Forensic Science International is an international journal publishing original contributions in the many different scientifi c disciplines pertaining to the forensic sciences. Fields include forensic pathology and histochemistry, chemistry, biochemistry and toxicology (including drugs, alcohol, etc.), biology (including the identifi cation of hairs and fi bres), serology, odontology, psychiatry, anthropology, the physical sciences, fi rearms, and document examination, as well as investigations of value to public health in its broadest sense, and the important marginal area where science and medicine interact with the law. Review Articles and Preliminary Communications (where brief accounts of important new work may be announced with less delay than is inevitable with major papers) may be accepted after correspondence with the appropriate Editor. Case Reports will be accepted only if they contain some important new information for the readers.
Submission of Articles: Manuscripts prepared in accordance with Instructions to Authors should be sent to the Editor-in-Chief or one of the Associate Editors
according to their area of expertise as listed below. If there is any doubt, a preliminary letter, telefax or telephone enquiry should be made to the Editor-in-Chief or his Assistant.
EDITOR-IN-CHIEF
P. Saukko – (for: Experimental Forensic Pathology, Traffi c
Medicine and subjects not listed elsewhere) Department of Forensic Medicine, University of Turku, SF-20520 Turku, Finland Tel.: (+358) 2 3337543; Fax: (+358) 2 3337600; E-mail: psaukko@utu.fi
A. Carracedo – (for: Forensic Genetics)
Instituto de Medicina Legal, Facultad de Medicina,
15705 Santiago de Compostela, Galicia, Spain Fax: (+34) 981 580336
Cristina Cattaneo – (for: Anthropology and Osteology)
Instituto de Medicina Legal, Universit`a degli Studi Via Mangiagalli 37, 20133 Milano, Italy
Tel: 0039 2 5031 5678; Fax: 0039 2 5031 5724
O.H. Drummer – (for: Toxicology)
Department of Forensic Medicine, Victorian Institute of Forensic Medicine
57–83 Kavanagh Street, Southbank 3006, Victoria, Australia Tel.: (+61)-3-9684-4334;
Fax: (+61)-3-9682-7353
Assistant Editor: M.A. LeBeau (Quantico, VA, USA)
Tel: (+1) 703 632 7408; [email protected]
C. Jackowski (for: Forensic Imaging)
Institut für Rechtsmedizin Medizinische Fakultät Universität Bern Bühlstrasse 20 3012 Bern, Switzerland Tel.: +41 (0)31 631 8411 Fax : +41 (0)31 631 38 33
P. Margot – (for: Questioned Documents, with the assistance of A. Khanmy and
W. Mazzela; and for Physical Science: ballistics, tool marks, contact traces, drugs analysis, fi ngerprints and identifi cation, etc.)
Ecole de Sciences Criminelles (ESC), UNIL-BCH, CH-1015 Lausanne, Switzerland Fax: (+41) 21 692 4605
Assistant Editor: P. Esseiva (Lausanne, Switzerland)
Tel: (+41) 21 692 4652; [email protected]
Martin Hall – (for: Forensic Entomology)
Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK
Tel: 0044 207 942 5715 Fax: 0044 207 942 5229
G. Willems – (for: Odontology)
Katholieke Universiteit Leuven, School of Dentistry, Oral Pathology and Maxillo-Facial Surgery, Departments of Orthodontics and Forensic Odontology, Kapucijnenvoer 7, B-3000 Leuven, Belgium
Tel: +32 16 33.24.59; Fax: +32 16 33.24.35 ASSOCIATE EDITORS
J. Amendt (Frankfurt, Germany) P. Beh (Hong Kong, China) P. Buzzini (Morgantown, WV, USA) H. Chung (Seoul, Korea)
J. Clement (Melbourne, Australia) S.D. Cohle (Grand Rapids, MI, USA) S. Cordner (South Melbourne, Australia) P. Dickens (Buxton, UK)
H. Druid (Stockholm, Sweden) A. Eriksson (Umeå, Sweden) J.A.J. Ferris (Auckland, New Zealand) M.C. Fishbein (Encino, USA)
G. L. de la Grandmaison (Garches, France) C. Henssge (Essen, Germany)
M.A. Huestis (Baltimore, MD, USA) A.W. Jones (Linköping, Sweden)
H. Kalimo (Helsinki, Finland) Y. Katsumata (Kashiwa, Japan) B. Kneubuehl (Thun, Switzerland) G. Lau (Singapore)
S. Leadbeatter (Cardiff, UK) C. Lennard (Canberra, Australia)
A. Luna Maldonado (Espinardo (Murcia), Spain) B. Madea (Bonn, Germany)
H. Maeda (Osaka, Japan)
D. Meuwly (The Hague, The Netherlands) C. Neumann (Pennsylvania, USA) S. Pollak (Freiburg i. Br., Germany) M.S. Pollanen (Toronto, Canada) D. Pounder (Dundee, UK) K. Püschel (Hamburg, Germany) G. Quatrehomme (Nice, France)
R. Ramotowski (Washington, DC, USA) J. Robertson (Canberra, Australia) C. Roux (Sydney, Australia) I. Sääksjärvi, (Turku, Finland) J. Stevens (Exeter, UK) M. Steyn (Pretoria, South Africa) F. Tagliaro (Verona, Italy) T. Takatori (Chiba, Japan) A. Thierauf (Freiburg, Germany) D. Ubelaker (Washington D.C., USA) D.N. Vieira (Coimbra, Portugal) J. Wells (Miami, FL, USA) P. Wiltshire (London, UK)
X. Xu (Shantou, People’s Republic of China) J. Zieba-Palus (Krakow, Poland)
CHAIRMAN FORENSIC SCIENCE INTERNATIONAL: P. Saukko (Turku, Finland)
Content
Profiles of pregabalin and gabapentin abuse by
postmortem toxicology
Margareeta Häkkinen, Erkki Vuori, Eija Kalso, Merja Gergov, Ilkka
Ojanperä
p1
–
6
Published online: May 3, 2014
Demonstration of spread-on peel-off consumer
products for sampling surfaces contaminated with
pesticides and chemical warfare agent signatures
Deborah L. Behringer, Deborah L. Smith, Vanessa R. Katona, Alan T. Lewis
Jr., Laura A. Hernon-Kenny, Michael D. Crenshaw
p7
–
14
Published online: May 5, 2014
Elderly arrestees in police custody cells:
implementation of detention and medical decision
on fitness to be detained
Aurélie Beaufrère, Otmane Belmenouar, Patrick Chariot
p15
–
19
Published online: May 5, 2014
Instar determination in forensically useful
beetles
Necrodes littoralis
(Silphidae)
and
Creophilus maxillosus
(Staphylinidae)
Katarzy a Frąt zak, Szy o Matuszewski
p20
–
26
Published online: May 2, 2014
Evaluation of a homogenous enzyme immunoassay
for the detection of synthetic cannabinoids in urine
Allan J. Barnes, Sheena Young, Eliani Spinelli, Thomas M. Martin, Kevin L.
Klette, Marilyn A. Huestis
p27
–
34
Terrestrial laser scanning and a degenerated
cylinder model to determine gross morphological
change of cadavers under conditions of natural
decomposition
Xiao Zhang, Craig L. Glennie, Sibyl R. Bucheli, Natalie K. Lindgren, Aaron
M. Lynne
p35
–
45
Published online: May 13, 2014
Assessment and forensic application of
laser-induced breakdown spectroscopy (LIBS) for the
discrimination of Australian window glass
Moteaa M. El-Deftar, Naomi Speers, Stephen Eggins, Simon Foster, James
Robertson, Chris Lennard
p46
–
54
Published online: May 7, 2014
Analysis of chain saw lubricating oils commonly
used i Thaila d s southe o de p ovi es fo
forensic science purpose
Aree Choodum, Kijja Tripuwanard, Niamh Nic Daeid
p60
–
68
Published online: May 3, 2014
Identification of scanner models by comparison of
scanned hologram images
Shigeru Sugawara
p69
–
83
Published online: April 30, 2014
Cross-reaction of propyl and butyl alcohol
glucuronides with an ethyl glucuronide enzyme
immunoassay
Torsten Arndt, Reinhild Beyreiß, Stefanie Schröfel, Karsten Stemmerich
p84
–
86
Morphine and codeine concentrations in human
urine following controlled poppy seeds
administration of known opiate content
Michael L. Smith, Daniel C. Nichols, Paula Underwood, Zachary Fuller,
Matthew A. Moser, Charles LoDico, David A. Gorelick, Matthew N.
Newmeyer, Marta Concheiro, Marilyn A. Huestis
p87
–
90
Published online: May 12, 2014
Evaluating the utility of hexapod species for
calculating a confidence interval about a succession
based postmortem interval estimate
Anne E. Perez, Neal H. Haskell, Jeffrey D. Wells
p91
–
95
Published online: May 19, 2014
Development of a GC
–
MS method for
methamphetamine detection in
Calliphora
vomitoria
L. (Diptera: Calliphoridae)
Paola A. Magni, Tommaso Pacini, Marco Pazzi, Marco Vincenti, Ian R.
