tissues the host plant provides relatively uniform and protected environment
enabling the endophytes to avoid microbial competition and extreme
environmental conditions such as fluctuations of temperature and moisture
(Ramamoorthy et al. 2001).
The endophytic fungi are easy to culture in vitro and can be applied as
seed treatments or on transplants, reducing the inoculums levels required
(Sikora 1992; Sikora & Schuster 1999). Another advantage is that once
developed, farmers will not need to apply the control products themselves as this
may be done by public or private organizations engaged in commercial tissue
culture production. Also fungal endophytes can easily be inoculated into tomato
strategy. The use of endophytic fungi from both environmental and economic
point of view has a major advantage over other biological control agents that are
applied directly to the soil. The latter, due to the high levels of inoculums is
needed to treat the soil, are more costly, have to be applied more frequently, and
their efficacy is often strongly influenced by environmental factors. Another
advantage is that endophytic fungi live in plant tissue, thereby reducing the risk of
side effects on non-target organisms (Niere et al. 2002). Once the endophytic
fungi has established and colonized the plant tissues they can be used as
biocontrol agents potential for controlling the root knot nematode in the tomato
plants.
In spite of these advantages of endophytes over other biological control
agents, the potential of fungal endophytes in pest and disease management in
crops remains largely unexplored. Mutualistic endophytic fungi (MEF) can
therefore be defined as fungus that live some time in their lifecycle in a plant
tissues without producing symptoms of a disease, but simultaneously demonstrate
antagonistic activity towards one or more pest or disease affecting the root system
(Sikora et al. 2003). It is assumed that mutualistic endophytes have evolved from
plant pathogenic fungi and that most if not all higher plants host endophytic fungi
(Isaac 1992). Majority of endophytic species which have been successfully
identified are Ascomycetes, Deutromycetes with few Basidiomycetes and
Oomycetes (Isaac 1992). Among the best studied endophytes are intercellular
symbionts in the family Clavicipitaceae found in many cool season grasses which
are known to benefit the host with improved tolerance to heavy metals, increased
drought resistance, systemic resistance to pests and pathogens and enhanced
growth (Arnold et al. 2003).
Endophytes are known to confer resistance to their host against pathogens
through a number of mechanisms that include competitive exclusion, parasitism,
metabolites production and induced resistance. Due to this, they are potential pest
control tools and scientists are using beneficial endophytes as biological control
agents against crop pest such as nematodes, borers and plant pathogenic fungi
(IITA 1998). Their presence has been proven in all plants investigated such as
Mutualistic endophytic fungi have been shown to biologically control root
knot nematodes of tomatoes (IITA 1998). The root knot nematodes attack tomato
plants through the roots, therefore biological enhancement of the tomato plant
using mutualistic fungal endophytes will increase plant resistance to infection
(Sikora & Pocasangre 2004). Endophytes are well adapted to the life inside the
plant and share the same ecological niche with endoparasitic nematodes, thus they
are effective at the exact site of the pest or disease attack
(Sikora & Pocasangre 2004).
2.11 Plant Tissue Colonization Process by Endophytic Fungi
The process of colonization of plant tissues by endophytic fungi are
complex and include host recognition, spore germination, penetration and
colonization. Endophytes penetrate their host plants through natural openings or
wounds or actively using hydrolytic cellulases and pectinases
(Hallmann et al. 1997b), forming inconspicuous infection within healthy plant
tissues for all or part of their life cycles. Plant wounding induced by biotic factors
such as plant-parasitic nematodes also constitute a major factor for the entry of the
endophytic microorganisms (Hallmann etal. 1998).
For many years endophytic microorganisms colonizing plant tissues have
been thought to be weekly virulent pathogens (Sinclair & Cerkauskas 1996). The
distinction between endophytic infection and latent infection is that in latent
infections, the host plant does not show any symptoms, with the infection
persisting latently until symptoms are prompted to appear by environmental or
nutritional stress conditions. The state of host plant and the pathogen may also
provide signals for symptom expression. Since the production of disease
symptoms is dependent upon the interaction between the host, parasite and the
environment over time endophytic colonization is considered not to cause any
disease (Sinclair & Cerkauskas 1996).
To detect endophyte colonization of plants, several methods for in situ
detection of fungal endophytes in plant tissues have been developed. A simple
method involves microscopic examination of differentially stained samples of
consuming and less reliable since histological staining is not endophyte specific
(Hahn et al. 2003). Other methods for in situ detection of endophytes include the
use of monoclonal antibodies (Hiat et al. 1997; Hiat et al. 1999) tissue printing
immunoblotting (Gwinn et al. 1991) tissue print immunoassay
(Hahn et al. 2003), electron microscopy (Sardi et al. 1992) and autoradiography
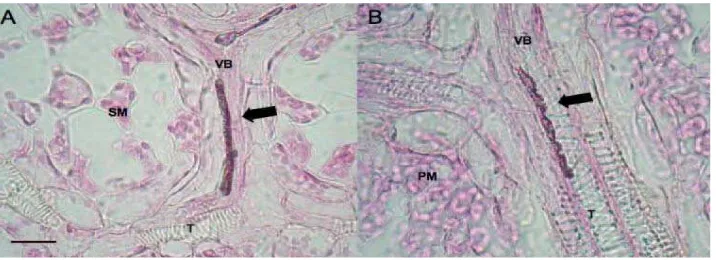
[image:37.612.143.501.214.344.2](You et al. 1995).
