Hepatitis B
Gontar Alamsyah SiregarDivision of Gastroentero-Hepatology, Department of Internal Medicine, Faculty of Medicine, University of Sumatera Utara / RSUP H. Adam Malik Medan
Introduction
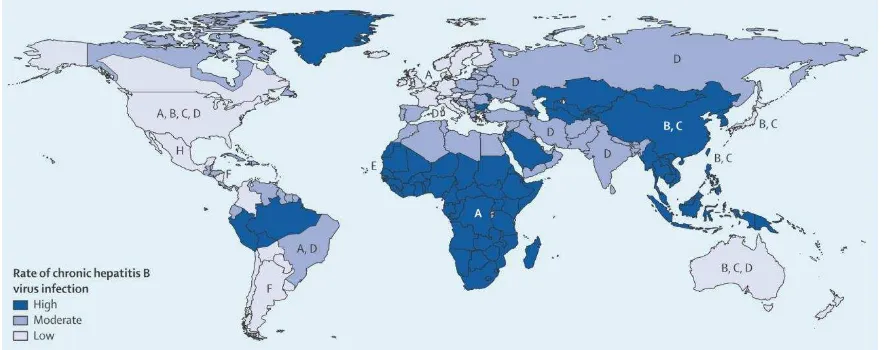
Figure 1. Geographical distribution of major hepatitis B virus and worldwide frequency of CHB 2
Life Cycle and Natural History of HBV
HBV is a small, partially double-stranded DNA virus that belongs to the family Hepadnaviridae. The virion is comprised of a core particle containing the viral genome, nucleocapsid protein and polymerase, as well as a lipoprotein envelope composed of viral antigens. Broadly, HBV is classified into four serotypes (adr, adw, ayr and ayw) based on antigenic determinants of the hepatitis B surface antigen (HBsAg), and eight genotypes (A to H) based on its nucleotide sequence. The genotypes have distinct geographic distributions, and an increasing body of evidence suggests it may also influence disease severity and response to treatment. 5, 6
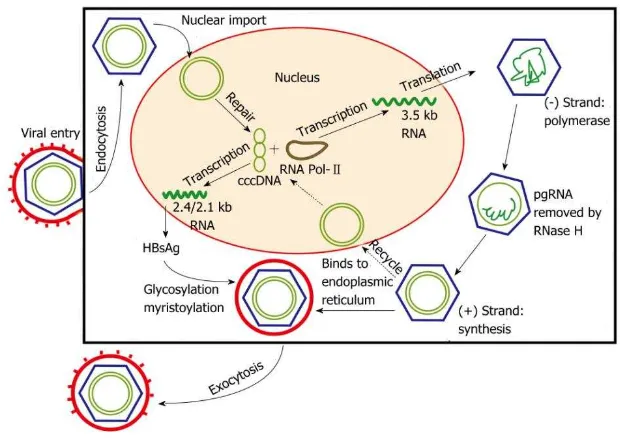
mediate viral persistence, or secreted through exocytosis as Dane particles to infect other hepatocytes. A greater understanding of the viral cycle of HBV will enable new therapeutic strategies. 5
Figure 2. Hepatitis B viral replication cycle 5
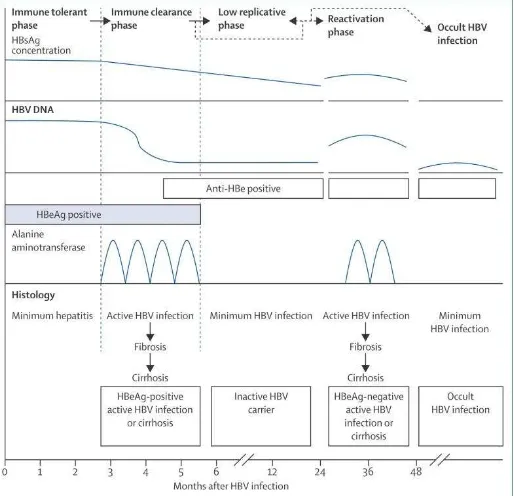
Figure 3. Schematic of the natural phases of CHB 2 Goals of treatment for chronic HBV infection
It is now clear that active HBV replication is the key driver of liver injury and disease progression, thus sustained viral suppression is of paramount importance. Therefore, the primary aim of chronic hepatitis B treatment is to permanently suppress HBV replication. This will decrease the infectivity and pathogenicity of the virus, thereby reducing hepatic necroinflammation. Clinically, the short-term goal of treatment is to achieve ‘initial response’ in terms of HBeAg seroconversion and/or HBV-DNA suppression, ALT normalization, and prevention of hepatic decompensation, and to ensure ‘maintained/sustained response’ to reduce hepatic necroinflammation and fibrosis during/after therapy. The ultimate long-term goal of therapy is to prevent hepatic decompensation, reduce or prevent progression to cirrhosis and/or HCC, and prolong survival. 7
Currently available treatments
Interferon
marrow suppression, depression, and exacerbation or unmasking of autoimmune illnesses. Interferon is contraindicated for patients with liver failure, and should be used with caution in patients with compensated cirrhosis. A 1 year course of pegylated interferon with or without lamivudine in HBeAg-positive patients resulted in HBeAg seroconversion in 29–32% of patients and HBsAg loss in 3–7% of patients 24 weeks after completion of treatment. On follow-up, HBeAg seroconversion was durable in 80% of patients, and HBsAg loss occurred in 58% of patients infected with genotype A and 11% of those infected with other genotypes who had HbeAg seroconversion. In HBeAg-negative patients, a 1 year course of pegylated interferon with or without lamivudine resulted in sustained response, which was defined as normalisation of alanine aminotransferase concentrations and suppression of HBV DNA below 10 000 IU/mL in 25% of patients and HBsAg loss in 9% of patients 3 years after completion of treatment. Addition of lamivudine to pegylated interferon did not improve responses. 2