Dadour
p96
–
101
Published online: May 19, 2014
Effects of methamphetamine and its primary
human metabolite,
p-
hydroxymethamphetamine,
on the development of the Australian
blowfly
Calliphora stygia
Christina Mullany, Paul A. Keller, Ari S. Nugraha, James F. Wallman
p102
–
111
Published online: May 19, 2014
Postmortem volumetric CT data analysis of
pulmonary air/gas content with regard to the cause
of death for investigating terminal respiratory
function in forensic autopsy
Nozomi Sogawa, Tomomi Michiue, Takaki Ishikawa, Osamu Kawamoto,
Shigeki Oritani, Hitoshi Maeda
Published online: May 24, 2014
Age estimation in U-20 football players using 3.0
tesla MRI of the clavicle
Volker Vieth, Ronald Schulz, Paul Brinkmeier, Jiri Dvorak, Andreas
Schmeling
p118
–
122
Published online: May 20, 2014
Spine injury following a low-energy trauma in
ankylosing spondylitis: A study of two cases
Frederic Savall, Fatima-Zohra Mokrane, Fabrice Dedouit, Caroline
Capuani, Céline Guilbeau-Frugier, Daniel Rougé, Norbert Telmon
p123
–
126
Published online: May 28, 2014
The transferability of diatoms to clothing and the
methods appropriate for their collection and
analysis in forensic geoscience
Kirstie R. Scott, Ruth M. Morgan, Vivienne J. Jones, Nigel G. Cameron
p127
–
137
Published online: May 23, 2014
Lethal hepatocellular necrosis associated with
herbal polypharmacy in a patient with chronic
hepatitis B infection
John D. Gilbert, Ian F. Musgrave, Claire Hoban, Roger W. Byard
p138
–
140
Published online: June 2, 2014
Effect of massing on larval growth rate
Aidan P. Johnson, James F. Wallman
p141
–
149
Published online: May 17, 2014
Norcocaine in human hair as a biomarker of heavy
cocaine use in a high risk population
S. Poon, J. Gareri, P. Walasek, G. Koren
Published in issue: August 2014
Intuitive presentation of clinical forensic data using
anonymous and person-specific 3D reference
manikins
Martin Urschler, Johannes Höller, Alexander Bornik, Tobias Paul, Michael
Giretzlehner, Horst Bischof, Kathrin Yen, Eva Scheurer
p155
–
166
Published online: May 29, 2014
Factors leading to the degradation/loss of insulin in
postmortem blood samples
Cora Wunder, Gerold F. Kauert, Stefan W. Toennes
p173
–
177
Published online: June 11, 2014
Utility of urinary ethyl glucuronide analysis in
post-mortem toxicology when investigating
alcohol-related deaths
M. Sundström, A.W. Jones, I. Ojanperä
p178
–
182
Published online: June 2, 2014
DNA evidence: Current perspective and future
challenges in India
Sunil K. Verma, Gajendra K. Goswami
p183
–
189
Published online: May 30, 2014
An RNA-based analysis of changes in biodiversity
indices in response to
Sus scrofa
domesticus
decomposition
R.C. Bergmann, T.K. Ralebitso-Senior, T.J.U. Thompson
p190
–
194
Epifluorescence analysis of hacksaw marks on bone:
Highlighting unique individual characteristics
Caroline Capuani, Céline Guilbeau-Frugier, Marie Bernadette Delisle,
Daniel Rougé, Norbert Telmon
p195
–
202
Published online: June 7, 2014
Prevalence of medicinal drugs in suspected
impaired drivers and a comparison with the use in
the general Dutch population
Karlijn D.B. Bezemer, Beitske E. Smink, Rianne van Maanen, Miranda
Verschraagen, Johan J. de Gier
p203
–
211
Published online: June 12, 2014
Sampling of illicit drugs for quantitative analysis
–
Part III: Sampling plans and sample preparations
T. Csesztregi, M. Bovens, L. Dujourdy, A. Franc, J. Nagy
p212
–
219
Published online: April 28, 2014
Detection of recent holding of firearms: Improving
the sensitivity of the PDT test
Joseph Almog, Karni L. Bar-Or, Amihud Leifer, Yair Delbar, Yinon
Harush-Brosh
p55
–
59
Published online: May 7, 2014
Morphological variations of the anterior thoracic
skeleton and their forensic significance:
Radiographic findings in a Spanish autopsy sample
P. James Macaluso Jr., Joaquín Lucena
p220.e1
–
220.e7
Published online: May 20, 2014
Forensic aspect of cremations on wooden pyre
Veronique Alunni, Gilles Grevin, Luc Buchet, Gérald Quatrehomme
p167
–
172
A body, a dog, and a fistful of scats
Ignasi Galtés, María Ángeles Gallego, Dolors Giménez, Verònica Padilla,
Mercè Subirana, Carles Martín-Fumadó, Jordi Medallo
e1
–
e4
Published online: April 16, 2014
Quantitative analysis of quazepam and its
metabolites in human blood, urine, and bile by
liquid chromatography
–
tandem mass spectrometry
Jing Zhou, Koji Yamaguchi, Youkichi Ohno
e5
–
e12
Published online: May 3, 2014
The identification of an impurity product,
4,6-dimethyl-3,5-diphenylpyridin-2-one in an
amphetamine importation seizure, a potential
route specific by-product for amphetamine
synthesized by the APAAN to P2P, Leuckart route
Joh D. Power, Joh O’Brie , Bria Tal ot, Mi hael Barry, Pier e
Kavanagh
e13
–
e19
Published online: May 5, 2014
Further occurrences of
Dohrniphora cornuta
(Bigot)
(Diptera, Phoridae) in forensic cases indicate likely
importance of this species in future cases
R. Henry L. Disney, Ana Garcia-Rojo, Anders Lindström, John D. Manlove
e20
–
e22
Published online: May 23, 2014
Unintentional lethal overdose with metildigoxin in
a 36-week-old infant
–
post mortem tissue
distribution of metildigoxin and its metabolites by
liquid chromatography tandem mass spectrometry
Cornelius Hess, Christopher Brockmann, Elke Doberentz, Burkhard
Madea, Frank Musshoff
e23
–
e27
Post-
o te β
-hydroxybutyrate determination in
synovial fluid
Cristian Palmiere, Dominique Werner
e28
–
e30
Published online: May 2, 2014
E atu to sa pli g of illi it d ugs fo ua titative
analysis
–
Part II. Study of particle size and its
i flue e o ass edu tio (Fo e si S ie e
International 234C (2014) 174
–
180)
M. Bovens, T. Csesztregi, A. Franc, J. Nagy, L. Dujourdy
p221
Published online: June 11, 2014
Inside Front Cover- Editorial Board
IFC
Effects
of
methamphetamine
and
its
primary
human
metabolite,
p-
hydroxymethamphetamine,
on
the
development
of
the
Australian
blow
fl
y
Calliphora
stygia
Christina
Mullany
a,
Paul
A.
Keller
b,
Ari
S.
Nugraha
b,
James
F.
Wallman
a,*
aInstituteforConservationBiologyandEnvironmentalManagement,SchoolofBiologicalSciences,UniversityofWollongong,NSW2522,Australia bSchoolofChemistry,UniversityofWollongong,NSW2522,Australia
ARTICLE INFO
Articlehistory:
Received22April2013
Receivedinrevisedform6April2014
Accepted8May2014
Availableonline20May2014
Keywords:
Forensicentomology
Entomotoxicology Calliphorastygia
Insectdevelopment
Methamphetamine
Postmorteminterval
ABSTRACT
Thelarvaeofnecrophagousflyspeciesareusedasforensictoolsforthedeterminationoftheminimum postmorteminterval(PMI).However,anyingesteddrugsincorpsesmayaffectlarvaldevelopment,thus leading to incorrect estimates of the periodof infestation. This study investigated the effects of methamphetamine and itsmetabolite, p-hydroxymethamphetamine, on theforensically important AustralianblowflyCalliphorastygia.Itwasfoundthatthepresenceofthedrugssignificantlyaccelerated larvalgrowthandincreasedthesizeofalllifestages.Furthermore,drug-exposedsamplesremainedas pupaeforupto78hlongerthancontrols.ThesefindingssuggestthatestimatesoftheminimumPMIof methamphetamine-dosedcorpsescouldbeincorrectifthealteredgrowthofC.stygiaisnotconsidered. Differenttemperatures,drugconcentrationsandsubstratetypesarealsolikelytoaffectthedevelopment ofthisblowfly.Pendingfurtherresearch,theapplicationofC.stygiatotheentomologicalanalysisof methamphetamine-relatedfatalitiesshouldbeappropriatelyqualified.
ã2014ElsevierIrelandLtd.Allrightsreserved.
1.Introduction
Analysis of entomologicalevidence can greatlyenhance the scopeofdeathinvestigationswheresuchevidenceispresent[1].A primaryobjectiveofforensicexaminationsuponfindingacorpseis thedetermination of theminimumpostmortem interval (PMI). Thisisoftenmadebystudyinganyinfestinginsects[2],especially
flies (Diptera),and involves an understandingof the biological characteristicsofthespecies present incarrion,including their development[3]. By determiningtheage ofthese insects, it is possibletodeterminetheirtime ofcolonisation,and hence,the minimum PMI [2]. Although the developmental rates of many forensically important species are known [4,5], bioclimatic influenceshavebeenshowntoaffectgrowthratesofflyspecies
[6,7].These factorsincludetemperature,atmospheric humidity, larvaldensityandbodylocation.Anotableenvironmentaleffectof forensicimportanceisthepresenceofdrugsinacorpseandtheir subsequenteffectsoninsectphysiology.Thisisknownasforensic entomotoxicologyandfailuretoconsidersuchfactorsmayleadto errorsinminimumPMIestimates[8–10].Preliminarystudieshave
shownthatillicitdrugsinfluencethedevelopmentofvariousfly species [11–15]. In particular, the larvae of some species have shownaccelerated growthupon exposuretostimulants[12,16]. However, few investigations have been undertaken into the physiologicaleffectsofdrugsofabuseonAustralianflies.Ifthese endemicspeciesaretobesuccessfullyusedtocorrectlyestimate minimum PMI, it is imperative to understand the effect of antemortem drug use and abuse on the growth rate in the postmortemperiod[17].
Theworldwideabundanceandsocialpopularityof metham-phetamine make it a prime candidate for entomotoxicological analysis.Thepotentialforaddictionoroverdoseforfirst-timeusers isoneofthehighestofallavailabledrugsduetoitsextremeand enduringphysiologicaleffects[18–20].Inpreviousexperiments, wholeanimalshavebeenusedtosimulatethepharmacokineticsof methamphetamineinhumans[12,20–22],however,thereisstill somedebateastowhetherthisisoptimalasithasbeenreported that drugmetabolism is species-specific[23]. Furthermore,the metabolicbreakdownandexcretion ratesof methamphetamine are also species-dependent. When compared to laboratory rodents,methamphetamineismetabolised ataslower rateand lesseffectivelyinhumans[23].Asaresult,sustained administra-tion of drugs is required to achieve plasma, urine or tissue concentrations comparable to humans, which in turn produce
*Correspondingauthor.Tel.:+61242214911;fax:+61242214135.
E-mailaddress:[email protected](J.F. Wallman).
http://dx.doi.org/10.1016/j.forsciint.2014.05.003
0379-0738/ã2014ElsevierIrelandLtd.Allrightsreserved.
ForensicScienceInternational241(2014)102–111
ContentslistsavailableatScienceDirect
Forensic
Science
International
moremetabolites.Asallofthesemetaboliteshavethepotentialto affectlarvalgrowth, theeffects of metabolitesof interestcould easilybeoverrated.Theprimarymetabolicproductof metham-phetamine degradation in humans is p- hydroxymethamphet-amine,whichaccountsfor15–50%ofallmetabolitesexcretedin urine [23]. It is itself a potent hallucinogen, and contributes significantlytothehalf-lifeofmethamphetamine[18].However,in non-humananimals,theprimarymetabolicproductsof metham-phetaminebreakdownhavebeenrecordedasp -hydroxynorephe-drineandp-hydroxymethamphetamine(rats),norephedrineand benzoicacidconjugates(guineapigs),andamphetamine(rabbits)
[12,23].
Withtheexceptionofamphetamine[24],nopublishedstudies haveinvestigatedtheeffectsofthesecompoundsonthegrowth ratesofblowflyspecies.Ascertainmetabolitesarealwayspresent in overdose victims [20], necrophagous insects feeding on a cadaverwillingestthem.Theeffectoftheseproductsoninsect metabolicactionneedstobesubstantiatedinordertoprovidea validminimumPMIestimatefordrug-inducedfatalities.
Theeasterngoldenhairedblowfly,Calliphorastygia(Fabricius) (Diptera:Calliphoridae),isamongthemostforensicallyimportant speciesineasternAustralia[25].Itisoneofthefirstspeciespresent at a corpse,usually arriving within hoursto oviposit.Previous studiesontheeffects ofdrugs onthis specieshavefocused on morphine [26,27], with no investigation having been done on stimulantsortheirmetabolites.Wereporthereforthefirsttime the effects of methamphetamine and p -hydroxymethamphet-amineonthegrowthrateoftheblowflyC.stygia,withtheaimof determiningthesuitabilityofthisspeciesasamodelforestimates ofminimumPMIinscenariosinwhichthecorpseiscontaminated withtheseillicitdrugs.