Figure 3. Light micrographs of stained endophytic mycelium inside plant tissue showing intercellular colonization by endophytic fungi. A, B. Mycelium(arrow) running along the host vascular bundle (VB) x1000. PM: palisade mesophyll, SM: spongy mesophyll, T: tracheids Bars = 10μm. (Source; Review Iberoam Micology 2007)
Majority of endophytic fungi isolated from healthy tomato tissues belong
to the genus Fusarium, followed by Acremonium, others include soil fungi
belonging to the genera Penicillium, Aspergillus and Gongronella also
Trichoderma which has biological potential is usually isolated(Niere et al. 2002).
The most dominant species is Fusarium oxysporum, which has been
reported as an endophyte of many crop plants including banana, tomato, rice and
maize and is an effective colonizer of plant roots (Niere et al. 2002). However,
Fusarium sp are also notorious as causal agent of Fusarium wilt of many crops
these are distinguished as specialised forms and physiological races, but majority
of isolates of F. oxysporum are non-pathogenic (Niere et al. 2002). Two fungal
endophytes F. oxysporum and Fusarium solani when added to tissue culture
plants were found to be highly effective in immobilizing root knot nematodes
2.12 Interaction between Endophytic Fungi and Plant Parasitic Nematodes Inhibitory effects against some species of migratory and sedentary
endoparasites occur in grasses infected by Neotyphodium endophytes
(West et al. 1988; Kimmons et al. 1990). Neotyphodium species infect aerial
tissues, not roots. Therefore the inhibitory effects observed in the infected plants
were interpreted as a result of fungal alkaloids being translocated to roots.
Non pathogenic Fusarium oxysporum isolated from roots are other groups
of endophytic fungi known to be implicated in the antinematode activity. Culture
filtrates of F. oxysporum have an inhibitory effect on Meloidogyne incognita
suggesting that fungal toxins could be the mechanism of interaction
(Hallmann & Sikora 1996). However the mechanism of Fusarium inhibition of
nematodes appears to be more complex than toxin operated system.
2.13 Antagonistic Mechanisms of Endophytic Fungi Against RKN
Various mechanisms of action by endophytic fungi have been suggested
(Clay 1987). Antibiosis which is the production of toxic metabolites
(Hallmann & Sikora 1994a; Hallmann & Sikora 1994b;
Siddiqui & Ehteshamul-Haque 2001; Li et al. 2002), changes in the host plant
physiology (West et al. 1988; Assuero et al. 2000; Elmi et al. 2000) and the
induction of the general plant defense responses (Kimmons et al. 1990;
Fuchs et al. 1999; Siddiqui and Shaukat 2003b).
2.13.1 Antibiosis: The production of toxic compounds is an important mechanism of action of beneficial endophytic organisms against plant parasitic
nematodes. Grass endophytes mainly those belonging to Neotyphodium sp
produces a wide range of metabolites both in culture and in plants.
The production of alkaloids toxic to both insects and herbivores by grass
endophytes has been documented (Breen 1994). These toxins have been isolated
successfully from pure cultures of grass endophytes. Infection of tall fascue plants
by N. coenophialum resulted in both qualitative and quantitative differences in the
production of volatile compounds between endophyte-infected and endophyte free
The ability of the endophyte infected plants to produce biologically active
compounds depends on the location and concentration of endophyte in plants.
Distribution of these compounds in the plant may also vary depending on the
compound itself and the season. Toxins produced in endophyte-infected plants
may be translocated elsewhere and exuded into the surrounding soil, affecting the
nematode population.
Although toxic metabolites produced by most endophytic fungi in culture
may show antagonistic activity against nematodes in vitro, the role of these
compounds in nematode reduction in plants can only be shown if they are present
in detectable concentrations in plant tissues. Secondary metabolites from
endophytic isolates obtained from tomato cultivars have been shown to have
inactivating or killing effects on the root knot nematode and mortality rates of up
to 80-90% have been recorded (Niere et al. 2002). Majority of isolates that
produce nematoxic or entomotoxic metabolites are F. oxysporum, others include
F. solani, F. concentricum and Acremonium sp (Niere et al. 2002).
Both the type and quantity of secondary metabolites produced in
endophyte infected plants might depend on the fungal genotype. For example tall
fescue endophytes grown in vitro differed in the production of ergot alkaloid
(Bacon 1988). Hill et al. (1990) also found that different isolates of
A. coenophialum from tall fescue plants differed in the amounts and types of
ergopeptine alkaloids produced. The host plant may also affect the production
and concentration of the secondary metabolites and therefore its very important to
determine a compatible host-endophyte-genotype combinations inorder to
maximize the benefits of the association (Hill et al. 1990; Breen 1994;
Siddiqui & Shaukat 2003a).
