-2O8
Simanungkalit MedJ
Univ IndonEffects
of Ethanol
on Isolated Hepatocytes
:
Alteration
in
Cell
Surface
and
Intracellular
ATP
Nelson Simanungkalit
Abstrak
Ethanol nerupakan salah satu to.rin yang sangat berbahal'a terhadap hati. Walau studi rcnrang keracunan ethanol telah banl'ak penguruh ethanol terhadap perrnukaan sel hati dan keduu lerhadap konsentrasi ATP intrasel. Petnaparan ethanol terhadap sel hati rikus yanp diisolttsi nenimbulkan terbentuknya penonjolan di pernukaannya, sedangkan konsentrasi ATP intrasel ,ilenurun secara benrrukntr (p < 0,05).
Abstract
Orrc o.f the urost serious lrcput()toci.t og,ents to the liver is ethanol. Alrhough irs ro.riciry' hos been investigated, the to.tic tnechanisnr itself retnains conrroy'ersial. The ains of the present w,ork are to investigare the effect of etlnnol on the su.rface of freshly isolated hepaîrtc1.tes qfter incubatiotr v,ith ethanol, and its itdluence on cytosolic ATP-concentration. Ittcuburion with ethanol led to the specific fornation of ret'ersible blebs otr rhe surface of hepatoc)'les and a signif cant decrease (p < 0,05) o.f c1'tosolic ATP-concentratiott.
Ke y v, o rd s : he pal o I o B)', e I han o I t o r i c i t1', ble b fo r n nt i o tt
Liver is
the main organ which
metabolizes
ethanol.Although
theliver
is at the beginning
resistant againstthe
influence
of ethanol,
the
continous uptake
of
ethanol
in
high
doses can damageliver
cell producing
apathological
state such as"cirrhosis". Many
studieson ethanol-toxicity
has beencarried out,
however its
mechanism
is
still
not
clear,
The
present paper
will
describetwo
studies about the influenceof
ethanol on
hepatocytes.Firstly,
its influence to the cell
surface aspictured by
scarnningelectron n.ricroscopy and
second-ly
its influence
on theintracellular
ATP concentration.
MATERIALS
AND METHODS
Sprague-Dawley rats (ca.
22Og)
were obtained
from
Savo, nredizinische Versuchstierzuchten GmbH,
Kissl egg/Al I gaeu, Germany.Isolated
hepatocytes
were
prepared by
col-lagenase perfr,rsion.lC"ll uiobility,
asjudged by trypan
blue exclusion,'
was
between
85 To
95%
for all
preparations.
Following
isolation,
a
suspension
of
hepatocytes
containing
I -
1.5
million
cells/ml
was transfered to plastic vials (10ml). Ham's
F-l2
medium
and ethanol of different concentrations (0.3 - 2.6mol/l)
were added, so
that the
final volume
was
1.0ml in all
experiments. The
contents were
n.rixedgently,
and thevials were kept
in
awaterbath at temperature
of
25"
Cfor
30 min.Scanning
electron microscopy
I
to
1.5million
hepatocytes
for
electron microscopic
scanning were
fixed
in 1%
glutaraldehyde
in
0. 1mol
cocodylate-buffer, washed washing (2 times) with
cocodylate-buffer for
15 min
each,
followed
by
thefixation with 0.5%
Os
(VIII)-oxide
for lh.
Dehydra-tion
was carriedout
using 50-,
70-
and IOO%alcohol
for
5min
each. Theair dried sanrples were then put on
"leit-Tabs" (Plano),
evaporated
with
gold-palladium
(Sputter coater,Bio-Rad)
and examinedwith
JSM, U3 - scanningelectron microscope.
Vol 3, No 4, October-December 1994
Determination of the ATP content of
hepatocytes
The
ATP
content
of
isolated
hepatocytes
was measured3'4using Auto-Clinilumina
t (LB
g52TI
16)from Berthold, Wildbad.