2.Materialsandmethods
Methamphetamine and its primary human metabolite, p-hydroxymethamphetamine, wereappliedto thefood sourceof C.stygialarvaetosimulatepostmortemconditionsin metham-phetamine overdosevictims. Eggs werecollected fromblowfly cultures and assigned randomly to one of ten groups (nine treatments and a control). Calliphora stygia specimens were cultured through a minimum of one generation (maximum of
five generations) from pupae obtained from Sheldon’s Bait (Parawa,Australia). Flieswere keptin plastic cages withmesh lidsat 23C, and exposedtoa photoperiodof 12:12light:dark. Waterandgranulatedsugarwereprovidedadlibitum.
Sheep'sliverwascutinto3cm3piecesandsuppliedtocultures
tofacilitateovarianmaturationinfemaleflies.Thecultures,once sufficientlymatured,werepresentedwithfreshlivercoveredwith athinlayerofcottonwooltoencourageoviposition.Cageswere checkedeverytwohoursandeggstransferredtoaPetridish.Eggs werecountedinto90groupsof150each.Tenexperimentalgroups wereestablishedforfeedinglarvae:(1)control,no methamphet-amine (MA) or p-hydroxymethamphetamine (p-OHMA); (2) 0.1mg/kg methamphetamine (0.1 MA); (3) 1.0mg/kg metham-phetamine(1.0MA);(4)10mg/kgmethamphetamine(10MA);(5) 0.1mg/kg methamphetamine:0.015mg/kg p -hydroxymetham-phetamine(0.1MA:0.015p-OHMA);(6)1.0mg/kg methamphet-amine:0.15mg/kg p-hydroxymethamphetamine (1.0 MA:0.15 p-OHMA); (7) 10mg/kg methamphetamine:1.5mg/kg p -hydroxy-methamphetamine (10 MA:1.5 p-OHMA); (8) 0.015mg/kg p -hydroxymethamphetamine (0.015 p-OHMA); (9) 0.15mg/kg p -hydroxymethamphetamine(0.15p-OHMA);and(10)1.5mg/kg p-hydroxymethamphetamine(1.5p-OHMA).
The control batch containing no methamphetamine was prepared by mixing 15mL of distilled water into 1.5kg of kangaroo mince. Treatments of 10mg/kg,1mg/kg and 0.1mg/
kgmethamphetaminewerepreparedbydissolving15mg,1.5mg and 0.15mg, respectively,of ()-methamphetamine hydrochlo-ridein15mLdistilledwaterandmixinginto1.5kgofkangaroo mince.p-Hydroxymethamphetamineconcentrationsof1.5mg/kg, 0.15mg/kgand0.015mg/kgwerepreparedbydissolving2.25mg, 0.225mgand0.0225mgofp-hydroxymethamphetamine, respec-tively, in 15mL of distilled water and mixing into 1.5kg of kangaroomince.Concentrationsofeachratioofdrug:metabolite were prepared bymixing 2.25mg, 0.225mg, and 0.0225mg of p-hydroxymethamphetaminewith1.5mg,0.15mgand0.015mgof methamphetamine,respectively, in15mLofdistilledwater and 1.5kgofkangaroomincetosimulatethreepostmortemratiosof drugand metaboliteconcentrations.These concentrationswere deemedsuitableforinvestigationsbasedoncommonlyreported self-administereddosesof()-methamphetaminehydrochloride thathaveconsequentlyledtotoxicbodilyconcentrationsofthe druganddeathofmethamphetamineusers[18,28–30].
Each treatment was mixed byhand for 10min,followed by 5minofmixingwithablendertoensureanevendistributionof drug,liquidandmeat,orliquidandmeatonlyforcontrolbatches. Toavoidcontamination,newglovesandmixingcontainerswere usedforeachtreatment,andtheblenderheadrinsedinethanol andflushedwithnearboilingwaterfor10minbetweenuses.Meat batchesweresplitinto150gportionsandplacedintoplasticweigh boats.Batcheswerestoredat20C,andmeatportionsdefrosted asrequiredtoreplenishthelarvalfoodsource.
()-Methamphetamine hydrochloride (99.71.3%) and p-hydroxyamphetaminehydrochloride(p-OHAM)(99.71.3%)were obtainedunderlicensefromtheNationalMeasurementInstitute (NSW,Australia).p-Hydroxyamphetaminehydrochloridewas con-vertedtotheprimaryhumanmetaboliteofmethamphetamine, p-hydroxymethamphetaminehydrochloride,priortoinsectstudies.
Asolutionofp-hydroxyamphetaminehydrochloride(8.49mg) inMilli-Qwater(1mL)wasadjustedtopH12bythedrop-wise addition of sodium hydroxide (10%). The solution was then extracted with CH2Cl2 (210mL), and the combined organic
layers concentrated to give p-hydroxyamphetamine (3.4mg), which was used without further purification. Boc2O (5.4mg)
wasaddedtoasolutionofp-hydroxyamphetamine(3.4mg)inTHF (0.004mL)andEt3N(2.7mg)andthereactionstirredatRTunder
N2 for 24h. CH2Cl2 (2mL) was then added and the mixture
sonicated and the organic layer extracted. This process was repeatedtwiceandthecombinedorganiclayersdried(MgSO4)and
concentratedtogivep-OHAM-Boc(6.3mg,80%yield).Confi rma-tionofthesuccessofthechemicaltransformationcamefrommass spectrometricanalysis,withtheESI-MSspectrumshowingapeak at m/z374 (M+H+), assigned totheprotonated mass of the
p -OHAM-Boc.
Asolutionofp-OHAMP-diBoc(14.3mg)indryTHF(0.5mL)was addeddrop-wiseover5mintoamixtureofNaH(1.45mg,2.42mg from60%NaHstock,1.5eq)indryTHF(1mL)ina5mLflaskunder N2gasandat0C.MeI(0.08mL,17.33mg,3eq)wasthenadded
drop-wiseintothemixture,whichwasstirredfor48hatRT,and thereactionmonitoredbyESIMS.Thereactionwasquenchedwith water (2mL), and the reaction extracted with CH2Cl2, the
combined organic layers dried (MgSO4)and then concentrated
anddriedundervacuumtoobtainN -methyl-4-hydroxymetham-phetamine(14.2mg,96%)asanUVactivepaleyellowpowder;1H
NMR(CDCl3,500MHz),7.14(d,3J=7.3Hz,2H,H20,H40),6.84(d,3J
=7.3Hz,2H,H30andH50),3.78(s,3H,NCH
3),3.39(d,2J=15.1Hz,
H1A),3.33(m,1H,H2),2.80(d,2J=15.1Hz,H1B),1.33(m,3H,H3); 13CNMRCDCl
3,125MHz),158.8(C40),130.4(C20,C60),127.8(C10),
114.3 (C30, C50), 57.4 (C2), 55.3 (NCH
3), 38.6 (C1), 15.6 (C3);
ESIMS,m/z166(M+H+).
Each subset of 150 eggs, collected as above, was placed immediatelyontoasmallpieceofcottonwoolandallocatedto
oneof the tengroups (ninereplicatesof eachgroup in total). Weighboats containing spiked meatwereplaced into 850mL rectangular plastic containers with mesh lids, lined with a shallowlayer(5mmdeep)ofwheatenchaff.Thechaffprovideda medium for the larvae to crawl into when they had finished feeding. All larvae were reared at 23C in a temperature-controlled incubator (Thermoline Scientific, Australia) and exposedtoaphotoperiodof12:12light:dark.Thedayonwhich eggs were laid, collected and distributed to an experimental groupwasdesignatedasday0.Thesamplingdesignofourstudy followed that of George et al. [27], in order to facilitate comparisonwiththeprevioustoxicologicalworkonthisblowfly. Sampleswerethereforecomparedatfourtimepoints.Thefirst two comparison stages took place on days 4 and 7 of experimentation, during the larval stage. Three replicates of eachgroupwereremovedattheday4and7comparisoninterval. Larvae were extracted from the meat and starved for 4h to encourage expulsionof thecontents of the crop.Larvae were killed and fixed by placing in boiling water for 60s and individually washed in near-boiling water for 30s to ensure thatalladhesivesubstratewasremoved.Larvaewerethenstored in80%ethanol,afterwhichtheirlength,widthandweightwere measured.The posteriorspiracles of eachspecimenwere also inspected to record developmental instar. To determine the persistence of methamphetamine and p- hydroxymethamphet-amineinthelarvalfoodsource,1gsamplesof kangaroomince were taken from each group for later analysis using high performanceliquidchromatography.
The third comparison, incorporating a third set of three replicates perexperimental group, was madeduring the pupal stage.Observationsweremadeevery4htoaccuratelyrecordthe dayandhour(wheresuitable)atwhichpupariation(theprocessof puparium formation) began and concluded in each replicate container.Commencementofpupariationwasrecordedatthefirst indicationofcolourchangeoftheprepupafromwhitetoorange. Conclusion of pupariation was denoted by all samples having progressedfromorange todark brown, whereuponthe length, width and weight of all samples were measured. Following measurement,pupaewerereturnedtotheiroriginalcontainersfor eclosion.
The fourthcomparison was madeonceadults had emerged. Observations were made every 4h to record as accurately as possible when emergence began and concluded. The day and averagehourofinitialeclosionwererecorded,aswerethedayand hourofaverageeclosion(P50value).Adultswereremovedfrom plasticcontainersandplacedinafreezerfor5mintoslowtheir movement. Flies were then killed by asphyxiation with ethyl
acetateandstoredin80%ethanol.Measurementsofadultweight weremadewithin4hofcollection.Theleftwingandrearleftleg werethenremovedforlateranalysis.
Larvaeandpupaewereviewedunderadissectingmicroscope (MZ16A,LeicaMicrosystems,Germany).Afibreopticlightsource (CLS150X,LeicaMicrosystems,Germany)wasusedtoilluminate samples on a contrasting background to assist analysis. Each specimenwasphotographedwithadigitalcamera(DFC259,Leica Microsystems, Germany) and Leica Application Suite V3.8 software(LeicaMicrosystems, Germany)employed tomeasure length andwidth parametersto thenearest 0.001mm. Larvae wereviewedlaterally,andtheirlengthsmeasuredbetweenthe mostdistalpointoftheheadandthemostposteriorabdominal segment (Fig. 1(a)). Larval width was measured across the intersectionofthefifthandsixthabdominalsegments. Ultrasen-sitivescales(ML204,MettlerToledo,Switzerland)andLabXdirect balance2.1software(MettlerToledo,USA)wereutilisedtorecord the weightsof each sampleto the closest 0.1mg. Pupae were viewedventrallyandtheirlengthsmeasuredbetweenthemost posterior to most anterior points. Width measurements were obtainedbymeasuringsamplesacrosstheintersectionofthefirst andsecondabdominalsegments(Fig.1(b)).Adultswereremoved fromethanolandallowedtodryfor10minbeforebeingweighed. Wing and leg samples were viewed under the dissecting microscopeand photographed. For each sample, thelength of thecosta(oneoftheperipheralwingveins)andtibia(oneofthe sectionsoftheleg)weremeasuredtogiveanindicationofadult size and to determine if any differences existed between treatments due to drug exposure during earlier life stages (Fig.1(c,d)).
