IN VITRO
ELIMINATION OF VIRUSES ON SHALLOT
(
Allium cepa
var.
aggregatum
) USING THERMOTHERAPY
AND CHEMOTHERAPY
PRABAWATI HYUNITA PUTRI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
AUTHOR’S STATEMENT ON THESIS AND ITS SOURCE OF
INFORMATION AND DELEGATION OF COPYRIGHT*
I declare that this thesis entitled In Vitro Elimination of Viruses on Shallot (Allium cepa var. aggregatum) Using Thermotherapy and Chemotherapy is
my own and authentic work under supervision of a thesis commitee and it is not yet submitted to any universities for any degree fulfillment. Source of information, both published or unpublished by its authors, used for quotations in this thesis is already cited appropriately and present in thesis‟s Literature Cited chapter.
I hereby delegate my thesis copyright to the Bogor Agricultural University.
Bogor, January 2017
Prabawati Hyunita Putri
Student ID number: A352130141
______________________
RINGKASAN
PRABAWATI HYUNITA PUTRI. Eliminasi Virus Pada Bawang Merah (Allium cepa var. aggregatum) Secara In Vitro Menggunakan Termoterapi dan Kemoterapi. Dibimbing oleh SRI HENDRASTUTI HIDAYAT dan DINY DINARTI.
Bawang merah merupakan salah satu komoditas hortikultura yang penting di Indonesia. Sentra produksi bawang merah di Indonesia terutama di darah Jawa Tengah, Jawa Timur, Jawa Barat, dan Nusa Tenggara Barat. Sebagian besar petani di Indonesia menggunakan umbi sebagai bahan perbanyakan vegetatif. Infeksi virus sangat mudah ditularkan melalui satu generasi ke generasi selanjutnya dan dari satu daerah ke daerah lainnya sehingga dikhawatirkan menurunkan kualitas dan hasil produksi. Infeksi virus pada umbi bawang merah di Indonesia telah dilaporkan, walaupun informasinya masih sangat terbatas.
Penelitian ini bertujuan mengembangkan metode eliminasi virus pada bawang merah menggunakan kombinasi termoterapi dan kemoterapi dengan beberapa ukuran eksplan kultur ujung tunas. Penelitian dilaksanakan mulai Januari 2015 sampai dengan Juni 2016, bertempat di Laboratorium Virologi Tumbuhan, Departemen Proteksi Tanaman dan Laboratorium Kultur Jaringan 3, Departemen Agronomi dan Hortikultura, Fakultas Pertanian, Institut Pertanian Bogor. Umbi benih bawang merah terdiri atas dua kultivar, yaitu „Bima Curut‟ dan „Bima Brebes‟, diperoleh dari penangkar benih di desa Tenguli, Brebes, Jawa Tengah. Eksplan berupa ujung tunas diisolasi menjadi tiga ukuran yang berbeda (1 mm, 2 mm, dan 3 mm). Masing-masing eksplan diberi perlakuan suhu (30 oC,
37 oC, dan 25 oC sebagai kontrol) dan diinkubasi selama 4 minggu. Termoterapi
dilakukan pada kondisi homogen dan heterogen. Plantlet hasil termoterapi yang masih terinfeksi virus kemudian digunakan sebagai sumber eksplan untuk kemoterapi. Ujung tunas berukuran 2 mm digunakan sebagai eksplan dan ditumbuhkan pada media yang mengandung ribavirin (10 mg L-1, 25 mg L-1, dan kontrol 0 mg L-1), selanjutnya diinkubasi pada suhu 30 oC. Deteksi virus setelah
perlakuan menggunakan RT-PCR dengan primer spesifik Carlavirus, Poyvirus, dan Allexivirus.
Benih umbi bawang merah yang diperoleh dari penangkar benih diseleksi lebih dahulu melalui deteksi virus dengan metode dot immunobinding assay (DIBA) menggunakan antibodi OYDV, SLV, dan GCLV. Sebanyak 100 sampel umbi diambil secara acak pada masing-masing kultivar dan ditanam pada styrofoam yang telah diberi air di bawahnya. Setelah 7 hari diambil secara acak 50 sampel daun yang tumbuh dari masing-masing kultivar. Hasil deteksi awal umbi benih bawang merah menunjukkan bahwa kedua kultivar 100 % positif terinfeksi OYDV. Sampel „Bima Curut‟ yang bereaksi positif terhadap antibodi SLV dan GCLV berturut- turut sebanyak 100% dan 90%, sedangkan sampel „Bima Brebes‟ berturut- berturut sebanyak 96% dan 96%. Infeksi OYDV dominan dibandingkan dengan SLV dan GCLV. Hasil deteksi ini mengindikasikan bahwa infeksi virus pada pertanaman bawang merah di Indonesia sangat tinggi.
sedangkan ukuran eksplan tidak berpengaruh nyata. Pada kultivar Bima Curut, kombinasi perlakuan termoterapi terbaik terhadap pertumbuhan eksplan adalah ukuran eksplan 1 mm dengan kondisi heterogen, sedangkan pada kultivar Bima Brebes adalah kombinasi ukuran eksplan 2 mm dengan suhu heterogen. Konfirmasi melalui RT-PCR pada plantlet hasil termoterapi menunjukkan bahwa semakin kecil ukuran maka tingkat keberhasilan eliminasi semakin tinggi. Seluruh plantlet (100%) yang berasal dari ukuran eksplan 1 mm bebas virus pada semua perlakuan. Tingkat eliminasi pada kultivar Bima Curut dengan ukuran eksplan 2 mm dan 3 mm pada suhu 30 °C terhadap Potyvirus adalah 67%, sedangkan terhadap Carlavirus berturut-turut adalah 33% dan 67%. Tingkat eliminasi pada kultivar Bima Curut dengan ukuran eksplan 2 mm dan 3 mm pada suhu heterogen terhadap Potyvirus adalah 67%, sedangkan terhadap Carlavirus adalah 50%. Tingkat eliminasi pada kultivar Bima Brebes dengan ukuran eksplan 2 mm dan 3 mm terhadap Potyvirus pada suhu 30 °C berturut-turut 33% dan 50%, sedangkan terhadap Carlavirus berturut-turut adalah 50% dan 67%. Tingkat eliminasi pada kultivar Bima Brebes dengan ukuran eksplan 2 mm dan 3 mm pada suhu heterogen terhadap Potyvirus adalah 50% dan 67%, sedangkan terhadap Carlavirus adalah 50% dan 67%. Kultur ujung tunas berukuran 1 mm terbukti dapat mengeliminasi virus hingga 100%. Namun, kultur ujung tunas ini memerlukan keahlian dan membutuhkan waktu yang lama dalam mengisolasinya sebelum dapat digunakan sebagai eksplan. Oleh karena itu, perlakuan menggunakan ukuran eksplan 2 mm yang dikombinasikan dengan suhu heterogen merupakan perlakuan terbaik untuk ukuran eksplan yang lebih besar karena dapat meningkatkan eliminasi virus serta tidak mengganggu pertumbuhan plantlet.
Kombinasi perlakuan termoterapi dan kemoterapi pada konsetrasi ribavirin lebih dari 10 mg L-1 pada kultivar Bima Curut tidak menghasilkan
plantlet yang hidup. Perlakuan ribavirin 25 mg L-1 pada kultivar Bima Brebes
menghasilkan plantlet yang tumbuh, namun vitrous. Konfirmasi dengan RT-PCR pada plantlet setelah perlakuan kemoterapi memperlihatkan bahwa Potyvirus dan Carlavirus masih terdeteksi pada semua plantlet. Hal ini mengindikasikan bahwa ribavirin 10 mg L-1 yang dikombinasikan dengan termoterapi belum mampu mengeliminasi virus.
Umbi benih bebas virus diperlukan untuk meningkatkan produksi bawang merah dan menekan insidensi penyakit di pertanaman bawang merah di Indonesia. Perlakuaan eliminasi virus menggunakan kultur ujung tunas dengan ukuran eksplan 1 mm terbukti menghasilkan plantlet bebas virus. Eliminasi virus menggunakan ukuran eksplan ynag lebih besar (2 mm) membutuhkan kombinasi dengan termoterapi pada kondisi suhu heterogen. Kedua metode tersebut merupakan metode yang efisien dalam memperoleh umbi bebas virus.
SUMMARY
PRABAWATI HYUNITA PUTRI. In vitro Elimination of Viruses on Shallot (Allium cepa var. aggregatum) Using Thermotherapy and Chemotherapy. Supervised by SRI HENDRASTUTI HIDAYAT and DINY DINARTI.
Shallot is one of important horticultural product in Indonesia. Main production area in Indonesia are located in Central Java, East Java, Wst Java, and West Nusa Tenggara. Most shallot‟s farmers in Indonesia use bulb as propagative material. Viral diseases are easily transmitted via infected bulb from one generation to the next and from one region to another, causing considerable quality and yield loss. Viral disease on shallot in Indonesia has been reported, although the information is very limited.