A
l0O
pl
aliquot
wastaken
from
thecell
suspension(l ml) for
theviability
deter-mination.
The remaining
vials
content 900
pl
wasput
in crushed ice
for
5 minutes to enhance thesedimention
of the cells. To remove the supernatant, the
cell
suspen-sion
was washed 3times
eachwith
1ml
physiological
saline.
After centrifugation for
5min
(200
xg)
at 4oC,the supernatant was
carefully
pipetted out
and900
pl
TCA
(57o) was addedto
thecells.
Thecell
suspension was thenhomogenized
using a BransonSonifier.
After
centrifugation
at 3000
x
gfor
15 min at 4oC,50
pl
of
supernatant was
diluted 1:41
(vol/vol)
with
physiologi-cal saline.
The
measurement was done 5 secondsafter
60pl
[image:2.595.47.546.352.683.2]of the
diluted solution
wasnrixed with
ATP-reagent.
Figure
I.
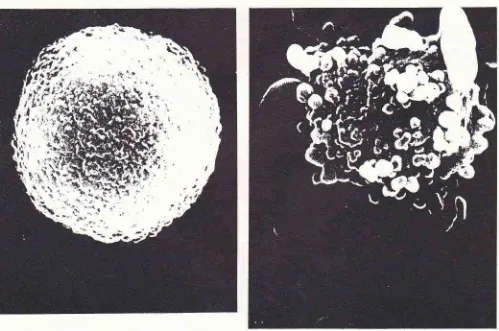
Sccurtritrq1 elcctron nicrogruph of a nonuol,.freshll, isolated hepatocl'teM u gn i.fi
ut
i o trs : -\U)OEthanol Effects ott Hepatocytes 2O9
Diluted
5
mmol/l
ATP-NazHz .3HzO was
used
asATP-standard.
During the
measurement,
the
sample were stored atcold
temperature.RESULTS
Freshly isolated
hepatocytes
were examined
with
ascanning electron microscope. The
shapeof a
normal
hepatocyteis mostly
round oroval
with
a rough surface(Figure 1).
Freshly isolated hepatocytes,
exposedto ethanol
of a dose
ranging from
0.3. to 2.6mol/|,
produced blebs on thecell
surfaces.Observation by
scanningelectron
microscopy showed blebs of various
sizeson
thecell
surface,
with
laige sized blebs resembling
cucumbrs
(Figure
2).Figure 2. Scanning electron nticrograph of a.freshls. isolated hepatocyte in the presence ofO.65
nol
ethanol/2lO
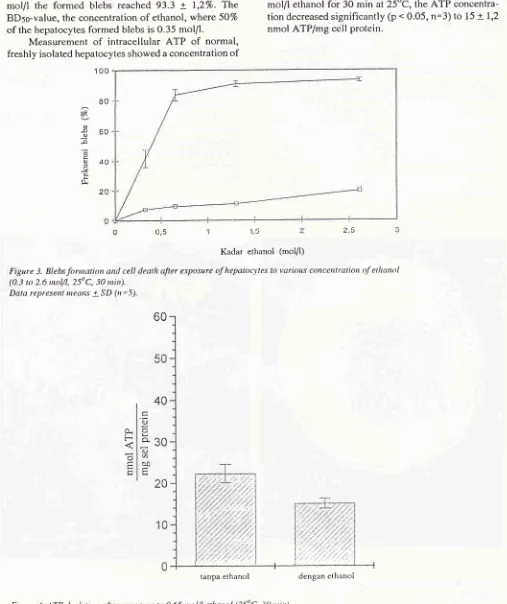
SimanungkalitAs
shown
in
Figure 3, the blebbing
caused by
ethanol were dose
dependent.
At
concentration
2,6mol/l
the formed blebs
reached
93.3
+
1,27o.The
BDso-vafue, the concentration
of
ethanol,
where 5O7oof
the hepatocytesformed
blebsis 0.35
mol/I.