2.13.2 Changes in host physiology: Endophyte infected plants have improved physiological responses to nematode parasitism; endophyte infected
tall fescue plants has been associated with enhanced root growth and osmotic
adjustments in growing points of the plant, thereby reducing the effects of
Endophytes have also been shown to influence photosynthesis rate in host
plants as seen in tall fescue plants infected by N. coenophialum photosynthesised
faster and flowered earlier than the non-infected ones (Newman et al. 2003), also
endophyte infected tall fescue plants exhibited higher survival and flowering
frequency (Hill et al. 1991). Such attributes of endophyte infection confer an
ecological advantage to the endophyte infected plants, enabling their survival and
dorminance over endophyte free plants.
2.13.3 Induced resistance: Induction of systemic resistance by non-pathogenic microorganisms against pests and diseases is well documented
phenomenon (Rammamorthy et al. 2001; Compant 2005a). For example
non-pathogenic F. oxysporum isolates induced resistance in tomato plants to
F. oxysporum f.sp. lycopersici Jarvis et Shoem, when inoculated prior to infection
by the pathogen. Induced systemic resistance (ISR) can be defined as the
resistance in plants induced by localized infection or treatment with microbial
components or their products, or chemicals compounds
(Rammamorthy et al. 2001). ISR can be differed from systemic acquired
resistance (SAR). SAR develops in plants in response to both biotic (pathogen
attack) and abiotic factors (Chemicals) and depends on the accumulation of the
salicylic acid (Van Loon et al. 1998), the onset of SAR is characterized by the
expression of the genes for the PR-proteins such as PR-1, PR-2, Chitinase and
peroxidase (M’Piga et al. 1987; Rammamorthy et al. 2001; Jeun et al. 2004).
ISR on the other hand is dependent on the jasmonic acid and phenylpropanoid
pathways (Pieterse et al. 1998; Van Loon et al. 1998; Rammamorthy et al.
2001) ISR leads to the synthesis of plant defence products including peroxidases,
polyphenol oxidases and phenylalanine ammonia-lyases (PAL). Polyphenol
oxydase catalyses the formation of lignin through polymerization of phenols while
PAL are involved in synthesis of phytoalexins and phenolic compounds.
2.13.4 Competition: Competition for plant space and resources may occur between resident endophytes and incoming plant pathogens this could eventually
III. MATERIALS AND METHODS
3.1 Time and Location of Study
This research was conducted in Nematology Laboratory Department of
Plant Protection, Faculty of Agriculture and in greenhouse at Cikabayan, Bogor
Agricultural University, Bogor West Java Province, Indonesia from February to
August 2009.
3.2 Exploration of Endophytic Fungi
3.2.1 Isolation and identification of endophytic fungi
Healthy and nematode infected tomato plant samples were collected for
isolation of endophytic fungi from tomato plant roots in highland and lowland
areas. The method used for isolation of endophytic fungi was that proposed by
(Rodrigues 1994) that has already been modified. Tomato plant roots were
washed thoroughly using flowing water to remove all the soil particles from the
roots. Sterilization of the root surface was done by dipping the roots in 70%
ethanol for one minute, and then to 1% NaOCl for three minutes after which the
roots were rinsed three times with sterile water then dried on dry sterile blotting
paper. The roots were then cut into small pieces and placed on petri dishes
already filled with PDA under laminar airflow, in each petri dish three root pieces
were placed and replicated three times then incubated at room temperature and
observed for a period of one week, after which the fungal colonies that developed
from the cut root tissues were purified on new PDA media and the isolates that
developed were tested for their pathogenicity potential then identified based on
colony colour and morphology as well as observation of microscopic features
using identification keys according to (Watanabe 2002).
3.2.2 Selection of endophytic fungi based on pathogenicity test
To ensure that the isolated endophytic fungi were not pathogenic and
produce disease symptoms to the host plants later after inoculation then there was
done by growing tomato seeds on the petri dish containing pure colonies of the
isolated fungi. The fungal colonies where tomato seeds grew were proved to be
non pathogenic while colonies where there were no growth at all or growth was
inhibited were proved to be potential pathogenic isolates, selection of endophytic
and pathogenic isolates was based on the pathogenicity test, the seeds were also
grown on a control petri dish filled only with PDA. At this stage 12 out of the 20
isolates were proved to be potential isolates of endophytic fungi and were used for
further inoculation. The resulting isolates of endophytic fungi included both
sporulating and non-sporulating fungi. These isolates were further used for
in planta test