The objective of this research was to develop method for elimination of virus infection on shallot bulbs using combination of thermotherapy and chemotherapy with different explant size in shoot tip culture. Research was conducted in Laboratory of Plant Virology, Department of Plant Protection and Laboratory of Tissue Culture 3, Department of Agronomy and Horticulture, Faculty of Agriculture, Bogor Agricultural University from January 2015 until June 2016. Two cultivars were used in this study, i.e „Bima Curut‟ and „Bima Brebes‟. The virus-infected bulbs material used in this study was obtained from Tenguli village, Brebes, Cental Java. The shoot tips from each samples were isolated onto three sizes (1 mm, 2 mm, and 3 mm). Each size will then be treated using 2 level of temperature (30 oC, 37 oC, and control at 25 oC) and incubated for 4 weeks. Thermotherapy was conducted using homogenous and heterogenous condition. Plantlets from thermotherapy that remained infected by viruses then used as source of explant for chemotherapy. Explants 2 mm in length were excised then growth in medium containing ribavirin (0 mg L-1 as control, 10 mg
L-1, and 25 mg L-1) followed by incubation at 30 oC for 4 weeks. Detection of
virus infection after treatment was conducted using reverse transcription-polymerase chain reaction (RT-PCR) with universal primer for Potyvirus, Carlavirus, and Allexivirus.
Screening of bulb samples were done by dot immunobinding assay (DIBA) using antibody to Onion yellow dwarf virus (OYDV), Shallot latent virus (SLV), and Garlic common latent virus (GCLV). One hundred samples of each cultivar were chosen randomly and growth in tray using styrofoam with water below. Fifty leaf samples from each cultivars were collected randomly for virus detection. Samples giving positive reaction to viral antibodies were then used as materials for further in vitro virus elimination. Infection of OYDV was detected 100% from both cultivars. Samples „Bima Curut‟ were positive to SLV and GCLV 100% and 90%, respectively. Meanwhile, samples „Bima Brebes‟ were positive to SLV and GCLV 96% and 96%, respectively. These result indicated that virus infection in shallot has been increasing. In general, the dominant virus infection on shallot bulbs was OYDV, whereas the least one was GCLV.
significant. Based on plantlets growth, the best treatment was using 1 mm-explant treated at heterogenous condition for cv. Bima Curut, meanwhile for cv. Bima Brebes the best treatment was using 2 mm-explant treated at heterogenous condition. Confirmation of virus infection using RT-PCR in plantlets resulted from thermotherapy showed that smaller explant size enhance the efficiency of viral elimination. Plantlets derived from 1 mm-explant shown negative result to viruses for both treated and untreated plantlets from both cultivars. Efficiency of viral elimination on cv. Bima Curut plantlets derived from 2 mm-explant and 3 mm-explant using 30 °C to Potyvirus was about 67%, while to Carlavirus was about 33% and 67%, respectively. In heterogenous condition, efficiency of viral elimination on cv. Bima Curut plantlets derived from 2 explant and 3 mm-explant was 67 dan 50%, respectively. Efficiency of viral elimination on cv. Bima Brebes plantlets derived from 2 mm-explant and 3 mm-explant using 30 °C to Potyvirus was about 33%, while to Carlavirus about 33% for both explant size. Meanwhile, in heterogenous condition efficiency of viral elimination on cv. Bima Brebes plantlets derived from 2 mm-explant and 3 mm-explant was 50 and 67% to Potyvirus, and to Carlavirus about 50 and 67%, respectively. In general, shoot tip culture using 1 mm-explant size could eliminate viruses until 100%. However, these techniques need expertise and time consuming. Therefore, treatment using 2 mm-explant at heterogenous condition is the best combination for bigger explant size to decrease viral concentration and did not interfere plantlets growth.
Chemotherapy using ribavirin in growth medium caused inhibition on plantlet growth, particularly using concentration of 25 mg L-1. Although plantlets
grew on medium containing 10 mg L-1 ribavirin but they remained contain
Potyvirus and Carlavirus. These results indicated that treatment combination of ribavirin and thermotherapy could not eliminate viruses.
Virus-free bulbs are needed to decrease disease incidence in the field and increase yield in Indonesia. Shoot tip culture with 1 mm-explant size resulted in virus-free plantlets. When bigger size of explants (2 mm) was used, it required treatment combination of thermotherapy at heterogenous condition.
© 2017, Bogor Agricultural University
All Right Reserved
It is prohibited to make any quotations from part or whole of this thesis without citing the author or the copyright holder. Quotation is allowed as long as for education, research, scientific writing, scientific report, research proposal or scientific review purposes only; those quotations should not produce any adverse effects to Bogor Agricultural University.
No part of this thesis may be reproduced or transmitted in any forms or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system without express written permission from Bogor
Thesis
in partial fulfillment of the requirements for the degree of Master of Science
at the
Phytopathology Study Program
IN VITRO
ELIMINATION OF VIRUSES ON SHALLOT
(
Allium cepa
var.
aggregatum
) USING THERMOTERAPY AND
CHEMOTHERAPY
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2017
PREFACE
Praise and great gratitude to Allah subhanahu wa ta’ala who always gives His bless to me for finishing this thesis entitled “In vitro Elimination of Viruses in Shallot (Allium cepa var. aggregatum) Using Thermotherapy and Chemotherapy”. This thesis is a requirement in accomplishing the Master of Science Program at Phytopathology Study Program, Graduate School, Bogor Agricultural University. I would like to thank all individuals who gave contribution so that this thesis can be finished. I would like to express my deep gratitude to Prof Sri Hendrastuti Hidayat, PhD and Dr Diny Dinarti for their support and guidance during lab work and thesis writing. My sincere grateful to Dr Efi Toding Tondok and Dr Giyanto for their suggestion during thesis defense.
I would also like to thank Domestic Graduate School Scholarship Organizer from Ministry of Research, Technology and Higher Education, Republic of Indonesia for the graduate scholarship in Graduate School of Bogor Agricultural University. My sincere gratitude to ACIAR who funded this research through ACIAR Project (HORT 2009/056 Project) “Sustainable productivity improvements in alliums and solanaceous vegetable crops in Indonesia and sub-tropical Australia”.
The greatest thanks and appreciation to my beloved parents, Bapak Kusnanto and Ibuk Sriyani, and Adek Dwi Setia Agusti Putri. They always gave me their pray, support, love, patience, and motivation during research and finishing the thesis. Thank you so much.
I addressed sincere gratefulness to big family of Phytopathology class of 2013, Plant Virology Laboratory members, Tissue Culture 3 Laboratory members, Ngecemes, Gottingers, Sari Nurulita, Aqlima, Tyas Ayu Lestari, Ankardiansyah Pandu Pradana and Rizki Haerunisa for their support during thesis writing, and for all relevant parties who I can not mention one by one. May Allah bless you all.
Hopefully this thesis can be useful and give positive contribution to the development of science.