Measurement
of
intracellular
ATP
of
normal,
freshly
isolated hepatocytes showed aconcentration
of
Med J Utriv Indon
22.3
+
2.2 nmol ATP/mg cell protein (Figure 4).
If
freshly
isolated
hepatocytes
were exposed
to
0.65mol/l
ethanol
for
30
min
at25"C,
theATP
concentra-tion
decreasedsignificantly
(p
.
0.05,n:3)
to
15+
1,2nmol ATP/mg cell
protein.
100
80 èR
q
€60
F I u40
=
-v,
0)
rl
0,5
Kadar ethanol (mol/l)
Figu.re 3. Blebs fonnation and cell death afier erposure of hepatocyles to various cot'tccntratio,l o.f ethanol (0.3 to 2.6 ttrol/l,25oC, 30 nùn).
Data represent means + SD (tr:5).
60
[image:3.595.47.554.126.730.2]40
Figure 4. ATP depletion afier etposure ro 0.65
nol/
elhanol (25oC, 30 tttitt). Data repre.sent ileans + SD(rt:3)
15
O.
F
o
4)
o
q30
OJ
0 è0
20
Vol 3, No 4, October-Deceuber t994
DISCUSSION
Cell
surfaceblebbing
is anearly
indication of hypoxic
and
oxic
injury to
hepatocytes.t'5'6'7Ethunol
caused
epato-
phal-
extra-re
dif-ferent.
Scanning
elçctron micrograph showed
largesized blebs resembling
to
cucumbers.
It
seemed thatthe blebs are typical depending
on
the
hepatotoxic
agent.
Another important observation was the
revers-ibility
of
the blebs.
The
blebs
dissapearedwithin
30 minutes.Ethanol interaot
with
biological
membrun"r.t2
This interaction affects physical
andchemical
proper-ties
of
membranes andmay
causeinhibition of
trans-membrane signal in g processes.Other authors
considered
that
acetaldehyde
anintermediate product
during
ethanol metabolism,
andalteration
of NAD/NADH
ratio
were responsible
for
the
cell
damage. l3'14'15'16Several authors
suggested that the blebsformation was
associatedwith the
risein
cytosolic
Ca2+.6'7'ti Since exposureof freshly isolated
hepatocytes
to
ethanol
caused
blebbing
it
was
sug-ges^ted
that ethanol may also
cause arise
in cytosolic
Ca2*.
In
our
experiments
cytosoli
c
Ca2* was
not
measured,
but
there was anevidence
of
an increaseof
cytosolic
Ca2* dueto
ethanol
toxicity.
Further,
it
wasreported that the rise was temporary. lE Observations
in
this
study
showed that the blebs werereversible.
Thereseems to be a
relationship
between the blebsformation
and the
rise
in cytosolic Ca2*.
As the
concentration
of
the cytosolic
Ca2*
wasincreased,
ATP consumption can be
expected
to
in-crease too sinceit
isknown
thattransportation of
2mol
Ca2*
from intra to extracell
consumes
I
mol of ATp.
The
results
of
this
study
confirmed this
expectation;
the
ATP
concentration
decreasedsignificantly
(p
<0.05,
n=3) from22.3
+2.2nmol ATP/mg cellprotein.
Whether this
ATP
depletion
wasonly caused
of
Ca2*-ATP-ase-Puntp
or
not
still
needfurther
investigation,
since
ethanol
inhibits
glycolysis.
leCONCLUSIONS
In
vitro
exposure
of
freshly
isolated hepatocytes
ex-posed
to
ethanol
causeblebbing
at the surface
of
the hepatocytes.The produced blebs were reversible
andsome
of
them had atypical
shape,resembling
acucum-ber.
Exposure to 0.65
mol
ethanol/l
for
30min caused
a significant decrease(p
<0,05)
of intracellular
ATP.
Ethanol Effects on Hepalocytes
2ll
Aknowledgement
Aknowledgement are
due
to
the Bureau
for
Assess-ment and
Application
of
Technology
of
Republic
of
Indonesia
aswell
asProf.
Dr.G.S.Rao
for giving
meaccess
to
do
research
at
the
Institut
der Klinische
Biochemie
derUniversitaet Bonn, Germany.
I
am alsograteful to
Mr, J. Bedorf
at theInstitut fur
experimen-talle
Pathologie der
Universitaet Bonn for
his help
in
taking photographs
from
scanning electron
micro-scope.
REFERENCES
1. Eckel
J,
Rao GS, Rao ML, BreuerH. Uptake
of
L-tri-iodothyronine by isolated rat liver cells. Biochem
I
1979; 182:473-91.2. Rao GS, Lemoch H, Kessler H, Damm I, Eiermann V, Koll
S, Zarbock J, Usadel KH. Prevention of Phalloidin-induced Lesions on Isolated Rat Hepatocytes
by
Novel Synthetic Analogues of Somatostatin. Klin Wochenschr 1986; 64. 3. De Luca M, Mc Elroy WD. In Methods of Enzymology (Ed:SP Colowick, NO Kaplan) 1978;57:3.
4. De Luca
M.
Firefly Luciferase. Advances Enzymol 1969; 44:3'l-68.5. Lemasters IJ, Ji S, Thurman RG. Centrilobular injury
fol-lowing hypoxia in isolated, perfused rat Iiver, Science 198
l;
213:661-3.6. Jewell SA, Bellomo G, Thor H, Orrenius S. Bleb Formation during Drug Metabolism is Caused by Disturbances in Thiol and Calciunr Ion Horneostasis. Science 1982;217:1257-9. 7. Lernaster JJ, Stemkowski CI, Ji S, Thurman RGJ. Cell Biol
1983;97:17 8-86.
8. Rao GS, L.ernoch H, Usadel KH. Behandlung mit
Somatos-tatin schiitzt
die
Rattenleberzelle gegen Lâsionen durch Phalloidin, Âthanol und Dirnethylsulfoxid.V. Freiburger
Kolloqium, AT'TEMPTO Verlag Tùbingen GmbH 1982.
9. Weiss E, Sterz
I,
FrimrnerM,
Kroker R. Electron Micros-copy of holated Rat Hepatocytes before and after Treatment with Phalloidin. Beirr Parh Bd 1973;150:345.10. Frimmer
M.
What we
have learnedfrom
phalloidin.Toxycology Letters 1987;3 5 :169 -82.
11. Nicotera P, Hartzell P, Baldi C, Svenson S, Bellomo G, Orrenius S. Cystamine Induces
Toxicity
in
Hepatocytes through the Elevation of Cytosolic Ca:* and the Stimulationol
a Nonlysosonral Proteolytic System. J Biol Chem 1986; 261:31 (5).12. Taraschi
lE,
Rubin E. Biology of Disease Effects of Ethanolon the
Chernical and Structural Propertiesof
BiologicMernbranes. Laboratory In vesti gation I 9 8 5 ; 5
I
(2): l2O l_ab Invest 41:393.13. Sorrell MF, Tuma DJ. Effect of alcohol on hepatic rnetabo-lism: selected aspects. Clin Sci 1919;51 481-9.
14. Sorrell
MF,
TumaDI.
The Functional Implicationsof
Acetaldehyde Binding to Cell Constituents. Ann New York
212
Sinanungkalir15. Lieber C. Alcohol and liver: 1984 update. Hepatology 1984;
1234-û.
16. lvtrezey
E.
Metabolic effectsof
alcohol. Fed Proc 1985;44:134-8.
17. Thor H, Hartzell P, Orrenius S.
I
Biol
Chem 1985; 259: 6612-5.Med
J
Univ Indon18. Hqek JB, Thomas AP, Rubin R, Rubin E. Ethanol-induced Mobilization of Calcium by Activation of Phosphoinositide specific Phospholipase C in Intact Hepatocytes. I Biol Chem
1987;262:.2.