Following measurement, maggots, puparia and adults were individuallygroundusingamortarandpestleandcombinedwith 800
mL
ofdistilledwater(maggotsandadultswereallowedtodry for24hpriortohomogenisation).Cellulardebriswasremovedby precipitationinmethanol(AjaxFinechem,Australia)and centri-fugation(Model5412D, EppendorfSouthPacific,Australia). The solution was run through filter paper to ensure that all large contaminantswereremoved.Smallercontaminantswereremoved by HPLC specific 4mm syringe filters with 0.45mm
polytetra-fluoroethylenemembranes.Sampledmeatwasgroundinamortar andpestleandcombinedwith1000
mL
ofdistilledwater.Cellular debriswasremovedbyprecipitationinmethanoland centrifuga-tionandfilteredasforlarvae,puparialandadultsamples.Aslittle isknownaboutthepharmacokineticsofdrugsofabuseingestedby insects, two standard solutions of methamphetamine were preparedbeforeanyanalysisofsampleswasattemptedinorder todeterminewhichformofmethamphetamine,ifany,waspresentFig.1.(a)LarvaeofCalliphorastygia,showinglengthandwidthmeasurements;(b)C.stygiapupa,showinglengthandwidthmeasurements;(c)leftwingofC.stygia,showing
costallengthmeasurement;(d)rearleftlegofC.stygia,showingtibiallengthmeasurement.
[image:13.595.113.482.564.720.2]inC.stygiasamples.Thefirstwaspreparedbydissolving7mgof ()-methamphetaminehydrochloride in5mLof distilledwater, thus leaving the hydrochloride salt intact. The second was preparedbyneutralising7mgof ()-methamphetamine hydro-chloridewithsodiumhydroxide.Thesolutionwasthen filtered with an HPLC specific syringe filter, and mixed with 5mL methanol.Standardsforp-hydroxymethamphetamine hydrochlo-ridewerepreparedinthesamemanner.Thepresenceorabsenceof methamphetamine was confirmed by high performance liquid chromatography(HPLC)underisocraticconditions.Solventswere acetonitrile(190grade)andwaterandethylamine.Solventswere sonicated in an ultrasonic cleaner (Ultrasonics, Australia) for 20minpriortouse.AnalytesweredetectedbyUVlightat254nm. Thepresenceorabsenceofmethamphetamineorp- hydroxyme-thamphetaminewasrecordedforeachsample.
StatisticalanalysiswasundertakenusingJMPv7forWindows (SAS,USA).NormalityofthedatawasevaluatedusingQ–Qplotsand Kolmogorov–Smirnov normalitytests.Measuredsample param-eters (length, width and weight) were analysed using nested ANOVAs,whichallowedidentificationofsignificancebothbetween groups,and betweenthereplicates ofgroups.Survivorship was investigatedatthelarvaland adultcomparison stages. Kruskal-Wallistestswereusedtodeterminewhethertherehadbeenany appreciablelossinsamplenumbersasaresultoftheirexposureto drugcompounds.Significantresultswerefurtherexaminedwitha Tukey–Kramertesttoassesswhereanydifferenceslay.
3.Results
HPLCchromatogramsqualitativelydeterminedtheabsenceof methamphetaminecompoundsinthecontrolmeat.Thepresence ofmethamphetamineand/orp-hydroxymethamphetamineinthe treatment groups was confirmed by HPLC–UV analysis. These compoundswouldthereforehavebeeningestedbyfeedinglarvae. Developmentratesoflarvaeweredeterminedbyincreasesin thelength and width of sampled specimens. Weight measure-mentswerealsotakentoidentifyanyunusualgrowthpatterns.No obviousdifferencesinaveragetemperaturewerenotedbetween groupsorreplicates.
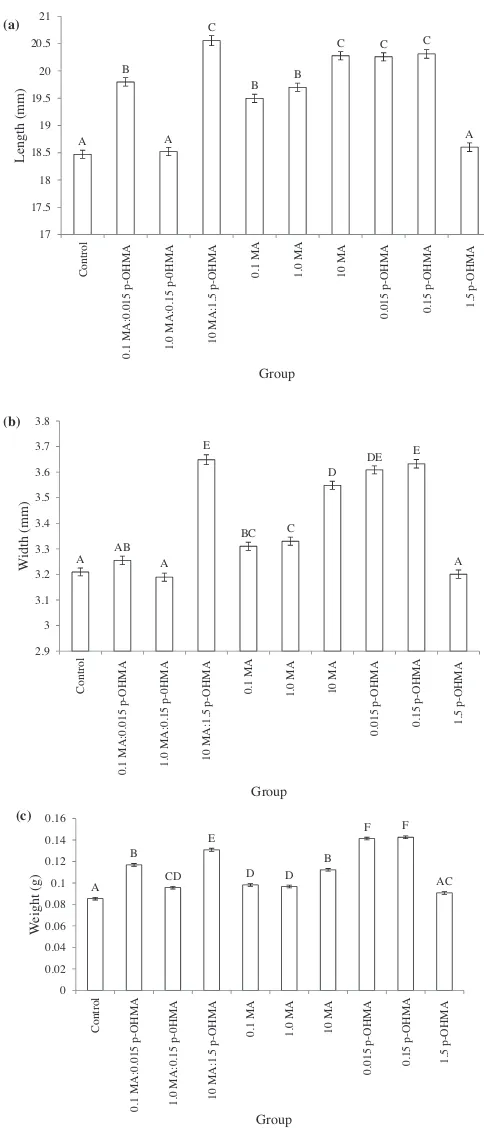
3.1.Comparisonofday4larvae
AnestedANOVAofmeanlarvallengthafterfourdaysofgrowth identified significant differences between treatment types (F9, 3485=40.75,p< 0.0001)(Fig.2(a)).
AposthocTukey–Kramertestdeterminedthatthemeanlengths ofeachofthepuremethamphetaminetreatments(0.1MA,1.0MA and10MA)weresignificantly greaterthanthecontrolgroup,aswas themeanlengthoflarvaeexposedtothetwolowerconcentrations ofpuremetabolite(0.015p-OHMAand0.15p-OHMA).Similarly,the twolowermethamphetamine:metaboliteratios(0.01MA:0.015 p-OHMAand1.0MA:0.15p-OHMA)producedlarvaeofsignificantly greaterlengththanthecontrol.Thetreatmentwiththehighest concentrationofp-hydroxymethamphetamine(1.5p-OHMA)and theintermediateratioofmethamphetamine:p- hydroxymetham-phetamine(1.0MA:1.5p-OHMA)werenotsignificantlydifferentin meanlengthfromthecontrolgroup.Significantdifferenceswere also seen between replicates within groups (F20, 3485=16.96,
p< 0.0001).AposthocTukey–Kramertestrevealedtherewasno significantdifferencein meanlengthbetweenreplicateswithin eachgroupexceptforinthecontroland0.15p-OHMAtreatment,for whichallreplicatesweresignificantlydifferentfromeachother.
Similartrendswereobservedinanalysesofmeanreplicatewidth (Fig.2(b)).AnestedANOVAshowedsignificantdifferencesbetween groups(F9,3485=136.29, p< 0.0001). Themean width of larvae exposedtomethamphetamine(0.1MA,1.0MAand 10MA)was
A B
A C
B B
C C C
A 17 17.5 18 18.5 19 19.5 20 20.5 21 Co ntr o l 0 .1 MA :0. 0 1 5 p-O H MA 1.0 MA :0. 1 5 p-0H MA 10 MA:1. 5 p-O HMA 0. 1 MA 1. 0 MA 10 MA 0. 015 p -OHMA 0. 15 p-O HMA 1. 5 p -O H MA Length (m m ) Group (b) (a) (c)
A AB A E BC C D DE E A 2.9 3 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Control 0. 1 MA :0.01 5 p-O HMA 1.0 M A :0 .1 5 p-0 H M A 10 MA:1.5 p
-OHMA 0.1 MA 1.0 MA 10
M A 0.015 p-OHMA 0. 15 p -O HMA 1.5 p-O HMA W idth (m m ) Group A B CD E D D B F F AC 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 Co ntr o l 0.1 MA :0. 0 1 5 p-O HMA 1.0 MA :0. 1 5 p -0H MA 10 MA:1. 5 p-O H MA
0.1 MA 1.0 MA 10 MA
0.
01
5
p-OHMA
0.15 p-OHMA 1.5 p
-O H MA W eight (g) Group
Fig. 2.Meanlength(a),width(b)andweight(c)(SE)ofday4larvae,exposed
duringdevelopmenttodifferentconcentrationsofmethamphetamineand/or
p-hydroxymethamphetamine.Experimentalgroupsarecontrol;0.1mg/kg
metham-phetamine:0.015mg/kgp-OHMA(0.1MA:0.015p-OHMA);1.0mg/kg
methamphet-amine:0.15mg/kg p-OHMA (1.0 MA:0.15 p-OHMA); 10mg/kg
methamphetamine:0.015mg/kgp-OHMA(10MA:1.5p-OHMA);0.1mg/kg
meth-amphetamine (0.1 MA); 1.0mg/kg methamphetamine (1.0 MA); 10mg/kg
methamphetamine(10MA);0.015mg/kgp-OHMA(0.015p-OHMA);0.15mg/kg
p-OHMA(0.15p-OHMA);1.5mg/kgp-OHMA(1.5p-OHMA).Groupsnotconnected
bythesameletteraresignificantlydifferent.
[image:14.595.314.556.54.618.2]significantlygreaterthanthecontrol.Similarly,themeanwidthsof larvaeinthehighestratiotreatment(10MA:1.5p-OHMA)andthe low and intermediate p-hydroxymethamphetamine concentra-tions(0.015p-OHMAand0.15p-OHMA)weresignificantlygreater thanthecontrolgroup.Bycontrast,themeanwidthsofthelower ratiotreatments(0.1MA:0.015p-OHMAand1.0MA:0.15p-OHMA) and the highest p-hydroxymethamphetamine treatment (1.5 p-OHMA)didnotdiffersignificantlyfromthecontrol.Withthe exception of the control and 0.15 p-OHMA groups, where one replicatedifferedsignificantlyfromtheothertwo(F20,3485=54.07,
p< 0.0001), a post hoc Tukey–Kramer test did not identify significantdifferencesbetweenreplicateswithineachgroup.