TABLE OF CONTENTS
Shallots Production and Its Constraints in Indonesia 5
Onion yellow dwarf virus (OYDV) 6
Garlic common latent virus (GCLV) 6
Shallot latent virus (SLV) 6
Detection and Quantification of Major Viruses on Shallot 7
Tehnique for Virus Elimination 8
Chemoterapy 8
Thermotherapy 8
3 MATERIALS AND METHODS 10
Screening of Bulb Infected Viruses 10
Detection of Viruses 10
Dot immuno binding assay (DIBA) 10
Reverse Transcription Polymerase Chain Reaction (RT PCR)
method 11
Shoot Tip with Thermotherapy and Chemotherapy Treatment 12
Preparation of Tissue Culture Medium 12
Bulbs and Explants Sterilization 12
Thermotherapy Treatment 13
Chemotherapy Treatment 13
Experimental Design and Variable of Observation 13
4 RESULTS AND DISCUSSION 14
Screening of Bulbs-Infected Virus 14
The Effect of Thermotherapy and Explant Size to the Number of
Plantlets Survived 15
The Effect of Thermotherapy and Explant Size to Average Number
of Leaves 16
The Effect of Thermotherapy and Explant Size to Plantlets Height 18 The Effect of Thermotherapy and Explant Size to Average Number
The Efficiency of Viral Elimination by Thermotherapy 21 The Efficiency of Viral Elimination by Chemotherapy Following
Thermotherapy 23
General Discussion 25
5 CONCLUSIONS AND RECOMMENDATIONS 27
Conclusions 27
Recommendations 27
REFERENCES 28
APPENDIX 33
LIST OF TABELS
1 Research of elimination in the plant using meristem culture for
period 1991-2010 9
2 Primers used for amplification of Potyvirus, Carlavirus, and
Allexivirus 12
3 The average of virus infection (%) on shallots leaves samples 14 4 Virus incidence on plantlets cv. Bima Curut and cv. Bima Brebes
after thermotherapy 22
5 Effect of chemotherapy to plantlets growth 24
LIST OF FIGURES
1 Research flow chart 4
2 Growing bulb samples in plastic tray using styrofoam 10 3 Nitrocellulose membrane showing the reaction of samples to
antibodies (purple dots) in dot immunobinding assay. 14 4 Numbers of plantlets survived after thermotherapy on different
explant size (1, 2, 3 mm) of two shallot cultivars : (A) cv. Bima
Brebes and (B) cv. Bima Curut 15
5 Plantlets growth on the thermotherapy treatment 16 6 The effect of thermotherapy on average number of leaves cv. Bima
Curut (A and B) four weeks during treatment and (C and D) four
weeks after treatment 17
7 The effect of thermotherapy on average number of leaves cv. Bima Brebes (A and B) four weeks during treatment and (C and D) four
weeks after treatment 18
8 The effect of temperature (A) and explant size (B) on plantlets
height of cv. Bima Curut 19
9 Plantlets height and number of shoots following thermotherapy
application on cv. Bima Curut explants derived from different sizes 19 10 The effect of temperature (A) and explant size (B) on plantlets
height of cv. Bima Brebes 19
11 The effect of thermotherapy on average number of roots cv. Bima Brebes (A and B) four weeks during treatment and (C and D) four
weeks after treatment 20
12 The effect of thermotherapy on average number of roots cv. Bima Curut (A and B) four weeks during treatment and (C and D) four
weeks after treatment 21
13 Virus detection of Potyvirus in plantlets derived from 2 mm-explant (A) and Carlavirus in plantlets derived from 3
mm-explants (B) cv. Bima Curut and cv. Bima Brebes 23 14 Number of plantlets survived after chemotherapy. (A) cv. Bima
15 DNA amplification of Potyvirus (A) and Carlavirus (B) from plantlets of cv. Bima Curut and cv. Bima Brebes following
thermotherapy and chemotherapy 24
LIST OF APPENDIXES
1 Description of shallot cultivars used in this research 34 2 Analysis of t-test the effect of thermotherapy on life percetage
plantlets cv. Bima Curut and cv. Bima Brebes 34
3 Analysis of the effect of temperatures, explant size, and interaction
to an average number of leaves and plantlets height cv. Bima Curut 35
4 Analysis of the effect of temperature, explant size, and interaction to an average number of leaves and plantlets height cv. Bima
Brebes 36
5 Analysis of effect of temperature, explant size, and interaction to an
average number of roots cv. Bima Brebes 37
6 Analysis of effect of temperature, explant size, and interaction to an
average number of roots cv. Bima Curut 38
1
INTRODUCTION
Background
Shallot (Allium cepa var. aggregatum) is one of important horticultural comodity in Indonesia. Production center of shallot in Indonesia is located in West Java, Central Java, East Java, Yogyakarta, and West Nusa Tenggara. Although shallot can be propagated through generative or vegetative stages using true seeds or bulbs, respectively, most farmers in Indonesia use bulbs as vegetative propagative material. This is due to difficulties to produce true shallot seed (TSS) in Indonesia because of climate condition. Several pathogens, including viruses, can be transmitted through vegetative materials, such as shallot bulbs (Sharaeen et al. 2008). There are three genera of viruses that has been reported to infect shallot, i.e. Carlavirus (Shallot latent virus / SLV and Garlic common latent virus / GCLV), Potyvirus (Onion yellow dwarf virus / OYDV, Shallot yellow stripe virus / SYSV, and Leek yellow stripe virus / LYSV), and Allexivirus (Garlic virus B / GV-B, Garlic virus C / GV-C), and Garlic virus D / GV-D) (Sharaeen et al. 2008; Sevik and Ackura 2013).
Infection of LYSV, SLV, and OYDV on shallot in Indonesia has been reported from Lembang and Subang, West Java (Duriat and Sukarna 1990; Wulandari et al. 2002). SYSV was detected in shallot samples varieties Jawa and Brebes from Brebes, Central Java (Kurniawan and Suastika 2013). Recently, Kadwati and Hidayat (2015) reported, the infection of Potyvirus, Carlavirus, and Allexivirus on shallot from Brebes (Central Java) and Cirebon (West Java).All of these reports indicated that shallot disease caused by viruses has increasing recently in Indonesia.
Little is known about yield loss on Allium, shallots in particular, caused by virus infection. Walkey et al. (1989) reported the potency of OYDV infection to cause yield loss on garlic up to 88%. In France, weight of garlic clove was reduced about 56-84% as a result of mixed infection of OYDV and LYSV (Lot et al. 1998). Infection of OYDV in garlic and shallot in Turkey caused weight reduction up to 25-54% compared with healthy bulb (Sevik and Ackura 2013). All of these reports indicated that virus infection could reduce yield and decrease quality of the bulbs. Therefore, information regarding yield loss in shallot due to virus infection is important to know especially to determine its affect on bulbs quality.
(antiviral), electrotherapy (electrical current), and cryotherapy (cold temperature). Among them, thermotherapy and chemotherapy are the most common method used for virus elimination (Rout et al. 2006).
Thermotherapy consisted of two techniques i.e hot water treatment and hot air treatment. Ali et al. (2013) reported the use of meristem tip culture in combination with thermotherapy to eliminate viruses in potatoes and the technique had efficiency about 24.55%. Chemotherapy is a virus elimination technique using a substance named antiviral, such as ribavirin. Neelamathi et al. (2014) reported that the elimination of Sugarcane mosaic virus in sugarcane using ribavirin 10 mg L-1 had an efficiency up to 95%. Shoot tip culture - based
techniques combined with thermotherapy and chemotherapy need to be developed as a method to get virus-free bulbs in order to improve the quality of shallot seeds in Indonesia.
Problem Statement
Most of shallot growers in Indonesia using bulbs as propagative material. High virus infestation was detected from seed bulbs collected from shallot‟s production center in Indonesia. Bulb borne viruses may infect the plants since early stage and cause yield loss. The actual yield loss caused by virus infection has not been studied because virus-free bulbs is very difficult to obtain. Among other techniques available, virus-free bulb can be obtained using shoot tip culture. Tissue culture in combination with thermotherpy and chemotherapy may increase efficiency of virus elimination. These treatment has been known to eliminate viruses in some horticultural plant succesfully. Therefore, thermotherapy and chemotherapy might have a good potential to be applied on shallots. The use of virus-free bulbs as seeds source can suppress disease incidence on shallots in Indonesia.
Research Objectives
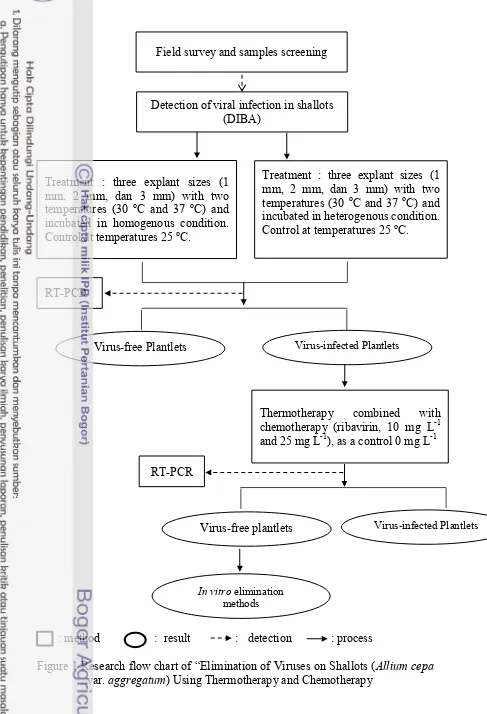
Objective of this research is to develop in vitro virus elimination method for shallot through thermotherapy (30 oC dan 37 oC) and chemotherapy (10 mg L-1 and 25 mg L-1) using different cultivars („Bima Curut‟ and „Bima Brebes‟) and
explant size (1 mm, 2 mm, and 3 mm) (Figure 1).
Research Hypothesis
1. Modification of explant may effect virus elimination efficiency in tissue culture method.
2. Combination of thermotherapy and chemotherapy could improve efficiency of virus elimination method.
Research Benefits
: method : result : detection : process
Figure 1 Research flow chart of “Elimination of Viruses on Shallots (Allium cepa var. aggregatum) Using Thermotherapy and Chemotherapy
Detection of viral infection in shallots (DIBA)
Treatment : three explant sizes (1 mm, 2 mm, dan 3 mm) with two temperatures (30 oC and 37 oC) and incubated in homogenous condition. Control at temperatures 25 oC.
Treatment : three explant sizes (1 mm, 2 mm, dan 3 mm) with two temperatures (30 oC and 37 oC) and
incubated in heterogenous condition. Control at temperatures 25 oC.
RT-PCR
Thermotherapy combined with chemotherapy (ribavirin, 10 mg L-1 and 25 mg L-1), as a control 0 mg L-1
RT-PCR
Virus-free Plantlets Virus-infected Plantlets
In vitro elimination
methods
2
LITERATURE REVIEWS
Shallot Production in Indonesia and Its Constraint
Shallot is a member of plant in family : Lilliaceae; belongs to genus : Allium L.; species : Allium cepa var. aggregatum. Different variety of shallot is grown by farmers across Indonesia. The most popular varieties grown in West Java are „Lodra‟, „Sumenep‟, „Batu‟, „Maja Merah‟, „Cigugur Merah‟, and „Ciniru‟; in Central Java are „Bima Curut‟, „Bima‟, „Bima Timor‟, „Bima Arjuna‟, „Kuning Tablet‟, „Kuning Gombong‟, and „Filipina‟ (Gunaeni et al. 2011).
Shallot production areas are found in all across Indonesia, but mainly in Java, mostly in the lowland. Shallots are grown at the beginning and at the end of the rainy season (October – November and March – July, respectively). A high bed of soil was built with 50 – 60 cm in height and 1.2 – 2 m in width, with a ditch between beds. The ditch is filled with water throughout growing period with water level 25 – 30 cm below the surface of the bed with water level 25 – 30 cm below the surface of the bed. Watering was done twice a day until 1 week before harvest (Permadi 1994).