Predictors of response in HBeAg-positive patients include high pretreatment alanine aminotransferase concentrations, low HBV DNA concentrations, and viral genotype A. The results of a 2013 study suggest that an on-treatment fall in HBsAg concentration is a strong predictor of response to interferon, but different stopping rules apply according to HBV genotype. 80 For HBeAg-negative patients, no consistent pretreatment predictor of response exists but a decrease in HBsAg concentration after the first 12–24 weeks of treatment is predictive of sustained response. Interferon lambda3/IL28B polymorphisms have also been reported to predict response in some but not all studies. 2
Nucleos(t)ide analogues
patients, a year of treatment with nucleos(t)ide analogues resulted in suppression of serum HBV DNA to undetectable concentrations in 51–93% of patients, normalised alanine aminotransferase in 62–78%, and HBsAg loss in less than 1%. Extension of the duration of treatment to 4–5 years maintained viral suppression in 67–99% of patients but the rate of HBsAg loss remained low (0–5%). 8, 9, 10
A major drawback with earlier nucleos(t)ide analogues is the high rate of antiviral resistance but the new drugs—entecavir and tenofovir—have low rates of resistance (1·2% and 0% after 5 years’ treatment). Antiviral resistance because of selection of drug-resistance mutations shown by increased serum HBV DNA concentrations can trigger flares of alanine aminotransferase and liver failure. Patients infected with HBV resistant to one drug are at increased risk of resistance to other drugs that share the same resistance mutations. Therefore, the choice of the initial drug is important to prevent multidrug resistance. 11, 12
Nucleos(t)ide analogues are given orally once daily, with dose adjustment for patients with impaired renal function. They are generally safe, but myopathy and neuropathy had been reported in patients taking telbivudine, and nephrotoxic effects and renal tubular dysfunction in those on adefovir or tenofovir. These uncommon adverse events might increase with duration of exposure. High pretreatment alanine aminotransferase is the most important predictor of response in HBeAg-positive patients. HBeAg-positive patients with normal or slightly raised alanine aminotransferase concentrations have lower rates of undetectable HBV DNA and HBeAg seroconversion. No predictors of response to nucleos(t)ide analogues including HBV or host IL28B genotypes have been identified for HBeAg-negative patients. 2
concentrations. After a median of 32 months, disease progression occurred in 7·8% and hepatocellular carcinoma in 3·9% of the treatment group compared with 17·7% and 7·4% in the control group. Other studies of patients receiving antiviral treatment in clinical practice have also shown a decrease in hepatocellular carcinoma; however, the risk persists in patients with advanced fibrosis or cirrhosis even after complete viral suppression or HBsAg seroclearance. 2