A nested ANOVA analysis identified significant differences between the larval groups for mean weight (F9, 3485=100.21,
p< 0.0001). The post hoc Tukey–Kramer test revealed similar trendstolengthandwidthcomparison.Averagelarvalweightsof the methamphetamine (0.1 MA,1.0 MA and 10 MA) and ratio treatments(0.1MA:0.015p-OHMA,1.0MA:0.15p-OHMAand10 MA:1.5p-OHMA)weresignificantlygreaterthanthecontrol.The mean weights of the two lower p-hydroxymethamphetamine treatments(0.015p-OHMAand 0.15p-OHMA)werealsosignifi -cantlygreaterthanthecontrol.Bycontrast,themeanlarvalweight of the highest p-hydroxymethamphetamine treatment (1.5p-OHMA) did not differ significantly from the control (Fig.2(c)).Significantdifferencesbetweenreplicateswithin treat-ments(F20,3485=102.67,p< 0.0001)wereidentifiedbyaposthoc Tukey–Kramertest. However,only one replicateof each of the controland0.1MAgroupsdifferedsignificantlyfromtheothertwo replicateswithinthegroup.
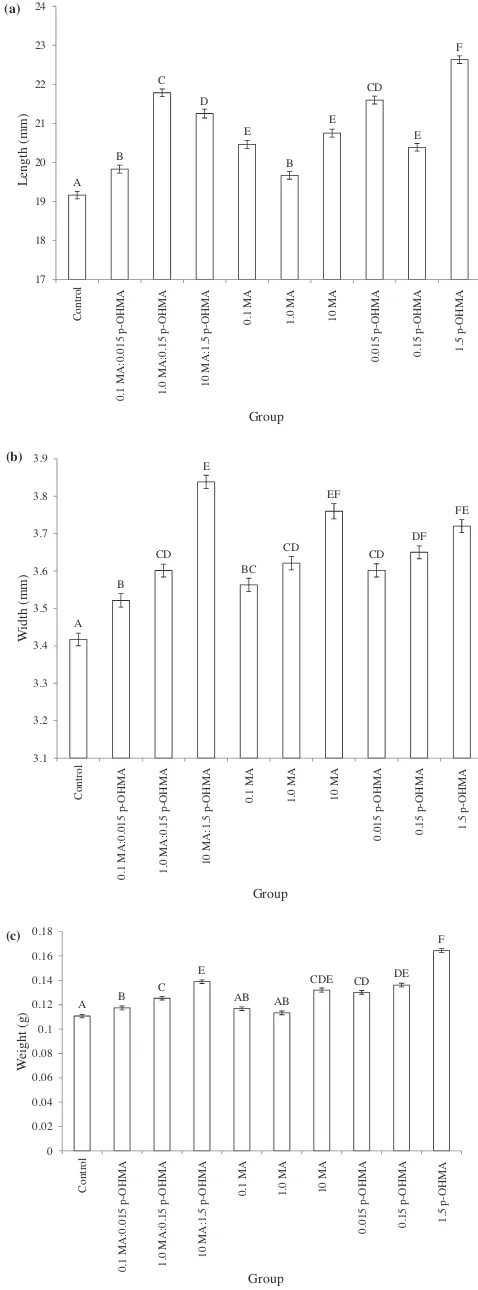
3.2.Comparisonofday7larvae
AnestedANOVAof meanlengthidentifiedsignificant differ-encesbetweentreatments(F9,3485=48.77,p< 0.001Theposthoc Tukey–Kramer test revealed that the mean lengths of larvae exposedtoanydrugcompoundweresignificantlygreaterthanthe control(Fig.3(a)).However,significantvariation between repli-cateswithingroupswasalsodetected(F20,3485=51.05,p< 0.001). Onereplicateofeachofthecontrol,intermediate methamphet-amineconcentration(1.0MA)andthehighestp -hydroxymetham-phetamineconcentration(1.5p-OHMA)wassignificantlydifferent fromtheothertwowithineachgroup.
Substantialvariationwasalsoseenbetweenthemeanreplicate widthsofday7larvae(F9,3080=19.28,p< 0.001)(Fig.3(b)).The mean widths of larvae exposed to any drug treatment were significantlygreaterthanthecontrolgroup.AnestedANOVAof replicatewithintreatment showedthat onereplicateofthe1.5 p-OHMA group differed significantly from the other replicates withinthetreatment(F20,3080=19.10,p< 0.001).
Similarly,nestedANOVAdeterminedthatthelarvaeexposedto methamphetamine compounds were significantly heavier on average than control samples (F9, 3080=65.45, p< 0.001) (Fig. 3(c)). Significant differences were also detected between replicateswithingroups(F20,3080=50.37,p< 0.001).Onereplicate ofeach ofthe1.5p-OHMAand 1.0MA:0.15p-OHMAtreatment groups,andtheintermediatemethamphetaminegroup(1.0MA) weresignificantlydifferentfromtheothertworeplicateswithin theirtreatments.Furthermore,eachreplicateofthecontrolwas significantlydifferentfromeveryother.
(a) (b) (c) A B C D E B E CD E F 17 18 19 20 21 22 23 24 Control 0.
1 MA:0.015 p-O
HMA 1.0 MA :0. 1 5 p -OH MA 10 MA:1.5 p-OHMA 0. 1 MA 1.
0 MA 10 M
A 0.015 p-OH MA 0. 15 p -O H MA 1.5 p-O HMA Length (m m ) Group A B CD E BC CD EF CD DF FE 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Co ntr o l 0.1 MA :0. 0 1 5 p-O HMA 1. 0 MA :0.15 p-O H MA 10 MA:1. 5 p-O H MA
0.1 MA 1.0 MA 10 MA
0. 015 p-OHMA 0. 15 p-O H MA 1. 5 p -O H MA Wi d th ( m m ) Group A B C E AB AB
CDE CD DE F 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 Co ntrol 0.1 MA :0.015 p-OHMA 1. 0 MA :0.15 p -OH MA 10 MA:1.5 p-OHMA 0. 1 MA 1.
0 MA 10 MA
0.
015 p-OHMA 0.15 p-O
H MA 1. 5 p -O H MA W eight (g) Group
Fig. 3. Meanlength(a),width(b)andweight(c)(SE)ofday7larvae,exposed
duringdevelopmenttodifferentconcentrationsofmethamphetamineand/or
p-hydroxymethamphetamine.Experimentalgroupsarecontrol;0.1mg/kg
metham-phetamine:0.015mg/kgp-OHMA(0.1MA:0.015p-OHMA);1.0mg/kg
methamphet-amine:0.15mg/kg p-OHMA (1.0 MA:0.15 p-OHMA); 10mg/kg
methamphetamine:0.015mg/kgp-OHMA(10MA:1.5p-OHMA);0.1mg/kg
meth-amphetamine (0.1 MA); 1.0mg/kg methamphetamine (1.0 MA); 10mg/kg
methamphetamine(10MA);0.015mg/kgp-OHMA(0.015p-OHMA);0.15mg/kg
p-OHMA(0.15p-OHMA);1.5mg/kgp-OHMA(1.5p-OHMA).Groupsnotconnected
bythesameletteraresignificantlydifferent.
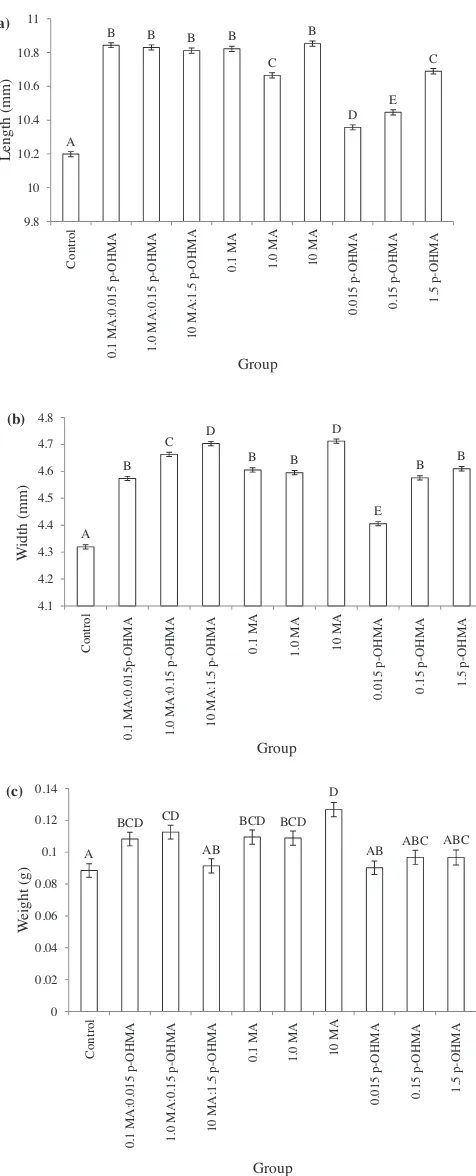
[image:15.595.38.277.59.704.2]3.3.Comparisonofpupae
Statisticalsignificancebetweenthemeanpupallengthsofeach treatment was detected by a nested ANOVA (F9, 3022=87.59,
p< 0.0001).AposthocTukey–Kramertestrevealedthatthemean lengthsofpupaeexposedtoanydrugcompoundweresignificantly longerthanthecontrol(Fig.4(a)).Significantvariationwasalso observed between replicates within each of the groups (F20, 3022=20.10, p< 0.0001). One replicate of the control and eachpuremethamphetaminetreatment(0.1MA,1.0MA,10MA) variedsignificantlyfromtheotherswithineachgroup.
A nested ANOVA of width showed significant differences between groups at the pupal stage (F9, 3022=94.23, p< 0.001) (Fig.4(b)).Themeanwidthsofpupaeexposedtoany metham-phetamineand/or metabolitetreatmentduring thelarval stage weresignificantgreater thanthecontrolsamples.Furthermore, nestedANOVAanalysisidentifiedsignificantvariabilitybetween the mean widths of individual replicates within groups (F20, 3022=97.87, p< 0.001). With the exception of the ratio treatments(0.1MA:0.015p-OHMA,1.0MA:0.15p-OHMAand10 MA:1.5p-OHMA),onereplicateineachtreatmentwassignificantly differentfromtheothertwo. Eachreplicate ofthe controlwas significantlydifferentfromtheremainingtworeplicates.
Whenaverage weightwas compared, significantdifferences were detected between treatments using a nested ANOVA (F9,3022=3.74,p< 0.001)(Fig.4(c)).AposthocTukey–Kramertest ofmean pupalweight showed thatallpuremethamphetamine treatments(0.1MA,1.0MAand10MA)andthetwolowerratio treatments(0.1MA:0.015p-OHMAand1.0MA:0.15p-OHMA)were significantlyheavierthanthecontrolgroup.Furthermore,signifi -cant differences between replicates within groups were also identified (F20,3022=3.68,p< 0.001). The highest methamphet-amine treatment (10 MA) had one replicate that differed significantlyfromtheothertwowithinthetreatment.
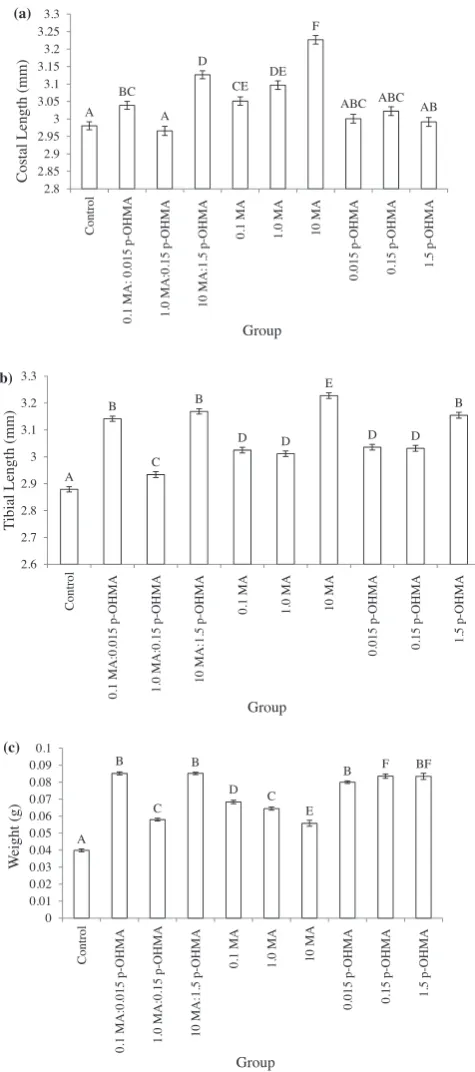
3.4.Comparisonofadults
Onceadultfliesemergedfromthepuparium,theleftwingand rearleftlegwereanalysedtoassessoverallflysize.Thecostalvein of the wingand the tibia of theleg were measured. A nested ANOVAshowed thatthereweresignificantdifferencesbetween themeancostallengthin eachgroup(F9,2755=15.17,p< 0.001) (Fig.5(a)).AposthocTukey–Kramertestidentifiedthatthepure methamphetaminetreatments(0.1MA,1.0MAand10MA),and thelowandhighratiotreatments(0.1MA:0.015p-OHMAand10 MA:1.5 p-OHMA) varied significantly from the control. No significantdifference wasseen betweenthemeancostal length ofreplicateswithingroups(F20,2755=0.14,p=0.87).
Significantdifferencesbetweenmeantibiallengthswerealso detectedbyanestedANOVA(F9,2755=40.22,p< 0.001)(Fig.5(b)). A post hoc Tukey–Kramer test showed that all treatments containing drug compounds produced flies with significantly greatermeantibiallengthsthanthecontrolgroup.Afurthernested ANOVAshowedasignificanteffectofreplicatewithingroup(F20, 2755=3.84,p=0.021)withaposthocTukey–Kramerrevealingone
replicateofeachofthelowestmethamphetaminetreatment(0.1 MA)andthecontrolgroupdifferingsignificantlyfromtheother tworeplicateswithinthesegroups.
The average weights of adult flies were determined to be statisticallydifferentby anestedANOVA(F9,2755=244.43,p< 0.001) (Fig. 5(c)). Apost hocTukey–Kramertestshowed that allflies exposed tomethamphetamineor p-hydroxymethamphetamineas larvae were significantlyheavier than the control samples. A nested ANOVA was used to investigate variability within treatments between replicates.Significantdifferenceswereidentified(F20,2755=11.22,
p< 0.001)andidentifiedbyaposthocTukey–Kramertobebetween
A
B B B B
C B D E C 9.8 10 10.2 10.4 10.6 10.8 11 Contro l 0.1 MA :0. 0 15 p-OHMA 1. 0 MA :0.15 p-OH MA 10 MA:1.5 p-OHMA 0.
1 MA 1.0 MA 10 MA
0.01
5
p-OH
MA
0.15 p-OHMA 1.
5 p -O H MA Length (m m ) Group (a) (b) A B C D B B D E B B 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Control 0.1 MA :0. 0 15p-OH M A 1.0 MA :0. 1 5 p-OH MA
10 MA:1.5
p-OHMA 0.1 MA 1.0 MA 10 MA
0. 01 5 p-OH M A 0. 15 p-OHMA 1.5 p-O HMA W idth (m m ) Group (c) A BCD CD AB BCD BCD D
AB ABC ABC
0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 Co n tr o l
0.1 MA:0.015 p-OHMA 1.0 MA:
0 .15 p-OHMA 10 MA:1.5 p-OHMA 0. 1 MA 1.
0 MA 10 M
A 0.01 5 p-OH MA 0. 15 p -OHMA 1. 5 p -O H MA W eight (g) Group
Fig. 4.Meanlength(a),width(b)andweight(c)(SE)ofpupae,exposedduring
developmenttodifferentconcentrationsofmethamphetamineand/or
p-hydro-xymethamphetamine.Experimentalgroupsarecontrol;0.1mg/kg
methamphet-amine:0.015mg/kg p-OHMA (0.1 MA:0.015 p-OHMA); 1.0mg/kg
methamphetamine:0.15mg/kgp-OHMA(1.0MA:0.15p-OHMA);10mg/kg
meth-amphetamine:0.015mg/kgp-OHMA (10MA:1.5 p-OHMA);0.1mg/kg
metham-phetamine (0.1 MA); 1.0mg/kg methamphetamine (1.0 MA); 10mg/kg
methamphetamine(10MA);0.015mg/kgp-OHMA(0.015p-OHMA);0.15mg/kg
p-OHMA(0.15p-OHMA);1.5mg/kgp-OHMA(1.5p-OHMA).Groupsnotconnected
bythesameletteraresignificantlydifferent.
[image:16.595.318.556.61.649.2]thereplicatesofthecontrol,0.1MA,1.0MA,10MAand0.15p-OHMA groups.Withinthecontroland10MAgroups,theaverageweightof eachreplicatewasstatisticallyindependent.For0.1M,1.0MAand
0.15 p-OHMA treatments, only one replicate was statistically significantfromtheothertworeplicatesofthetreatment.
3.5.Survivorship
Kruskal–Wallisanalysisofsurvivorshipshowednosignificant difference in the number of larvae surviving after four days (X92=3.54,p>0.432).However,attheday7intervaltherewasa
significantdifferencein survivorshipbetweendrugand control groups (X92=28.01, p =0.017).When compared to the control,
larval numbers had decreased in all pure methamphetamine (0.1MA,1.0MAand10MA),andratiotreatments(0.1MA:0.015 p-OHMA,1.0MA:0.15p-OHMAand10MA:1.5p-OHMA)butnotin the treatments feeding on substrates spiked with pure p-hydroxymethamphetamine(0.015p-OHMA,0.15p-OHMAand 1.5p-OHMA).Adultsurvivorshipwasalsosignificantlydifferent betweentreatments(X92=29.00,p< 0.001).Thenumberofadult
flies remaining in each drugged treatment was significantly smallerthanthenumberofsamplessurvivinginthecontrol.
3.6.Developmentrates
Changesingrowth rateduetothepresenceof methamphet-amineandp-hydroxymethamphetamineinthelarvalfoodsource weredeterminedbyrecording,tothenearesthour,whensamples in each replicate commenced and completed pupariation, and began and completedadult emergence.Calliphora stygia larvae exposedtoanyconcentrationofmethamphetamineorp- hydrox-ymethamphetamine proceeded to show accelerated growth, commencing pupariation significantly before control larvae (F2,10=144.03, p< 0.0001) (Fig. 6). This discrepancy was most obviousin the10 MAand 10MA:1.5 p-OHMA treatments. The initialcolourchangeoftheprepupaefromwhitetoorangebegan 44hearlierinthesetreatmentsthaninthecontrol.
Pupariationwasalsocompleteinallmethamphetamine-and metabolite-treated pupae significantly before the control (F2,10=293.47, p< 0.0001).Samplesthenremainedaspupaefor significantly longer in treatments containing drug compounds (F2,10=441.63,p< 0.0001),andemergedlaterthancontrolsamples (F2, 10=91.47, p< 0.0001). The 10 MA:1.5 p-OHMA treatment showedthegreatestincongruityfromthecontrol.Emergencein this treatment began 34h following the first control samples, equatingtoa78htotaldifferenceindevelopmentrate.
(a) A BC A D CE DE F
ABC ABC AB
2.8 2.85 2.9 2.95 3 3.05 3.1 3.15 3.2 3.25 3.3 Co ntr o l 0.1 MA
: 0.015 p-OH
MA
1.
0 MA
:0.15 p-OHMA
10 MA:1.5
p-OHMA 0.1 MA 1.0 MA
10 MA 0. 015 p-OHMA 0. 15 p -O H MA 1.5 p-OH MA
Costal Length (m
m ) Group (b) A B C B D D E D D B 2.6 2.7 2.8 2.9 3 3.1 3.2 3.3 C ont ro l 0. 1 MA :0.015 p-OHMA 1.0 M A :0 .1 5 p-O H M A 10 MA:1.5 p-OHMA 0. 1 MA 1.
0 MA 10 M
A 0. 01 5 p-OHMA 0.15 p-O H MA 1. 5 p-OH MA T
ibial Length (m
m ) Group (c) A B C B D C E
B F BF
0 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0.09 0.1 Control 0. 1 MA :0. 0 15 p-O HMA 1. 0 MA :0.15 p-OHMA 10 MA:1.5
p-OHMA 0.1 MA 1.0 MA 10 MA
0.01 5 p-OHMA 0. 15 p-O H MA 1.5 p-O H MA We ig h t ( g ) Group
Fig.5.Meancostallength(a),tibiallength(b)andweight(c)(SE)ofadults,
exposedduringdevelopmenttodifferentconcentrationsofmethamphetamine
and/orp-hydroxymethamphetamine.Experimentalgroupsarecontrol;0.1mg/kg
methamphetamine:0.015mg/kg p-OHMA (0.1 MA:0.015 p-OHMA); 1.0mg/kg
methamphetamine:0.15mg/kgp-OHMA(1.0MA:0.15p-OHMA);10mg/kg
meth-amphetamine:0.015mg/kg p-OHMA (10 MA:1.5 p-OHMA);0.1mg/kg
metham-phetamine (0.1 MA); 1.0mg/kg methamphetamine (1.0 MA); 10mg/kg
methamphetamine(10MA);0.015mg/kgp-OHMA(0.015p-OHMA);0.15mg/kg
p-OHMA(0.15p-OHMA);1.5mg/kgp-OHMA(1.5p-OHMA).Groupsnotconnected
bythesameletteraresignificantlydifferent.
Fig. 6.Developmentrates of Calliphorastygia samples exposed to different
concentrationsofmethamphetamineand/orp-hydroxymethamphetamine.
Exper-imentalgroupsare control;0.1mg/kgmethamphetamine:0.015mg/kgp-OHMA
(0.1MA:0.015p-OHMA);1.0mg/kgmethamphetamine:0.15mg/kgp-OHMA(1.0
MA:0.15p-OHMA);10mg/kgmethamphetamine:0.015mg/kgp-OHMA(10MA:1.5
p-OHMA);0.1mg/kgmethamphetamine(0.1MA);1.0mg/kgmethamphetamine
(1.0MA);10mg/kgmethamphetamine(10MA);0.015mg/kgp-OHMA(0.015
p-OHMA);0.15mg/kgp-OHMA(0.15p-OHMA);1.5mg/kgp-OHMA(1.5p-OHMA).
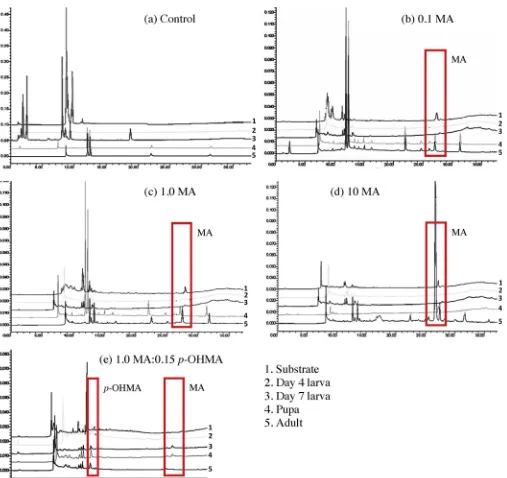
[image:17.595.41.279.113.650.2] [image:17.595.303.556.527.667.2]3.7.HPLCanalysis
Standardsformethamphetaminehydrochlorideandp- hydrox-ymethamphetamine hydrochloride were established for HPLC underisocraticconditions,withtheformer showingaretention timeof27.3minandthelatter14.2min.Analysisofsamplesofeach lifestagebyHPLCwithUVdetectionqualitativelyconfirmedthe absenceofmethamphetamineandp-hydroxymethamphetamine inthefeedingsubstrateofthecontrol(Fig.7).
Methamphetamine could not be detected in homogenised larvalsamplesoftreatments0.1MA,1.0MAor10MA.However, HPLCchromatogramsqualitativelyconfirmeditspresenceinpupal and adult samples (Fig. 7). p-Hydroxymethamphetamine was detected in larval, pupal and adult preparations of treatments
0.015p-OHMA,0.15p-OHMAand1.5p-OHMA.Inratiotreatments 0.1 MA:0.015p-OHMA,1.0 MA:0.15p-OHMA and10MA:1.5 p-OHMA,onlyp-hydroxymethamphetaminecouldbedetected.
4.Discussion
Inthisstudy,methamphetamine-spikedkangaroomeatwas utilisedtosimulatethepostmortemenvironmentofa metham-phetamineoverdose.Kangaroomincewasselectedinfavourofa live laboratoryanimal as the major andminor metabolites of methamphetamine and absorption and excretion rates are knowntovarybetweenvertebratespecies[20].Theapplication of drugor metabolitedirectlytothemeatensuredthatlarvae were exposed to known concentrations and types of drug
Fig.7.SelectedexamplesofHPLCtracesqualitativelyshowingthepresenceorabsenceofmethamphetamine(MA)andp-hydroxymethamphetamine(p-OHMA):(a)control;
(b)0.1MA;(c)1.0MA;(d)10MA;and(e)1.0MA:0.15p-OHMA.
[image:18.595.52.559.235.713.2]compound,a recognised issuein entomotoxicological research
[31]. Sustained mixingensured a homogenous distribution of methamphetamineand/orp-hydroxymethamphetamine.
Thesecompoundswerefoundtosignificantlyalterthesizeofall lifestagesat theconcentrations investigated.After fourdaysof growth,larvaeinalltreatments,withtheexceptionofthehighest p-hydroxymethamphetamine (1.5p-OHMA) and the intermediate ratiotreatments(1.0MA:0.15p-OHMA),werelonger,widerand heavierthanthecontrolsamples.After sevendays,samples exposed toanymethamphetaminetreatmenthaddevelopedintosignifi -cantlylonger,widerandheavierlarvaethanthecontrolmaggots.
Thesefindingscontrastwiththose ofGoffetal.[12] intheir studies of the flesh fly Sarcophaga ruficornis. When reared on methamphetamine-dosed rabbit tissues,individuals exposed to thehighestconcentrationsofthedrugweresignificantlyshorterin lengththan control samples.This may have resulted fromthe different metabolism of methamphetamine in flesh flies and blowfliesand highlights theinadvisabilityof extrapolating out-comesfromentomotoxicologicalstudiesbetweenspecies.
Inforensicpractice,thelengthsofthelargestlarvaesampled fromabodyareusedtoestimatetheageoftheoldestspecimens, andhence,giveanestimateoftheminimumPMI[32].Theresults ofthis studystronglysuggestthat,in amaggot-infestedcorpse containingmethamphetamine,thelengthof alarva ofC. stygia might not necessarily be a valid indication of its age. If this enhancedgrowthis notconsidered,estimatesof minimumPMI basedonthelarvalstagesofC.stygiacouldbeerroneous.
Furthermore, the presence of methamphetamine and p-hydroxymethamphetamineinthefeedingsubstratesignificantly affectedthegrowthratesofC.stygialarvaeattheconcentrations investigated. The onset of pupariation, and its duration, was significantlyalteredindrug-exposedtreatments.Calliphorastygia larvaeexposed tothese compounds as immaturesreachedthe pupalstageupto44hpriorto,andremainedaspupaeupto34h longerthan controls, a total divergenceof 78h. These findings againcontrastwiththoseofGoffetal.[12],whofoundtheduration ofthepupalperiodofS.ruficornistobesignificantlyshorterthan thecontrolforallsamplesexposedtomethamphetamineduring thelarvalstage.Additionally,thepreviousstudyonC.stygiaby Georgeetal.[27]foundthatitsdevelopmentwasunaffectedby puremorphineatsub-lethal,lethal,andtwice-lethaldoses.These contradictoryfindingsaremostlikelyduetothedifferenttypeof drugusedineachstudy,andtheeasewithwhichtheyareabsorbed bythetissuesofanorganism.
While morphineisapotentanalgesic[33],andrepressesthe centralnervoussystem [34], methamphetamineis a psychosti-mulant [35]. In humans,it immediately induces an increase in metabolismandthereleaseof‘pleasure’neurotransmitters[18]. Althoughbothtypesofdrugarewatersoluble,morphineispoorly solubilisedinlipids[33],whereasbothmethamphetamineand p-hydroxymethamphetaminearehighlylipidsoluble[36]. Metham-phetamine compounds may therefore be better suited to the internal environment of blowfly larvae, which have a high fat content[37].Itispossiblethatthesecompoundsareabletocross thelipid bi-layer of larval cells and accelerate metabolism.An increaseinmetabolicratecouldmanifestasanacceleratedrateof development,orenlargedlarvae,pupaeandadults,whichwereall observedinthecurrentstudy.
ComparedwiththefindingsofGeorgeetal.[27],asignificant differenceinsurvivorshipwasrecordedbetweendrugtreatments andcontrols.Therewas asignificantdecreaseinthenumberof larvaeinall methamphetamine-spikedtreatmentsafter 7days. Similarly,overallsurvival,calculatedonceallflieshademerged, showedthat there were significantly fewer flies in treatments exposedtomethamphetamineasimmatures. Thissuggeststhat methamphetamine compounds have a toxic effect onC. stygia
larvae at any concentration,and mayinfluence their ability to undergometamorphosis.
Methamphetamine and p-hydroxymethamphetamine were detected both in meat and C. stygia samples. HPLC analysis confirmed that methamphetamine and/or p- hydroxymetham-phetaminewere absentfrom thecontrolgroup, but presentin thelarvalfoodsourceoftheremainingtreatments.Consequently, larvaewouldhaveingestedthedrugcompounds.However,when larvaesampledatthefirstandsecondcomparisonintervalswere analysed with HPLC–UV, methamphetamine could not be detected. These findings differed from those of Wilson et al.
[38]intheirstudiesofCalliphoravicina.Whenrearedonskeletal musclefromasuicidaloverdoseofco-proxamolandamitriptyline, amitriptylineanditsactivehumanmetabolite,nortriptyline,were bothdetectedinC.vicinalarvae.
Thisnegativedetectionmayhavebeenduetothewayinwhich sampleswerepreparedforanalysis. Larvaewereground witha mortarandpestleandcombinedwithdistilledwatertosolubilise anycellularmaterialreleased.Thiswasarelativelycrudemethod of homogenization and the complete lysis of all cells, and the releaseofstoredmethamphetamine,wasnotguaranteed.Studies byKinnearetal.[39]haveshownthattheconcentrationoflipidsin C. stygia larvae increases sharply between days 3 and 6 of development, before decreasing after day 7. As larvae were sampled ondays 4 and day 7, within this period of increased lipid production, increased fat content, combined with poor homogenizationtechniques,couldberesponsiblefortheabsence ofmethamphetamineinlarvalpreparationsanalysedbyHPLC.
Itisalsopossiblethatdrugcompoundswerenotdetectedinlarval samplesduetothelowersensitivityofHPLC–UV,whencomparedto chemiluminescenceorfluorescencedetection[40].Takayamaetal., in their studieson methamphetaminedepositsin hair,wereunable to detectmethamphetamineinsmallhairsampleswhenUVdetection wasemployed.However,minuteamountsofthedruginsinglehairs were able to be isolated and detected by chemiluminescence techniques[41].Theuseofalternatedetectionmethodsmightyield moreconclusiveresultsinfuturestudies.
Although the results of the present studyare notable, it is recommended that further investigations at different temper-atures,and alternativeconcentrationsof methamphetamine,be carriedouttoformacomprehensivebankofdataagainstwhich forensiccasescan becompared.As corpsesare rarelyfoundin environmentswithstabletemperatures,agreaterunderstanding oftheeffectsofmethamphetamineatdifferenttemperaturescould assistwithinterpretingforensiccases.Similarly,thepostmortem concentrationsofdrugsinacorpsemayvaryaccordingtotissue type andlocation[42,43]and alsomaydifferfromthe concen-trationsatthetimeofdeathduetopostmortemredistributionby passivereleasesfromthedrugreservoirsof thegastrointestinal tract,lungsandmyocardium,oratlaterstages,fromtheautolysis ofcellsandputrefactionprocesses[44,45].Basiclipophilicdrugs, suchasmethamphetamine,appeartobeparticularlysusceptibleto postmortemredistributionprocesses [44].Itis alsoknownthat larvalgrowthcanbeinfluencedbytissuetypeandageinanimal models[46–48].Therefore,furtherinvestigationoftheeffectsof methamphetamine on blowfly larvae must be carried out at different concentrations and in different substrates for its influence to be conclusively understood. Of course, once a comprehensivesetofdataisavailableforC.stygia,theinfluence ofthisdrugonotherflyspeciesofforensicimportancewouldalso needtobeinvestigated.
5.Conclusions
These findings hold significance for forensic science with particular regard tominimum PMI calculations usingflies. The
alteredgrowthexhibitedinthisstudysuggeststhatanyestimateof minimumPMIbasedonthenormalratesofC.stygiadevelopment at23Ccouldbeoverestimatedbyupto44hwhenbasedonthe larval stage,and by up to78hwhen based onpupal samples. Indeed, because of the resultant developmental acceleration, cautionshouldbeexercisedwheneverC.stygiaisusedtoestimate the minimum PMI of methamphetamine-dosed corpses. The developmental changes of this species of blowfly are also yet unknownattemperatures,drugconcentrationsandinsubstrates otherthanthoseusedhere.Furtherresearchisthereforeessential tomorecomprehensivelyunderstandtheeffectsof methamphet-amineon blowfly development. Until then, C.stygia cannot be assumedtobeareliablemodelforagingcorpsescontainingthis drug.
Acknowledgements
Thisresearchwasmadepossiblebythefinancialsupportofthe AustralianResearchCouncil,theAustralianFederalPolice,theNew SouthWales PoliceForceand UOW’sInstitutefor Conservation BiologyandEnvironmentalManagement.ASNthanksUOWforthe provision of a UPA scholarship. We thank Melanie Archer (Victorian Institute of Forensic Medicine) for useful initial discussions,ThaoDangand GregoryTarrant(National Measure-mentInstitute)foradviceandsupplyoftheMAandp-OHMA,and AidanJohnson(UOW)forstatisticalassistance.
References
[1]C.P.Campobasso,F.Introna,Theforensicentomologistinthecontextofthe forensicpathologist'srole,ForensicSci.Int.120(2001)132–139.
[2]J.Amendt,C.S.Richards,C.P.Campobasso,R.Zehner,M.J.R.Hall,Forensic entomology:applicationsandlimitations,ForensicSci.Med.Pathol.7(2011) 379–392.
[3]J.Amendt,R.Krettek,R.Zehner,Forensicentomology,Naturwissenschaften91 (2004)51–65.
[4]M.A.O'Flynn,Thesuccessionandrateofdevelopmentofblowfliesincarrionin southernQueenslandandtheapplicationofthesedatatoforensicentomology, Aust.J.Entomol.22(1983)137–148.
[5]G.S. Anderson, Minimum and maximum development rates of some forensicallyimportant Calliphoridae (Diptera),J. Forensic Sci. 45(2000) 824–832.
[6]M.S.Archer,R.B.Bassed,C.A.Briggs,M.J.Lynch,Socialisolationanddelayed discoveryofbodiesinhouses:thevalueofforensicpathology,anthropology, odontologyandentomologyinthemedico-legalinvestigation,ForensicSci. Int.151(2005)259–265.
[7]S.E.Donovan,M.J.R.Hall,B.D.Turner,C.B.Moncrieff,Larvalgrowthratesofthe blowfly,Calliphoravicina,overarangeoftemperatures,Med.Vet.Entomol.20 (2006)106–114.
[8]M.L.Goff,W.D.Lord,Entomotoxicology:anewareaforforensicinvestigation, Am.J.ForensicMed.Pathol.15(1994)51–57.
[9]F.Introna,C.P.Campobasso,M.L.Goff,Entomotoxicology,ForensicSci.Int.120 (2001)42–47.
[10]L.M.L. Carvalho,Toxicology and forensic entomology,in: J. Amendt, C.P. Campobasso,M.L.Goff,M.Grassberger(Eds.),CurrentConceptsinForensic Entomology,Springer,Dordrecht,2010.
[11]M.Gosselin,V.DiFazio,S.M.R.Wille,M.D.M.RamírezFernandez,N.Samyn,B. Bourel,P.Rasmont,Methadonedeterminationinpupariaanditseffectonthe developmentofLuciliasericata(Diptera,Calliphoridae),ForensicSci.Int.209 (2011)154–159.
[12] M.L.Goff,W.A.Brown,A.I.Omori,Preliminaryobservationsoftheeffectof methamphetamine in decomposing tissues on the developmentrate of
Parasarcophagaruficornis(Diptera:Sarcophagidae)andimplicationsofthis effectontheestimationsofpostmortemintervals,J.ForensicSci.37(1992) 867–872.
[13]E.Musvasva,K.A.Williams,W.J.Muller,M.H.Villet,Preliminaryobservations ontheeffectsofhydrocortisoneandsodiummethohexitalondevelopmentof
Sarcophaga (Curranea) tibialis Macquart (Diptera: Sarcophagidae), and implications for estimating postmortem interval, Forensic Sci. Int.120 (2001)37–41.
[14]H.Kharbouche,M.Augsburger,D.Cherix,F.Sporkert,C.Giroud,C.Wyss,C. Champod,P.Mangin,Codeineaccumulationandeliminationinlarvae,pupae, andimagooftheblowflyLuciliasericataandeffectsonitsdevelopment,Int.J. Leg.Med.122(2008)205–211.
[15]S.Yan-Wei,L.Xiao-Shan,W.Hai-Yang,Z.Run-Jie,Effectsofmalathiononthe insectsuccessionandthedevelopmentofChrysomyamegacephala(Diptera:
Calliphoridae) in the field and implications for estimating postmortem interval,Am.J.ForensicMed.Pathol.31(2010)46–51.
[16]L.M.L.deCarvalho,A.Z.Linhares,F.A.B.Palhares,Theeffectofcocaineonthe development rate of immatures and adults of Chrysomya albiceps and
Chrysomyaputoria(Diptera:Calliphoridae)anditsimportancetopostmortem intervalestimate,ForensicSci.Int.220(2012)27–32.
[17]T.Nagata,K.Kimura,K.Hara,K.Kudo,Methamphetamineandamphetamine concentrationsinpostmortemrabbittissues,ForensicSci.Int.48(1990)39–47. [18]T.E. Albertson,R.W.Derlet,B.E.VanHoozen, Methamphetamineand the expandingcomplicationsofamphetamines,West.J.Med.170(1999)214–219. [19]S.Darke,S.Kaye,R.McKentin,J.Duflou,Majorphysicalandpsychological
harmsofmethamphetamineuse,DrugAlcoholRev.27(2008)253–262. [20]T.Yamamoto,R.Takano,T.Egashira,Y.Yamanaka,Metabolismof
metham-phetamine, amphetamine and p-hydroxymethamphetamine by rat-liver microsomalpreparationsinvitro,Xenobiotica14(1984)867–875. [21]I.delaPena,H.Ahn,J.Choi,C.Shin,J.Ryu,J.Cheong,Reinforcingeffectsof
methamphetamine in an animal model of attention-deficit/hyperactivity disorder-thespontaneouslyhypertensiverat,Behav.BrainFunct.6(2010)72. [22]G.D.Ellison,M.S.Eison,Continuousamphetamineintoxication:ananimal
modeloftheacutepsychoticepisode,Psychol.Med.13(1983)751–761. [23]J.Caldwell,L.G.Dring,R.T.Williams,Metabolismof[14C]methamphetaminein
man,theguineapigandtherat,Biochem.J.129(1972)11–22.
[24]M.R.Peace,ForensicEntomotoxicology:AStudyintheDepositionandEffects ofAmphetaminesandBarbituratesintheLarvaeoftheBlackBlowFly,Phormia regina,Ph.Dthesis,VirginiaCommonwealthUniversity,Virginia,2005. [25]G.W. Levot, Insect fauna used to estimate the post-mortem interval of
deceasedpersons,Gen.Appl.Ent.32(2003)31–39.
[26]S.l.Parry,S.M.Linton,P.S.Francis,M.J.O’Donnell,T.Toop,Accumulationand excretionofmorphinebyCalliphorastygia,anAustralianblowflyspeciesof forensicimportance,J.InsectPhysiol.57(2011)62–73.
[27]K.A.George,M.S.Archer,L.M.Green,X.A.Conlan,T.Toop,Effectofmorphineon thegrowthrateofCalliphorastygia(Fabricius)(Diptera:Calliphoridae)and possibleimplicationsforforensicentomology,ForensicSci.Int.193(2009)21–
25.
[28]S.B.Karch,B.G.Stephens,C.H.Ho,Methamphetamine-relateddeathsinSan Francisco,J.ForensicSci.44(1999)359–368.
[29]G.Rivière,W.B.Gentry,S.M.Owens,Dispositionofmethamphetamineandits metaboliteamphetamineinbrainandothertissuesinratsafterintravenous administration,J.Pharmacol.Exp.Ther.292(2000)1042–1047.
[30]O.H.Drummer,Postmortemtoxicologyofdrugsofabuse,ForensicSci.Int.142 (2004)101–113.
[31]M.Gosselin,S.M.R.Wille,M.delMarRamírezFernandez,V.DiFazio,N.Samyn, G. De Boeck, B. Bourel, Entomotoxicology, experimental set-up and interpretationforforensictoxicologists,ForensicSci.Int.208(2011)1–9. [32]B.Greenberg,J.C.Kunich,EntomologyandtheLaw,CambridgeUniversity
Press,Cambridge,2002.
[33]L.L.Christrup,Morphinemetabolites,ActaAnaesthesiol.Scand.41(1997)116–
122.
[34]S.Darke,W.Hall,S.Kaye,J.Ross,J.Duflou,Hairmorphineconcentrationsof fatalheroinoverdosecasesandlivingheroinusers,Addiction97(2002)977–
984.
[35]M. Ferrucci, L. Pasquali, A. Paparelli, S.Ruggieri, F.Fornai, Pathways of methamphetaminetoxicity,Ann.N.Y.Acad.Sci.1139(2008)177–185. [36]L.E.Gaudette,B.B.Brodie,Relationshipbetweenthelipidsolubilityofdrugs
andtheiroxidationbylivermicrosomes,Biochem.Pharmacol.2(1959)89–96. [37]L.L.Keeley,Endocrineregulationoffat-bodydevelopmentandfunction,Ann.
Rev.Entomol.23(1978)329–352.
[38]Z.Wilson,S.Hubbard,D.J.Pounder,Druganalysisinflylarvae,Am.J.Forensic Med.Pathol.14(1993)118–120.
[39]J.F.Kinnear,M.D.Martin,J.A.Thomson,G.J.Neufeld,Developmentalchangesin thelatelarvaofCalliphorastygiaI.Haemolymph,Aust.J.Biol.Sci.21(1968) 1033–1046.
[40]N. Takayama, R. Iio, S. Tanaka, S. Chinaka, K. Hayakawa, Analysis of methamphetamine and its metabolites in hair, Biomed. Chromatogr.17 (2003)74–82.
[41]N.Takayama,S.Tanaka,K.Hayakawa,Determinationofstimulantsinasingle humanhairsamplebyhigh-performance liquidchromatographicmethod withchemiluminescencedetection,Biomed.Chromatogr.11(1997)25–28. [42]C.P.Campobasso,M.Gherardi,M.Caligara,L.Sironi,F.Introna,Druganalysisin
blowflylarvaeinhumantissues:acomparativestudy,Int.J.Leg.Med.118 (2004)210–214.
[43]K.R.Williams,D.J.Pounder,Site-to-sitevariabilityofdrugconcentrationsin skeletalmuscle,Am.J.ForensicMed.Pathol.18(1997)246–250.
[44]F.Moriya,Y.Hashimoto,Redistribution ofmethamphetamineintheearly postmortemperiod,J.Anal.Toxicol.24(2000)153–154.
[45]A.L. Pelissier-Alicot, J.M. Gaulier, P.Champsaur, P. Marquet, Mechanisms underlyingpostmortemredistributionofdrugs:areview,J.Anal.Toxicol.27 (2003)533–544.
[46]G.Kaneshrajah,B.Turner,Calliphoravicinalarvaeatdifferentratesondifferent bodytissues,Int.J.Leg.Med.118(2004)242–244.
[47]J.F.Wallman,D.M.Day,Influenceofsubstratetissuetypeonlarvalgrowthin
CalliphoraaugurandLuciliacuprina(Diptera:Ca