Propagation of shallot can be done using generative and vegetative materials. Generative is using seeds produced by flower as propagative material, meanwhile vegetative is using bulbs. Seed-propagated shallots were difficult may be due to unfavourable condition of climate so that many farmers in Indonesia use bulbs as propagative materials. Farmers produce their own seeds from selected bulbs which are dried in the sun after harvested. They are stored in bunches called “para-para” in the kitchen above the stove. Seed producers were built special storagehouse which seeds were hung on bamboo racks. Below bamboo racks were built artificial drier which wood is burned to further dry and smoke the bulbs. Bulbs which is used as propagative material commonly stored for 2 until 3 months before planted in the field (Grubben 1994; Permadi 1994).
Shallots farmers in Indonesia has to deal with several contraints, among others are yield and quality of shallots production among farmers, poor propagation material, pest and disease control, storage losses, inappropriate fertilizer, and marketing. The health of the planting bulbs is an important factor that affect shallots production. As described earlier, production of seed-propagated shallot were not enough and about 70% of bulbs were proved to be infested with viruses. Bulbs-infested viruses caused vigour declining of the planting materials (Van Dijk 1993; Grubben 1994).
Major Viruses in Shallot
Major group of viruses that infect shallot are Potyvirus, Carlavirus, Allexivirus, and Nepovirus. There are four important species of viruses that has been reported infecting shallot i.e. OYDV, SLV, GCLV, and SYSV (Diekmann 1997; EPPO 2007).
Onion yellow dwarf virus (OYDV)
Onion yellow dwarf virus is a member of the genus Potyvirus, with flexious, filament-shaped particle. Virus particle approximately 772-823 nm in length and weight of coat protein about 34 kDa. Onion yellow dwarf virus is transmitted through insect vector, Myzus persicae (Aphididae), with non persistent manner. The viruses is also transmitted mechanically through vegetative propagation material, and can not be transmitted through pollen (Takaichi et al. 2001).
On shallots, OYDV produces symptom of a mild yellow chlorotic irregular stripes to bright yellow stripes. Infected leaf become curling downward, and shrinken. In addition, infected shallot become stunted and the bulb size smaller than healthy bulb. Mechanical inoculation of OYDV can cause local lession on Chenopodium murale. The common method for detection of OYDV is using serological method, i.e. indirect enzyme-linked immunosorbent assay (I-ELISA). At the beginning of plant growth OYDV is difficult to detect because the concentration of viruses is very low (Mishra et al. 2014).
Garlic common latent virus (GCLV)
One member from genus Carlavirus that infect shallots is Garlic common latent virus (GCLV). These virus belongs to the family Betafleviridae, and ordo Tymovirales. The virus particle is filamentous and flexious. The nucleic acid is single stranded RNA approximately 8.6 kb. Garlic common latent virus spread across several region around the world but it is not endemic (Barg et al. 1994, Barg et al. 1997). Single infection of GCLV produce no symptom or symptomless. Severe yellowing and mosaic was produced when shallot and garlic infected by combination of GCLV and Potyvirus, such as OYDV and LYSV. Carlavirus and Potyvirus may have a synergitic effect on symptom expression when infecting garlic and shallot (Takaichi et al. 1998).
Decoration tests in France showed the general presence in French garlic of a carlavirus not
Shallot latent virus (SLV)
Potyvirus will cause serious damage and yield losses. SLVs showed local symptomatic lesion in some indicator plants i.e Chenopodium spp., Celosia argentea, and Vicia faba. Systemic symptoms were showed on Nicotiana occidentalis and N. hesperis (Van Dijk 1993).
Detection And Quantification of Major Viruses on Shallots
Detection of viral diseases in the plant seldom using symptoms observation. The methods is not always reliable because more than one virus can cause similar symptoms in the plant and many non-biotics disorder, such as nutritional deficiency or drought, may causes symptoms similar to virus diseases. Furthermore, the diseases symptoms also depends on cultivars and growth stages of host plants (Naidu and Hughes 2009).
Nowadays, detection and quantification of virus particles in the plant can be done through two approach i.e. serological and nucleic acid-based methods. These two methods are reliable to identify viruses at all times. Serological methods for virus identifications are based on the virus coat protein properties. The basic principle in which the virus antigens are recognized by their specific antibodies (IgG) in association with colorimetric properties. This method is able to detect viruses in very low concentrations and can be used with viruses of different particle morphology. Because of its adaptability, high sensitivity, and economy in the use of reagents, serological methods are used in a wide range of situations, especially for detection a large number of samples in a relatively short period of time. Several serological methods that used in virus detection, i.e enzyme linked immunosorbant assay (ELISA) and dot immunobinding assay (DIBA) (Webster et al. 200; Naidu and Hughes 2009). DIBA methods was used in this research to detect virus diseases in samples.
Quantification and detection of virus particles using serology method had several weakness i.e cross reaction between antigens which closely related so give unspecific result, diversity among virus strain, and pH of reagent affected test result.
Molecular methods based on nuleid acid are widely used for detection and quantification viruses in the field. PCR is a techniques that has specifity and sensitivity to detect plant pathogens (Naidu and Hughes 2009). Molecular methods for detecting plant pathogens are based on the accurate design of primers and target sequences. Specific nucleotide regions are selected and primers specific for DNA or RNA targets can be easily designed. The target sequences then amplified using dNTP (nucleotide triphospate) and enzyme Taq polymerase. Revers transription polymerase chain reaction (RT-PCR) is a modification of PCR technique for enable amplification of RNA instead of DNA. For the case of RNA viruses, RNA were reversed into cDNA using enzyme transcriptase then amplified
using appropriate primers (Naidu and Hughes 2009; Martinelli et al. 2015).
polymerases, sample cross-contamination can give false positives, and the cost of equipment and reagents must be considered when selecting a molecular detection
method (Naidu and Hughes 2009; Martinelli et al. 2015)
Technique for Virus Elimination
Tissue culture is a method to isolate parts of plant (protoplasm, cell, tissue, and organ) based on totipotency and can be cultured in aseptic condition. Culture plant is carried out under control condition so that the parts of plant can be regenerated became a whole plant. Tissue culture widely used nowadays because it can give some benefits, i.e produce plant in large quantities in a short time and disease-free, seed production with desirable properties, and need a narrow place (Altman et al. 2005)
Variety of tissue culture techniques is available, among others is shoot tip culture. Initial explants in shoot tip culture is shoot tips which consisted of leaf primordia and one meristem dome. Those type of culture usually used to get virus-free plant. Some leaf primordia could be included because if explant is too small it difficult to do root regeneration (Rout et al. 2006; Panattoni et al. 2013). The successful of virus elimination determined by explant size used for tissue culture. In order to increase efficiency of virus elimination shoot tip culture usually is combined with some other treatments, such as thermoteraphy, cryotherapy, electrotherapy, and chemotherapy (Milasevic et al. 2012; Panattoni et al. 2013).
Chemotherapy
A method for virus elimination by application of drugs or chemical substances as antiviral agent is known as chemotherapy. There are several antiviral agents that may inhibit virus replication and used in chemotherapy, such as ribavirin, acylguanocine, acidotimidin, green malachit, 2-thiouracil (Milasevic et al. 2012). The most common antiviral used in tissue culture technique is ribavirin. Ribavirin can be used in plant meristem that cultured in vitro or cultured before shoot tip or meristem isolation (Panattoni et al 2013).
Ribavirin is a synthetic chemical that analog with guanocyne. Added 10-50 mg L-1 of ribavirin in the selective culture media prevent several viruses infection such as PVX and PVY in potato, CMV in meristem of Nicotiana rustika (Hadidi et al. 1998). Soliman et al. (2012) reported that 20 mg L-1 dose of antiviral can
eliminate OYDV in garlic about 85%. Ribavirin interferes formation of nucleid acid so that inhibit virus replication. Active form of ribavirin is triphospate which inhibit capping process at 5‟ RNA virus. Moreover, ribavirin inhibits virus replication in the initial phase by interfering RNA-dependant RNA polymerase and RNA helicase synthesis. In the final phase, ribavirin is known to interfere coat protein synthesis (Neelamathi et al. 2014; Panattoni et al. 2013).
Thermotherapy
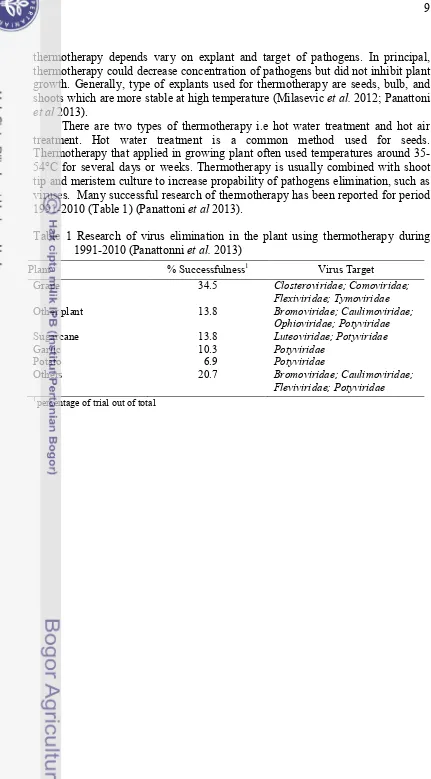
thermotherapy depends vary on explant and target of pathogens. In principal, thermotherapy could decrease concentration of pathogens but did not inhibit plant growth. Generally, type of explants used for thermotherapy are seeds, bulb, and shoots which are more stable at high temperature (Milasevic et al. 2012; Panattoni et al 2013).
There are two types of thermotherapy i.e hot water treatment and hot air treatment. Hot water treatment is a common method used for seeds. Thermotherapy that applied in growing plant often used temperatures around 35-54°C for several days or weeks. Thermotherapy is usually combined with shoot tip and meristem culture to increase propability of pathogens elimination, such as viruses. Many successful research of thermotherapy has been reported for period 1991-2010 (Table 1) (Panattoni et al 2013).
Table 1 Research of virus elimination in the plant using thermotherapy during 1991-2010 (Panattonni et al. 2013)
Plant % Successfulness1 Virus Target
Grape 34.5 Closteroviridae; Comoviridae;
Flexiviridae; Tymoviridae
Other plant 13.8 Bromoviridae; Caulimoviridae;
Ophioviridae; Potyviridae
Sugarcane 13.8 Luteoviridae; Potyviridae
Garlic 10.3 Potyviridae
Potato 6.9 Potyviridae
Others 20.7 Bromoviridae; Caulimoviridae;
Fleviviridae; Potyviridae
3 MATERIALS AND METHODS
Research activities were conducted in Laboratory of Tissue Culture 3, Department of Agronomy and Horticulture; and Laboratory of Plant Virology, Departement of Plant Protection, Faculty of Agriculture, Bogor Agricultural University from January 2015 to June 2016. The research was consisted of 4 main activities, i.e (1) collection of bulb samples from Tenguli village (Brebes, Central Java), (2) detection of viruses from bulb samples, (3) tissue culture with thermotherapy and chemotherapy treatment, and (4) detection of viruses in tissue culture plantlets after treatment.
Screening of Bulbs-infected Viruses
Bulb samples were collected from seed grower in Tenguli village, Brebes, Central Java. There are two cultivars that commonly used as seeds in Brebes, i.e. cv. Bima Curut and cv. Bima Brebes (Appendix 1). Bulb samples has been stored for 60 until 70 days in a dry place with low humidity named “para-para”. One hundred bulbs for each cultivars were grown in styrofoam with water below (Fig 2). The bulbs were then placed in a cool room with temperatures of 16 oC. After
one week, leaf samples were collected randomly, i.e. 50 leaves for each cultivar, and tested for virus incidence by dot immunobinding assay (DIBA) using three antibodies, i.e. OYDV, SLV, and GCLV. Bulbs gave positive reactions were then used as materials for tissue culture.
Figure 2 Growing bulb samples in plastic tray using styrofoam
Detection of Viruses
Two methods were used, i.e dot immunobinding assay (DIBA) for early detection of viruses from shallots bulb, and reverse transcription polymerase chain reaction (RT-PCR) for detection of viruses from plantlets after in vitro treatment.
DIBA Method
Membranes was then washed 5 times with H2O, each washed process was shaked
at 100 rpm for 5 min. After washing, the membranes were soaked into 2.5 μL first antibody solution (with 2% non-fat milk-TBST), then incubated at 4 oC overnight.
After washing 5 times for 5 min using TBST, the membranes was soaked on 2.5 μL conjugate antibody (with 2% non-fat milk-TBST), then incubated for 60 min. The membranes was soaked on 10 mL AP-buffer (Tris-HCl 0.1 M, NaCl 0.1 M, MgCl2 5 mM, H2O) containing 1 tablet nitro blue tetrazolium (NBT) and bromo
chloro indolil phosphate (BCIP). Positive reaction was qualitatively indicated by appearance of purple colour in nitrocelullose membrane on the spotted area. Reaction was stoped by soaking the membranes onto dH2O.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Method
Leaf samples was collected from each plantlet, then it was bulked, i.e. one sample consisted of 10 leaves. The RT-PCR method started with total RNA extraction, followed by cDNA synthesis, cDNA amplification, and visualization using gel electrophoresis.
Total RNA Extraction. Extraction of total RNA was conducted following
Doyle and Doyle (1987) method with some modification. Samples was weighed and 0.1 g of samples was ground in extraction buffer containing 1% merchaptoethanol (1:5 w/v). The samples were incubated at 65 °C for 30 min and inverted every 10 min. Chloroform/isoamyl alcohol (24:1, v/v, 500 µL) was added, then the tube was mixed well. The samples was centrifugated at 11 000 rpm for 15 min. The supernatant was transferred into a clean 1.5 mL tube and isopropanol (2/3 volume supernantant) was added for precipitation, then incubated in -80 °C for 2 hr. The supernatant containing isopropanol was sentrifugated at 11 000 rpm for 15 min. The pellets were washed with 500 µL 70% ethanol, then centrifugated at 8000 rpm for 5 min. The supernatant was discarded, and pellets was dried under room temperature. Dried pellets containing RNA were dissolved in 50 µL TE buffer (pH 8).
cDNA Synthesis. The total RNA was used for reverse transcription
reaction to form cDNA (complementary DNA) using reverse transcriptase enzyme. Reagent and volume per tube that used for this reaction consisted of: 2 µL 5x Buffer RT, 1 µL 0.1 M DTT, 1 µL dNTP mix, 0.5 µL RNAse inhibitor (40 U/µL), 0.5 µL M-MuLV (200 U/µL), 1 µL Poty 1 primer, and 1 µL nuclease-free water. The mixtures were added into clean tube, then 3 µL of total RNA was added. Reverse transcription reaction was done using automated thermal cycler (Gene Amp PCR System 9700; PE Applied Biosystem, USA) with one cycle program at temperature 65 oC for 5 min, 42 oC for 60 min, and 70 oC for 10 min to inactivate RNAse inhibitor. The synthesized cDNA was then used as DNA template in the amplification reaction.
cDNA Amplification and Visualization. Amplification reaction per tube
contains 12.5 µL Go Taq Green (Thermoscientific), 9.5 µL nuclease-free water, 10 µM for each primer (forward and reverse), and 1 µL of cDNA. Amplification of cDNA viruses was performed ini GeneAmp PCR System 9700 started with pre-heating cycle for 5 min at 94 oC, followed by 35 cycles of denaturation (20 sec at
94 oC), annealing (1 min at 54 oC), and extension (3 min at 72 oC). The last stage
was ended at 72 oC for 1 min and cooled down to 4 oC. The amplicon then
acid-EDTA) buffer. The electrophoresis was performed at 50 V for 50 min, then the gel was soaked on to 0.1% EtBr for 10 min, washed with H2O for 5 min, and
visualized under UV transilluminator. Three pairs of primers was used separately to amplify Potyvirus (U341/Poty1), Allexivirus (pGV3t/Poty1), and Carlavirus (AlcarF/Poty1) (Chen et al. 2001; Langeveld et al. 1991) (Table 2).
Table 2 Primers used for amplification of Potyvirus, Allexivirus, and Carlavirus
Primers Sequences1 Amplicon
Poty1 R: 5‟-GGATCCCGGGTTTTTTTTTTTTTTTTTV-3‟ Carlavirus
pGV3t F: 5‟-TGGNCNTGCTACCACAANGG-3‟ 950
Poty1 R: 5‟-GGATCCCGGGTTTTTTTTTTTTTTTTTV-3‟ Allexivirus
1F : forward; R : reverse
Shoot Tip Culture with Thermotherapy and ChemotherapyTreatment
Thermotherapy was applied through hot air treatment by incubating explants in growth chamber. Ribavirin (Virozole, Sigma Aldrich) was used as antiviral agent in chemotherapy.
Preparation of Tissue Culture Medium
Tissue culture medium consisted of shoot-inducing medium and root-inducing medium. Shoot-root-inducing medium was made by MS (Murashige-skoog) medium modified with B5 vitamin. Growth regulator hormones such as 2ip (2 mg L-1) and GA3 (0.3 mg L-1) was added onto MS medium. Distilated water was
added up to 1 L and pH was checked up to 5.8 - 6. Gelrite 2 g L-1 was added onto
medium and stired well. The medium was poured into 8 mL sterilized culture tube. Culture tube contains medium was autoclaved at 121 °C, 1 atm, for 15 min. The medium was then incubated for 3-6 days at 25 °C, and only medium-free contaminant were later used.
Bulbs and Explants Sterilization
The bulbs were prepared before treatment. The outer protective skin and necrotic stem base of the mature bulbs were removed. The bulbs were washed using detergent and rinsed with tap water. The bulbs was soaked into 2 g L-1
fungicide (Dithane 80 WP) and 2 g L-1 bactericide (Agrept 20 WP) and incubated
damage. The 5 mm-explants were excised into 3 mm, 2 mm, and 1 mm using sterile needles under binocular microscope. Three sizes of explant were used as a treatment for thermotherapy experiment. Each size of explants were cultured on basic MS medium and incubated for 4 days. Explants that contaminant-free were transferred into medium to induce shoot development and used later for thermotherapy.
Thermotherapy Treatment
Thermotherapy for this research is using hot air treatment. The treatment consisted of two temperatures, i.e. 30 oC, 37 oC, and control treatment at 25 oC.
There are two conditions for thermotherapy, i.e. incubation in homogenous condition and in heterogenous condition. For heterogenous condition, the explants were incubated in 25 oC for the first 2 weeks, then in 30 oC for the next week, then
in 37 oC for the following week. For homogenous condition, explants were
incubated in each temperature (30 oC, 37 oC, and 25 oC) for 4 weeks with a photoperiod of 16 h light (52 µm s-1 m-2). All plantlets survived from
thermotherapy treatment was tested for Potyvirus, Carlavirus, and Allexivirus using RT-PCR as described earlier.
Chemotherapy Treatment
Explant for chemotherapy was obtained from thermotherapy experiment, i.e. plantlets that remain infected by Potyvirus, Carlavirus, and Allexivirus. The shoot tips were excised from plantlets into 2 mm. The shoot tips then planted in the medium containing ribavirin (Virozole, Sigma aldrich) with 2 concentration level, i.e. 10 mg L-1, 25 mg L-1, and without ribavirin as a control medium. Those explants then incubated at 30 oC for 4 weeks with a photoperiod of 16 h light (52
µm s-1 m-2). After 4 weeks, plantlets were transferred into MS medium without
ribavirin and incubated at 25 oC.
Experimental Design and Variable of Observation
The first experiment was conducted using factorial completely block design with two factors, i.e explant size (1 mm, 2 mm, and 3 mm) and temperatures (30 oC and 37 oC, control 25 oC), 3 replications, 10 explants per
treatment. Second experiment was conducted using completely randomized design i.e thermotherapy combined with several concentration of ribavirin (10 mg L-1 and 25 mg L-1), and without ribavirin (0 mg L-1) as a control medium.
4 RESULTS AND DISCUSSION
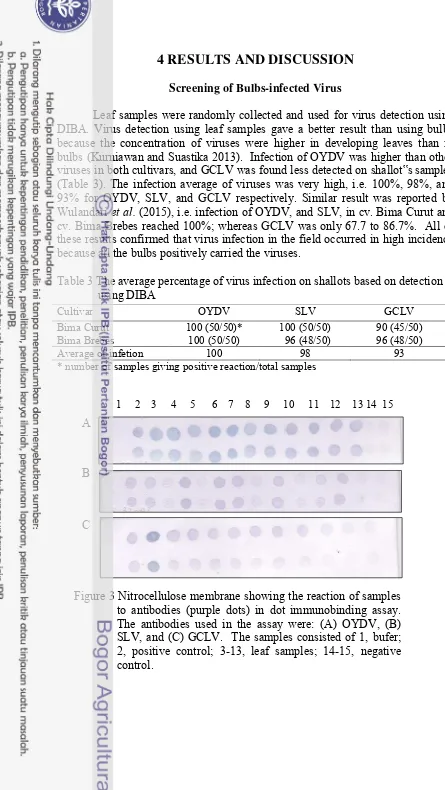
Screening of Bulbs-infected Virus
Leaf samples were randomly collected and used for virus detection using DIBA. Virus detection using leaf samples gave a better result than using bulbs because the concentration of viruses were higher in developing leaves than in bulbs (Kurniawan and Suastika 2013). Infection of OYDV was higher than other viruses in both cultivars, and GCLV was found less detected on shallot‟s samples (Table 3). The infection average of viruses was very high, i.e. 100%, 98%, and 93% for OYDV, SLV, and GCLV respectively. Similar result was reported by Wulandari et al. (2015), i.e. infection of OYDV, and SLV, in cv. Bima Curut and cv. Bima Brebes reached 100%; whereas GCLV was only 67.7 to 86.7%. All of these results confirmed that virus infection in the field occurred in high incidence because all the bulbs positively carried the viruses.
Table 3 The average percentage of virus infection on shallots based on detection using DIBA
Cultivar OYDV SLV GCLV
Bima Curut 100 (50/50)* 100 (50/50) 90 (45/50)
Bima Brebes 100 (50/50) 96 (48/50) 96 (48/50)
Average of infetion 100 98 93
* number of samples giving positive reaction/total samples
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
A
B
C
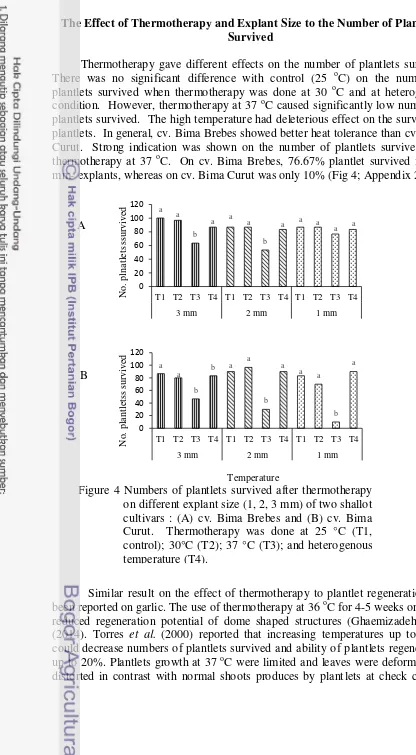
The Effect of Thermotherapy and Explant Size to the Number of Plantlets Survived
Thermotherapy gave different effects on the number of plantlets survived. There was no significant difference with control (25 oC) on the number of plantlets survived when thermotherapy was done at 30 oC and at heterogenous condition. However, thermotherapy at 37 oC caused significantly low number of plantlets survived. The high temperature had deleterious effect on the survival of plantlets. In general, cv. Bima Brebes showed better heat tolerance than cv. Bima Curut. Strong indication was shown on the number of plantlets survived after thermotherapy at 37 oC. On cv. Bima Brebes, 76.67% plantlet survived from 1 mm- explants, whereas on cv. Bima Curut was only 10% (Fig 4; Appendix 2).
Similar result on the effect of thermotherapy to plantlet regeneration had been reported on garlic. The use of thermotherapy at 36 oC for 4-5 weeks on garlic reduced regeneration potential of dome shaped structures (Ghaemizadeh et al. (2014). Torres et al. (2000) reported that increasing temperatures up to 40 oC could decrease numbers of plantlets survived and ability of plantlets regeneration up to 20%. Plantlets growth at 37 oC were limited and leaves were deformed and distorted in contrast with normal shoots produces by plantlets at check control.
Most of plantlets showed vitrified and the leaves colour became light green to yellow (Fig 5). These might be the result of deficiency in chlorophyl a and b which can inhibit the plantlet growth (Rasco and Patena 1997).
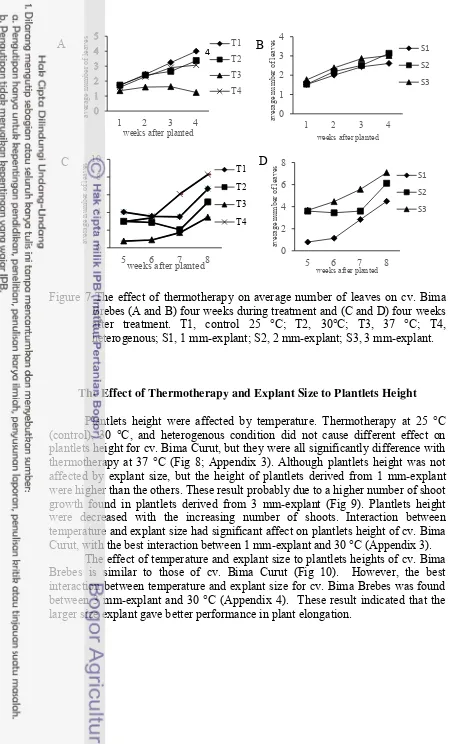
The Effect of Thermotherapy and Explant Size to Average Number of Leaves
The average number of leaves for both cultivars were affected by temperatures. Thermotherapy on cv. Bima Curut at 37 oC gave significantly difference result than those at control and other treatment. Plantlets at control and heterogenous condition had a highest average leaves among all. Plantlets at heterogenous were incubated for two weeks in control temperatures, which may ease response to gradual temperature changes. However, average number of leaves among treatment at 30 oC, heterogenous condition, and control were not significantly difference. A highest average number of leaves during treatment was control plantlets followed by plantlets at 30 oC. Average number of leaves on all temperatures on cv. Bima Curut were decreased at 8 weeks (Fig 6) (Appendix 3). It probably caused by many senescense leaves became dried up and therefore it could not count (Patena et al. 1991).
A
Figure 5 Plantlets growth on thermotherapy treatment. A, control; B, 30 oC; C, heterogenous condition; D & E, 37 oC (Bar = 0.5 cm).
B C
C
D C
The explant size had significant effect on average number of leaves during 1-2 weeks on cv. Bima Curut, but did not have significant difference started at 3 - 8 weeks (Appendix 3). These means that explant size alone did not affect leaves development on plantlets cv. Bima Curut. According to data, plantlets from 1 mm-explant in 4 weeks treatment were higher than plantlets from 3 mm and 2 mm (Fig 6). Although there was no significant difference between each explant, the smaller explant size produce leaves per plant better than larger explant.
Interaction between temperature and explant size were not significantly difference on cv. Bima Curut, except at 5-6 weeks (Appendix 3). These probably due to the treatment given at 5-6 weeks, i.e. all plantlets were transferred and incubated at 25 °C. Number of leaves on plantlets from treatment at 37 °C in all size were significantly difference. Although the plantlets was able to recovery when incubated at 25 °C, but the averages number of leaves remains low.
An average number of leaves on cv. Bima Brebes was also affected by temperatures, but not explant size. Leaves development on cv. Bima Brebes were not affected by interaction of explant size and temperatures (Appendixx 4), although untreated plantlets and plantlets treated at heterogenous condition had better growth. Average number of leaves on plantlets cv. Bima Brebes at 37 oC were decreased during treatment, but they were able to recover after 7 weeks. During treated in temperatures treatment, plantlets at 30 oC and heterogenous
condition were not significant difference with control. Plantlets from 3 mm-explant had the best leaf development followed by plantlets from 2 mm-mm-explant (Fig. 7). Comparison test using T-test student‟s showed that number of leaves between two cultivars, cv. Bima Curut and cv. Bima Brebes, were not different. Meanwhile, the mean values of explant size after treatment were significant difference between two cultivars (Appendix 7).
Figure 6 The effect of thermotherapy on average number of leaves on cv. Bima Curut (A and B) four weeks during treatment and (C and D) four weeks after treatment. T1, control 25 °C; T2, 30°C; T3, 37 °C; T4, heterogenous; S1, 1 mm-explant; S2, 2 mm-explant; S3, 3 mm-explant.
Figure 7 The effect of thermotherapy on average number of leaves on cv. Bima Brebes (A and B) four weeks during treatment and (C and D) four weeks after treatment. T1, control 25 °C; T2, 30°C; T3, 37 °C; T4, heterogenous; S1, 1 mm-explant; S2, 2 mm-explant; S3, 3 mm-explant.
The Effect of Thermotherapy and Explant Size to Plantlets Height
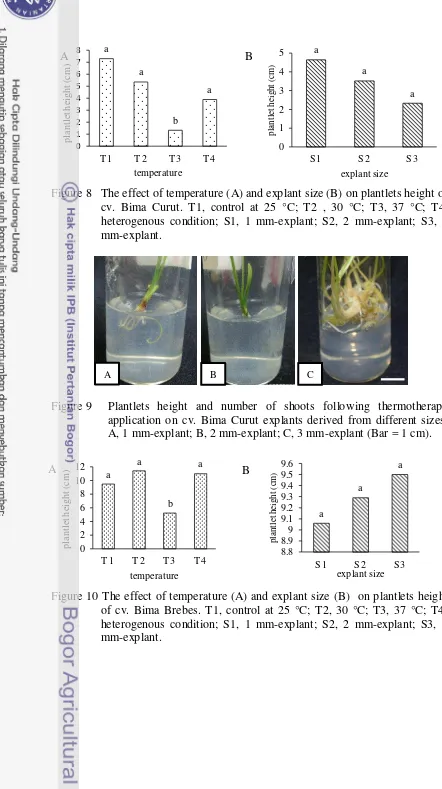
Plantlets height were affected by temperature. Thermotherapy at 25 °C (control), 30 °C, and heterogenous condition did not cause different effect on plantlets height for cv. Bima Curut, but they were all significantly difference with thermotherapy at 37 °C (Fig 8; Appendix 3). Although plantlets height was not affected by explant size, but the height of plantlets derived from 1 mm-explant were higher than the others. These result probably due to a higher number of shoot growth found in plantlets derived from 3 mm-explant (Fig 9). Plantlets height were decreased with the increasing number of shoots. Interaction between temperature and explant size had significant affect on plantlets height of cv. Bima Curut, with the best interaction between 1 mm-explant and 30 °C (Appendix 3).
The effect of temperature and explant size to plantlets heights of cv. Bima Brebes is similar to those of cv. Bima Curut (Fig 10). However, the best interaction between temperature and explant size for cv. Bima Brebes was found between 3 mm-explant and 30 °C (Appendix 4). These result indicated that the larger size explant gave better performance in plant elongation.
Figure 8 The effect of temperature (A) and explant size (B) on plantlets height of cv. Bima Curut. T1, control at 25 °C; T2 , 30 °C; T3, 37 °C; T4, heterogenous condition; S1, 1 mm-explant; S2, 2 mm-explant; S3, 3 mm-explant.
Figure 9 Plantlets height and number of shoots following thermotherapy application on cv. Bima Curut explants derived from different sizes. A, 1 mm-explant; B, 2 mm-explant; C, 3 mm-explant (Bar = 1 cm).
The Effect of Thermotherapy and Explant Size to Average Number of Roots
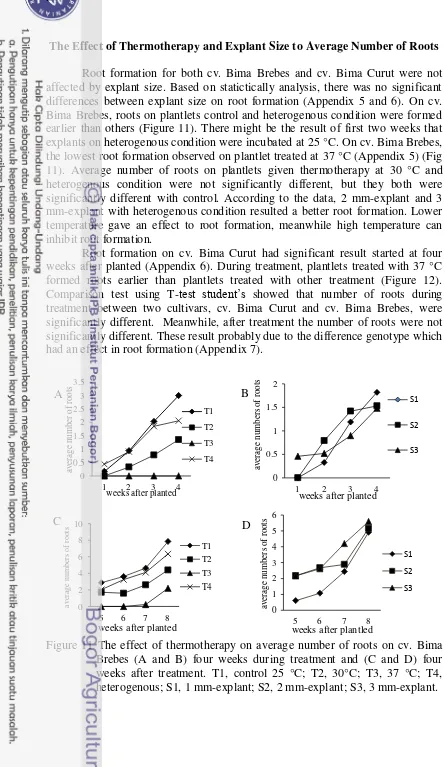
Root formation for both cv. Bima Brebes and cv. Bima Curut were not affected by explant size. Based on statictically analysis, there was no significant differences between explant size on root formation (Appendix 5 and 6). On cv. Bima Brebes, roots on plantlets control and heterogenous condition were formed earlier than others (Figure 11). There might be the result of first two weeks that explants on heterogenous condition were incubated at 25 °C. On cv. Bima Brebes, the lowest root formation observed on plantlet treated at 37 °C (Appendix 5) (Fig 11). Average number of roots on plantlets given thermotherapy at 30 °C and heterogenous condition were not significantly different, but they both were significantly different with control. According to the data, 2 mm-explant and 3 mm-explant with heterogenous condition resulted a better root formation. Lower temperature gave an effect to root formation, meanwhile high temperature can inhibit root formation.
Root formation on cv. Bima Curut had significant result started at four weeks after planted (Appendix 6). During treatment, plantlets treated with 37 °C formed roots earlier than plantlets treated with other treatment (Figure 12). Comparison test using T-test student‟s showed that number of roots during treatment between two cultivars, cv. Bima Curut and cv. Bima Brebes, were significantly different. Meanwhile, after treatment the number of roots were not significantly different. These result probably due to the difference genotype which had an effect in root formation (Appendix 7).
Figure 11 The effect of thermotherapy on average number of roots on cv. Bima Brebes (A and B) four weeks during treatment and (C and D) four weeks after treatment. T1, control 25 °C; T2, 30°C; T3, 37 °C; T4, heterogenous; S1, 1 mm-explant; S2, 2 mm-explant; S3, 3 mm-explant.
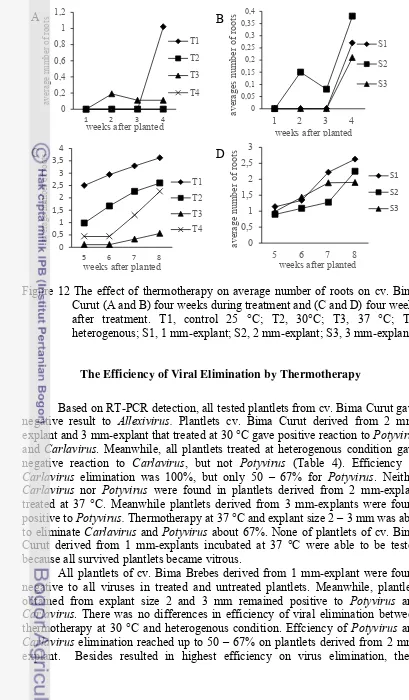
Figure 12 The effect of thermotherapy on average number of roots on cv. Bima Curut (A and B) four weeks during treatment and (C and D) four weeks after treatment. T1, control 25 °C; T2, 30°C; T3, 37 °C; T4, heterogenous; S1, 1 mm-explant; S2, 2 mm-explant; S3, 3 mm-explant.
The Efficiency of Viral Elimination by Thermotherapy
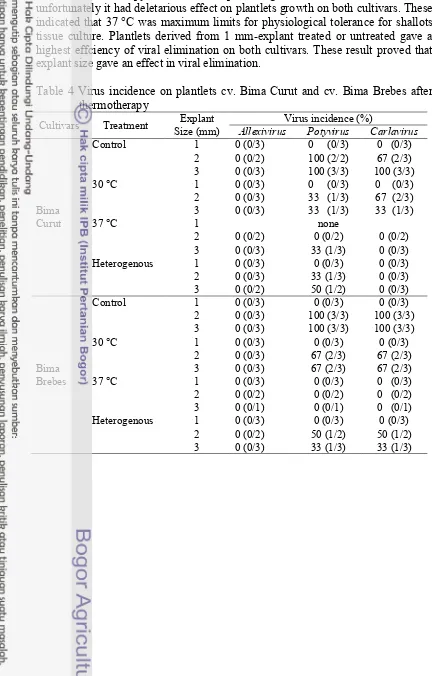
Based on RT-PCR detection, all tested plantlets from cv. Bima Curut gave negative result to Allexivirus. Plantlets cv. Bima Curut derived from 2 mm-explant and 3 mm-mm-explant that treated at 30 °C gave positive reaction to Potyvirus and Carlavirus. Meanwhile, all plantlets treated at heterogenous condition gave negative reaction to Carlavirus, but not Potyvirus (Table 4). Efficiency of Carlavirus elimination was 100%, but only 50 – 67% for Potyvirus. Neither Carlavirus nor Potyvirus were found in plantlets derived from 2 mm-explant treated at 37 °C. Meanwhile plantlets derived from 3 mm-explants were found positive to Potyvirus. Thermotherapy at 37 °C and explant size 2 – 3 mm was able to eliminate Carlavirus and Potyvirus about 67%. None of plantlets of cv. Bima Curut derived from 1 mm-explants incubated at 37 °C were able to be tested because all survived plantlets became vitrous.
treatment resulted a better performance on plantlets growth. Plantlets obtained from thermotherapy at 37 °C were virus-free (Table 4) (Fig 13).
Thermotherapy at 37 °C was a promising treatment for virus elimination, unfortunately it had deletarious effect on plantlets growth on both cultivars. These indicated that 37 °C was maximum limits for physiological tolerance for shallots tissue culture. Plantlets derived from 1 mm-explant treated or untreated gave a highest effciency of viral elimination on both cultivars. These result proved that explant size gave an effect in viral elimination.
Table 4 Virus incidence on plantlets cv. Bima Curut and cv. Bima Brebes after thermotherapy
Cultivars Treatment Size (mm) Explant Allexivirus Virus incidence (%) Potyvirus Carlavirus
The Efficiency of Viral Elimination by Chemotherapy Following Thermotherapy
Plantlets from previous thermotherapy experiments that remained virus positive was used as materials for chemotherapy. The chemical treatment using ribavirin is aimed to enhance viral eliminaton from previous thermotherapy treatment. Ribavirin treatment resulted differently for the two cultivars. Number of plantlets survived for cv. Bima Curut was higher on media containing ribavirin 10 mg L-1 than those on media without ribavirin (control media). On the other hand, ribavirin suppressed the growth of cv. Bima Brebes plantlets (Fig 14). None of cv. Bima Curut plantlets survived when treated with ribavirin 25 mg L-1, meanwhile there was one of cv. Bima Brebes plantlet survived for a short time
Table 5 Effect of chemotherapy to plantlets growth
Cultivar Treatment
(mg L-1)
Characteristic of plant growtha
Number of leaves Number of roots Plantlets
height (cm)
Means followed by the same letter are not significantly difference at 1% level using DMRT ** these letter signifies p < 0.01
Plantlets growth was affected by ribavirin for both cultivars (Table 5). Leaves and roots formation of cv. Bima Curut was affected significantly by ribavirin at 10 mg L-1 as well as plant heights. Higher concentration of ribavirin at 25 mg L-1 caused inhibition of plantlets growth. Similarly with cv. Bima Brebes, except roots formation was not affected significantly, and one plantlet was survived shortly on medium containing 25 mg L-1. Some virus-free plants can be obtained by adding ribavirin on the medium, but shoot grown in the medium containing 25 mg L-1 ribavirin was weak and died fast. Once the plantlets recovered from ribavirin, leaves was sampled for virus detection using RT-PCR. The result showed that all plantlets for both cv. Bima Curut and cv. Bima Brebes remained positive to Potyvirus and Carlavirus (Fig 15).
General Discussion
The used of virus-free bulbs as seed is needed in order to reduce virus incidence and increase shallot‟s productivity. Studies in another Allium plant, for instance garlic, showed significant difference on garlic‟s yield between healthy plants and infeceted plants as plant materials. For example, the use of healthy seeds of garlic in Serbia produced 75.3% of bulb mass, meanwhile infected seeds producing 59.11%. Cloves mass and water content per bulb were decreased 25.9% and 24% respectively compare to healthy plant (Bagi et al. 2012).
In this recent study on shallot, virus incidence was proven very high, although the leaves did not show any symptom or symptomless. Field symptoms of shallot disease were difficult to be found. Sometimes, the symptoms relates to the virus strain, host species susceptibility, and enviromental condition (Yanju et al. 2010). Infection of each viruses alone rarely show any symptom. According to Gunaeni et al. (2011) severe symptom is obvious on shallot leaves when OYDV infection occurred in mix infection with SLV and SYSV. Virus disease symptom that was found on leaves included chlorosis, yellow, and green stripe mosaic, and decreasing on a leaves size.
In vitro culture combined with thermotherapy and chemotherapy has been widely used to obtain virus-free plants. Despite of the ability to eliminate viruses, techniques that used in this study must not interfere on charactheristics of plantlets growth. On cv. Bima Curut, a smaller explant size gave a better leaves development than bigger size. According to Taskin et al. (2013) smaller explant size of garlic either free or carry a very low concentration of viruses so competition are less and plantlets are able to grow better. Furthermore, smaller explant size had a rapid cell proliferation so that enhancing leaves development.
A better result in plantlets growth on cv. Bima Brebes were showed by plantlets from bigger explant size. Fayek et al. (2009) described that the larger explant size in meristem culture gave a better plantlets growth. Furthermore, Ali et al. (2013) described that bigger explant size contained a higher concentration of metabolites and plant hormones so that it could grow better. AlMaarri et al. (2012) described that the highest elongation of potato plantlets were achieved by using 0.3 mm-explant than those using 0.1 mm-explant.
The root formation might be affected by genotype, exogenous plant hormones, and ability to form roots in different temperatures. Some genotype of plants are able to form roots earlier with the increasing of temperature. According Dinarti et al. (2011) lower temperature might be affecting in cell proliferation and concentration of endegenous gibberelin. Gibberelin plays a role in root development, breaking dormancy, and plant elongation. This subtances were easily broken to high temperature, so that lower temperature affecting in gibberelin‟s works in order to induce root formation and the rate of cell poliferation became high (Rahman et al.2003).
elimination, but these techniques need expertise and time consuming (Taskin et al. 2013). Shoot tip culture is easier techniques than meristem culture and is the most used method to produse virus-free plantets. These techniques was used because asumption that virus concentration is not uniform distributed in infected plant (Torres et al. 2000). Thus, combination with thermotherapy or another techniques will enhace efficiency of viral elimination.
At certain temperatures, thermotherapy disrupts movement protein which used for virus translocation in the plant. Thermotherapy was able to increase respiration, protein synthesis, cell cleavage, and cell growth. Therefore, temperatures that used as a treatment should be able to eliminate viruses and decrease the propability of explants damages (Neelamathi et al. 2014; Panattoni et al 2013). Furthermore, thermotherapy enhance defense mechanism of plant to viruses infection by activated gene silencing (Panattoni et al 2013).
Optimum temperature that used as thermotherapy treatment is a comparison between survival and viral elimination (Ghaemizadeh et al. 2014). The result in this recent study gave different results compared to research on garlic done by Torres et al. (2000). Thermotherapy using 37 °C was effective to eliminate viruses on garlic and had efficiency about 54% and did not interfere plantlets growth. Ghaemizadeh et al. (2014) was able to eliminate OYDV using thermotherapy at 36 °C combined with stem-disc dome culture and had efficiency about 77%.
The effect of chemotherapy on plantlets growth on infected shallot‟s plantlets was influenced by the concentration level of ribavirin. Ribavirin has been known as base analog (guanocyne) that interfere replication, both viruses or plant. Ribavirin inhibits RNA-capping enzyme leading to inefficient mRNA translation. Ribavirin might cause death or delay of cellular metabolic processes (Faccioli and Collalongo 2002; AlMaarri et al. 2012). Combination effects of thermotherapy and chemotherapy (Ribavirin higher than 10 mg L-1) did not give the expected results. These plant completely dried up turning brown and remained positive to viruses. These finding had in contrast with Fletcher et al. (1998). Virus-free plants could be obtained about 60 - 62% from thermotherapy at 38 °C combined with 50 mg L-1 ribavirin. The succesfull virus elimination using those combination
5 CONCLUSIONS AND RECOMMENDATIONS
Conclusions
The incidence of OYDV, GCLV, and SLV on shallot bulbs cv. Bima Brebes and cv. Bima Curut were very high, i.e 90 to 100%. Plantlets developed from 1-mm explant has a better chance to be virus free. The best treatment for bigger explant size was using 2 mm-explant in heterogenous condition. Thermotherapy at 37 ºC had deletarious effect on plantlet growth. The use of ribavirin needs further study, since treatment combination of thermotherapy and chemotherapy was not able to produce virus-free plantlets.
Recommendations