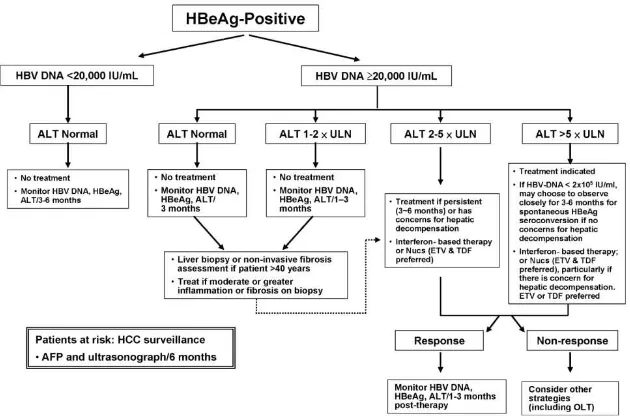
Indications for treatment
Figure 5. Algorithm for the management of hepatitis B (HBeAg)-negative patients with chronic hepatitis B 7
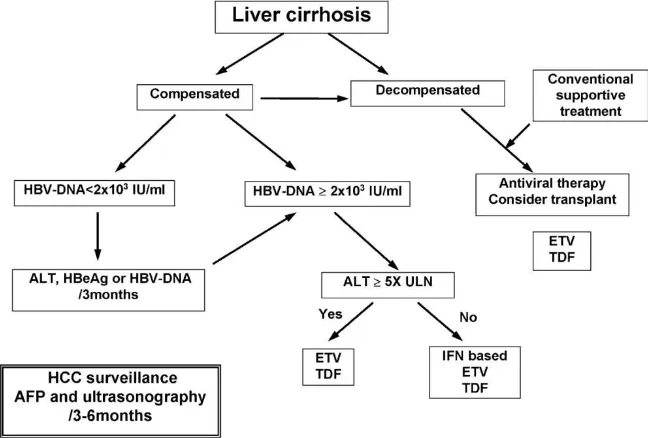
Figure 6. Algorithm for the management of chronic hepatitis B patients with liver cirrhosis 7
When to stop therapy?
The recommended duration of IFN-based therapy is finite, irrespective of whether or not response has been achieved. A 6–12 month observation period after the end of IFN-based therapy is also recommended to both detect a delayed response and establish whether the response is sustained, and thus whether retreatment or other therapy is required. 7, 13
4–6 months for HBeAg-positive patients and at least a year for HBeAg-negative patients. For Peg-IFN, the recommended duration is 12 months. 7, 13
For nucleos(t)ide analogues: In HBeAg-positive patients, treatment can be stopped when HBeAg serocon- version with undetectable HBV DNA has been maintained for at least 12 months. In HBeAg-negative patients, it is not clear how long treatment should be continued if HBsAg remains positive, but treatment discontinuation can be considered if patients have been treated for at least 2 years with undetectable HBV DNA documented on three separate occasions 6 months apart. In compliant patients with primary treatment failure at month 3 or suboptimal viral response at month 6, switch to a more potent drug or add a drug without cross resistance if LAM, LdT, or ADV was used. 7
References
1. WHO. Guidelines for The Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization. 2015.
2. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. The Lancet. 2014.
3. Lozano R, Naghavi M, Foreman K, al e. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study. Lancet. 2012;380:2095-128.
4. Hatzakis A, Van Damme P, Alcorn K, al e. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a ummit conference. J Viral Hepat. 2013;20:1-20.
5. Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection: Current treatment guidelines, challenges, and new developments. World J Gastroenterol. May 2014;20:6262-6278.
6. Dienstag JL. Hepatitis B Virus Infection. N Engl J Med. 2008;359:1486-1500.
7. liaw YF, Kao JH, Piratvisuth T, Chan LYH, Lesmana LA. Asian-Pacific co se sus state e t o the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561.
8. Chang T, Lai C, Kew SY, all e. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;511:422-30.
9. Marcellin P, Gane E, Buti M, al e. Regression of cirrhosis during treatment with tenofovir disoproxil
10. Tenney D, Rose R, Baldick C. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-14.
11. Liaw Y, Gane E, Leung N, al e. GLOBE Study Group. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-95.
12. Cooper R, Wiebe N, Smith N, al e. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496-505.
13. Liver EAftSot. EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection.