Bacillus pumilus-SPECIFIC BACTERIOPHAGE ISOLATED
FROM CIAPUS RIVER IN BOGOR, WEST JAVA
ANIK KUSMIATUN
POSTGRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
DECLARATION
I hereby declare that the thesis entitled Bacillus pumilus-Specific Bacteriophage Isolated from Ciapus River in Bogor, West Java is true of my work with the guidence of my academic supervisors and has not been submitted in any form to any university. Sources information from published and unpublished works of the other authors has been mentioned in the text and listed in the references of this thesis.
I hereby assign the copyright of my papers to the Bogor Agricultural University.
Bogor, September 2014
Anik Kusmiatun
SUMMARY
ANIK KUSMIATUN. Bacillus pumilus-Specific Bacteriophage Isolated from Ciapus River in Bogor, West Java. Supervised by SRI BUDIARTI and IMAN RUSMANA.
Diarrhea disease is part of the public health problems in developed countries. It is the main cause of mortality of diarrheal patients in Indonesia with Case Fatality Rate (CFR) 1.79 %. In 2012, UPT Public Health Center Cigudeg, Bogor reported that incident of diarrhea in Cigudeg was high (3180 patients). The main factors that predispose children to diarrhea in the area is contamination of pathogenic bacteria due to their poor sanitation. Salmonella sp, Shigella sp
Citrobacter, Vagococcus, Haemopillus, Micrococcus, and Streptococcus were isolated from faeces samples of children sufferiety with diarrhea disease in Cigudeg. Other bacteria i.e Enterococcus sp and Bacillus sp were isolated from water of their drainase system in the area. Interestingly, the Bacillus sp isolate was identified as Bacillus pumilus. It was a new report that B. pumilus found in an area with high number of diarrhea disease. This bacterium is a spore-forming bacterium commonly associated with food poisoning case. Characterization of its patogenicity was shown that B. pumilus produced haemolisin toxin that lyses red blood cells in culture media. Antibiotics were usually used to treat the disease caused by bacteria. Antibiotic typing test of B. pumilus showed that this isolate was resistant to ampicillin and clindamycin. It was a new report that B. pumilus
was resistant to these antibiotics.
An alternative way was by application bacteriophages (phages) as biocontrol agents to reduce B. pumilus in environment. In this study, the phages was isolated from Ciapus River using the double agar overlay method. It was found that the concentration of B. pumilus-phage indicated that naturally phage was found in this river.
RINGKASAN
ANIK KUSMIATUN. Bakteriofag Spesifik Bacillus pumilus Diisolasi dari Sungai Ciapus di Bogor, Jawa Barat. Dibimbing oleh SRI BUDIARTI dan IMAN RUSMANA.
Penyakit diare merupakan masalah kesehatan masyarakat di negara-negara berkembang. Penyakit ini menyebabkan kematian pasien diare di Indonesia dengan Case Fatality Rate (CFR) sebesar 1.79%. Pada tahun 2012, UPT Pusat Kesehatan Masyarakat Kecamatan Cigudeg, Bogor melaporkan bahwa jumlah penderita diare di Cigudeg cukup tinggi sebanyak 3180 pasien. Faktor utama penyebab diare pada anak-anak di daerah tersebut adalah kontaminasi bakteri patogen karena sanitasi lingkungan yang buruk. Salmonella sp, Shigella sp
Citrobacter,Vagococcus, Haemopillus, Micrococcus, dan Streptococcus diisolasi dari sampel tinja anak-anak penderita diare di Cigudeg. Bakteri lain yaitu
Enterococcus sp dan Bacillus sp diisolasi dari air lingkungan daerah Cigudeg. Menariknya, isolat Bacillus sp diidentifikasi sebagai Bacillus pumilus. Hal ini adalah laporan baru adanya B. pumilus yang ditemukan di daerah dengan jumlah penyakit diare tinggi. Bakteri ini merupakan bakteri pembentuk spora yang berkaitan dengan kasus keracunan makanan. Karakterisasi patogenisitas isolat B. pumilus dapat menghasilkan toksin haemolisin yang melisiskan sel darah merah dalam media kultur. Antibiotik biasa digunakan untuk mengobati penyakit yang disebabkan oleh bakteri. Uji antibiotik terhadap B. pumilus menunjukkan bahwa isolat ini resisten terhadap ampisilin dan klindamisin.
Cara alternatif untuk mengurangi B. pumilus yaitu dengan aplikasi bakteriofag (fag) sebagai agen biokontrol B. pumilus di lingkungan. Fag pada penelitian ini diisolasi dari Sungai Ciapus dengan menggunakan metode agar dua lapis (double agar overlay). Isolat fag tersebut dapat menginfeksi dan membunuh
B. pumilus melalui mekanisme lisis. Ukuran diameter plak FBa1 sebesar 2 mm, plak transparan dengan halo (cincin luar); plak FBa2 berukuran 1mm, plak transparan dengan halo; dan plak FBa3 berukuran 0,5 mm, plak transparan tanpa
halo. Konsentrasi isolat fag FBa1 adalah 10.2x108 PFU mL-1, fag FBa2 adalah 5.9x108 PFU mL-1, dan fag FBa3 adalah 8.5x108 PFU mL-1. Konsentrasi fag B. pumilus yang tinggi menunjukkan bahwa secara alami fag ditemukan di sungai ini.
Uji kisaran inang menunjukkan bahwa fag FBa1, FBa2, dan FBa3 memiliki aktivitas spesifik untuk Bacillus pumilus. Fag tersebut tidak menginfeksi sel-sel bakteri lainnya. Pengamatan morfologi fag FBa1 menggunakan Transmission Electron Microscope (TEM) dengan pewarnaan negatif menunjukkan kepala fag berbentuk ikosahedral tanpa ekor, diameter fag sebesar 166,67 nm, bentuk fag menyerupai partikel fag. Karakterisasi fag FBa1 dengan SDS-PAGE menunjukkan lima pita protein. Berat molekul masing-masing protein fag FBa1 adalah 70.9 kDa, 54.9 kDa, 33.8 kDa, 28.3 kDa, dan 21,4 kDa. Hasil penelitian ini menunjukkan prospek fag FBa1 untuk diaplikasikan sebagai agen biokontrol B. pumilus di lingkungan.
© Copyright by Bogor Agricultural University 2014
All rights reserved
No part or all of this thesis may be excerpted without inclution or mentioning the sources. Excerption only for research and education use, writing for scientific papers, reporting, critical writing or reviewing of a problem.
Bacillus pumilus-SPECIFIC BACTERIOPHAGE ISOLATED
FROM CIAPUS RIVER IN BOGOR, WEST JAVA
A thesis submitted as partial fulfillment of the requirement for the degree of Master of Science in Microbiology
POSTGRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
2014
Thesis External Examiner: Dr Drh Sri Murtini, MSi
ACKNOWLEDGEMENT
In the name of Allah The Most Gracious and The Most Merciful. The theme of this research was about bacteriophage, with the title Bacillus pumilus-Specific Bacteriophage Isolated from Ciapus River in Bogor, West Java.
I would like to gratitude to my supervisors, Dr dr Sri Budiarti and Dr Ir Iman Rusmana, MSi for their guidance, patience, and support during my study. I would like to thank to Prof Dr Anja Meryandini, MS; Prof Dr Okky Setyawati Dharmaputra; and Dr Drh Sri Murtini, MSi for their presence, patience and guidance in my thesis examination. I would like to thank to all lecturer for their guidance during my study in Microbiology Study Program.
I would also like to thank my parents, Mr. Saidi and Mrs. Siti Aminah; my sisters, Tutik Supriyati and Muslimah; my brothers, Juprianto and Jalaludin Sayuti, for their support and love that allowed me to continue my study.
I would also like to thank to Prof Dr Ir Suharsono, DEA and all staff of Research Center for Bioresources and Biotechnology (PPSHB), Bogor Agricultural University for their permission during my research in Biotechnology of Animal and Biomedical Laboratory. I would also like to thank to Mrs. Dewi, Mrs. Heni, Mr. Jaka, Mr. Aldi, and Mr. Pras for their guidance during my research in laboratory. I would also like to thank to Mrs. Ita and Labibah for their help to observed phage in TEM Laboratory, Eijkman Institute, Jakarta.
I would also like to thank to Bob Edwin Normande for help to isolated the host bacterium from Cigudeg, Bogor. My appreciation to all lab members, Tini, Novi, Rachmi, Ira, Fatin, Debby, Yeni, Leni, Ika, Lia, teteh Fitri for their knowledge sharing to me and friendship.
Finally I would like to thank Microbiology students 2012 for their support and friendship during my study in Bogor Agricultural University.
Bogor, September 2014
TABLE OF CONTENT
LIST OF TABLES x
LIST OF FIGURES x
INTRODUCTION 1
Background 1
Objectives 2
LITERATURE REVIEW 2
Bacillus pumilus 2
Lytic Bacteriophages 3
Phage Therapy 4
MATERIALS AND METHOD 4
Place and Duration of the Study 4
Identification of Host Bacterium 5
Water Samples Collection 5
Isolation and Purification of Phage 5
Phage Quantification 6
Host Range Determination 6
Morphological Observation of Phage using Transmission Electron
Microscope (TEM) 6 Analysis of Phage Proteins 6
RESULTS 6
Identification of Host Bacterium 6
Isolation and Purification of Phage 8
Phage Quantification 9
Host Range Determination 9
Morphological Observation of Phage using Transmission Electron
Microscope (TEM) 10
Analysis of Phage Proteins 10
DISCUSSIONS 11
CONCLUSIONS 13
LIST OF TABLES
1 Identification of host bacterium isolated from water in Cigudeg, Bogor 7 2 Antibiotic susceptibility test results of Bacillus pumilus 7
3 Detection of phage in water samples 8
4 Phage isolates from Ciapus river water, Bogor 8
5 Phage quantification of FBa1, FBa2, and FBa3 9
6 Host range of phage FBa1, FBa2, FBa3 9
LIST OF FIGURES
1 Photomicrograph of Bacillus pumilus 3
2 Bacillus pumilus isolate on blood agar 7
3 Morphology of plaque 8
4 Morphology of purified plaques 9
INTRODUCTION
Background
Diarrhea disease is part of the public health problems in developed countries. It is the main cause of mortality of diarrheal patients in Indonesia with Case Fatality Rate (CFR) 1.79 % (Kemenkes RI 2012). In 2012, UPT Public Health Center Cigudeg, Bogor reported that incident of diarrhea in Cigudeg was high (3180 patients). The main factors that predispose children to diarrhea in the area is contamination of pathogenic bacteria due to their poor sanitation. Salmonella sp,
Shigella sp, Citrobacter, Vagococcus, Haemopillus, Micrococcus, and
Streptococcus were isolated from faeces samples of children sufferiety diarrhea disease in Cigudeg, Bogor (Normande 2013). Other bacteria include Enterococcus
sp and Bacillus sp were isolated from water of their drainase system in the area. Interestingly, the Bacillus sp isolate was identified as Bacillus pumilus. It was a new report that B. pumilus found in an area with high number of diarrhea disease. Transmission of pathogenic bacteria that cause diarrhea are usually by faecal contaminated water or food to person contact (Gosh et al. 1991).
Bacillus pumilus is a spore-forming bacterium commonly associated with foodborne illness caused by intoxication. Suominen et al. (2001) reported that incidents of food poisoning with diarrhea symptoms was occurred after ingestion of foods containing large numbers of B. pumilus (105-107 cfu g-1). In 2007, From
et al. reported a food poisoning outbreak caused by B. pumilus in Norway.
Bacillus pumilus was found as contaminant from reheated rice in a Chinese restaurant. The acute symptoms developed after meal, the people experienced stomach cramps and diarrhea for several days. Large numbers of B. pumilus
strains produced a lipopeptides complex known as pumilacidins, a toxic compounds. This toxin can destroy cell membranes through production of selective cationic channels (Sheppard et al. 1991). The membrane destroying capacity of this toxin explains that food poisoning often associated with stomach cramps and diarrhea.
Antibiotics were used to treat bacterial infections. Antibiotic resistance in bacteria is a global health problem. Antibiotic resistance in bacteria occurs via either chromosomal mutations or foreign gene transfer by plasmids (Zhang et al. 2006). Transfer of resistant antibiotic gene between bacterial cells may cause a new antibiotic resistant bacterial strain. Irrational application of antibiotics can increase the antibiotics resistant, not only on pathogenic bacteria but also normal flora in human gastrointestinal. Budiarti (2011) reported that E. coli as intestinal normal flora isolated from faecal of new born children was resistant to antibiotic bacitracin and erytromycin. It was showed that normal flora E. coli in new born children has material genetic changes caused by transfer of the resistant E. coli
piperacillin-2
tazobactam, and tetracycline. These report give information that antibiotic resistant level in pathogenic and flora normal bacteria is very high. The phage therapy was used to overcome the problem of antibiotic resistance.
Bacteriophages (phages) are viruses that infect bacteria. Phages have a protein coat that encloses a nucleid acid (DNA or RNA). There are two life cycle types of phages, lytic and lysogenic phages (Snyder and Champness 2007). Lytic phage kills bacteria through lysis and releasing many particles of phages. Lytic phages provide opportunity as biocontrol agent to decrease pathogenic bacteria. Recently, the potential lytic phages could be used as a biocontrol agent of foodborne pathogens. For example, phage FB4 could lyse EPEC K1.1 and this phage expected as biocontrol to prevent foodborne disease in Indonesia (Budiarti
et al. 2011). Oral application in rat diet, phage FR38 to reduce Salmonella P38 had no effect on body weight, blood chemistry, kidney and liver functions in rat (Sartika et al. 2012). Phage FGCSSa1 isolated from sewage had potential use for biocontrol of Salmonella spp. in foods (Carey-Smith et al. 2006). Bacteriophage cocktail as a biocontrol agent of Salmonella enterica serovar Typhimurium and S. enterica serovar Enteritidis used in food process (Spricigo et al. 2013). Many phages infecting Bacillus spp. were isolated; i.e. phages FWLBc1 and FWLBc2 were isolated from B. cereus (Lee et al. 2011). Tsamsa phage isolated from B. antrhracis (Ganz et al. 2014). SPP1 phage was isolated from B. subtilis (Jakutytė et al. 2011). PhiAGATE phage was isolated from B. pumilus (Barylski et al. 2014). However B. pumilus phages have not been reported in Indonesia yet. It was very important to found a biocontrol agent to reduce B. pumilus in the environment.
Objectives
The objectives of this research were to isolate and characterize B. pumilus
bacteriophage isolated from water sample of Ciapus River in Bogor, West Java. The prospect of this research can be applied as a biocontrol agents of B.pumilus in the environment.
LITERATURE REVIEW
Bacillus pumilus
Bacillus pumilus is a Gram-positive, rod-shaped, aerobic, spore-forming bacteria (Figure 1). Bacillus pumilus can growth at pH 6-10 and temperature 10-50 oC (Vos et al. 2009). Bacillus pumilus has peptidoglycan layer that play a role in adhesion to host cells and other surfaces in the environment. Peptidoglycan is composed of teichoic and lipoteichoic acids. These acids are composed polyglycosyl phosphates i.e glycerol phosphate or ribitol phosphate (Kubler-Kielb
et al. 2004). Bacillus pumilus life cycle is similar to other Bacilli. It has three life cycle stages: sporangium, vegetative cell, or free spore (Sonenshein 2000).
3 growth. The spore will germinate to be a vegetative cell when the condition is favourable.
Figure 1 Photomicrograph of Bacillus pumilus viewed by phase-contrast microscopy. Bar = 2 µm. (Logan 2011)
Human infection by B. pumilus is rarely reported, however an outbreak of
B. pumilus was found in reheated rice. It was responsible for 3 cases of food poisoning outbreak. Bacillus pumilus produces a complex of lipopeptides called pumilacidins. This complex is toxic to epithelial cells. The symptoms of this infection were dizziness, headache, stomach cramps, and diarrhea (From et al. 2007). Parvathi et al. (2009) reported that B. pumilus produced protease and lipase. This bacterium had cesA and cesB genes encoded cereulide synthetase. Cereulide is a stable cyclic dodecadepsipeptide produced by some strains of B. cereus. This toxin has high toxicity to humans (Agata et al. 1995; Yokohama et al. 1999). Matarante et al. (2004) reported that hemolytic and lecithinase activities were virulence factors in B. pumilus.
Lytic Bacteriophages
Bacteriophages were discovered in 1915 by Towart and in 1917 by Felix
d’Herelle (Duckworth 1976). They reported a filterable entity that capable to destroyed bacterial culture. The destruction of the bacterial cell was noticeable at the appearance of plaques. Plaques are clear spots formed in bacterial colonies
during the rupturing of the cells. D’Herelle categorized these filterable entities as bacteriophages, meaning bacterial eaters.
4
host machinery for phage replication. The RNA polymerase of the host recognises strong phage promoters which results in the transcription of immediate early genes. The early genes encode enzymes involved in DNA synthesis such as DNA polymerase, primase, DNA ligase, and helicase (Snyder and Champness 2007). These enzymes help the phage DNA to replicate. The late genes encode proteins involved assembly of head and tail. After the phage particle is completed, the DNA is packaged into procapsids which are icosahedral protein shells. The mature phages then were released in a process known as lysis. Lytic phage involve an enzyme, known as an endolysin which degradates the bacterial peptidoglycan. Endolysins need a second lysis factor, a phage-encoded membrane protein called holin. The holin-endolysin is essential for host lysis (Young 2002).
Phage Therapy
Multidrug resistant bacteria are a serious health problem these days. Antibiotics are not bacteria specific and thus can result in distruption in the balance of normal flora, which are bacteria that are naturally found in our bodies (Giuliano et al. 1987). Phage therapy seems to be a good option for this problem. Phage therapy is use of a specific bacteriophage to a bacterial cell. The introduction of phage therapy was seen in the early 1930s (Levin and Bull 2004). Phage therapy today will be used in possible applications to control foodborne pathogens.
There are many research in the use of phage to control pathogenic bacteria. Phage therapy against bacterial pathogens, for example, to control Salmonella
5
Identification of Host Bacterium
Host bacterium was isolated from water located in Cigudeg, Bogor, Indonesia. Pathogenicity of Bacillus was determining using blood agar plates. Identification was performed using API 50CHB (bioMérieux, Marcy I`Etoile, France). The isolate was maintained aerobically on Tryptic Soy Agar (Difco), at 37 oC for 24 h. Antibiotics test with disk diffusion method was used to identify the susceptibility of the bacterium to ampicillin, amoxicillin, ciprofloxacin, and clindamycin (CLSI 2012). Antibiotics susceptibility was assessed by measuring the diameter (in millimeters) of the zone inhibition surrounding disks.
Water Samples Collection
Water samples were collected from Ciapus River in Bogor. Each sample (50 mL) from four station was stored in a sterile bottle at 4 oC overnigt. The crude sample was centrifugated at 5,000 rpm for 20 min to remove bacterial cells and debris. The supernatant was filtered through a 0.22 µm sterile filter and then stored at 4 oC. This supernatant was used as a sample to isolate phage.
Isolation and Purification of Phage
Specific phages were sometimes difficult to isolate directly so enrichment culture was used to multiply phages. Water sample of 4.5 mL was incubated overnight with 0.5 mL of Nutrient broth (Difco) and 0.5 mL of host bacteria culture (previously grown 24 h in Nutrient broth medium). The culture was centrifugated at 5000 rpm for 25 min and then filtered through a 0.22 µm sterile filter millipore. Stock solution of phage was stored in sterile tube at 4 oC.
The presence of a lytic phage in the filtrate was examined by using the double layer method with some modification (Carey-Smith et al. 2006). Stock solution of phage as much as 100 µL (dilutions of 10-5 to 10-8) was mixed with
Bacillus pumilus culture 100 µL (approximately 108 CFU mL-1), and incubated at 37 oC for 30 minutes. The culture bacterium with phage after incubation was mixed with 7 mL of 0.8 % soft-agar (42 oC). It was poured as an overlay onto Nutrient Agar (Difco) base plates, and incubated at 37 oC overnight. The presence of a lytic phage in the form of plaques was detected after incubation of the plate.
6
Phage Quantification
Phage quantification (plaque assay) was examined using double layer method as mentioned earlier. Stock solution of purified phage was serially diluted (10-1 to 10-8) in 0.85 % NaCl. Each dilution was subjected to plaque assay. Plaques were counted on the plate that contain 30-300 plaques and expressed as plaque forming unit per milliliter (PFU mL-1).
Phage titer (PFU mL-1) = number of plaques x 10 x reciprocal of counted dilution
Host Range Determination
Exponential phase cultures of the host bacteria (Bacillus pumilus, Proteus mirabilis, Photobacterium damselae, Salmonella sp., EPEC K1.1) were prepared, and agar overlays inoculated with a host (100 µL) and phage stocks (100 µL). The plates were incubated at 37 oC for 24 h and the plaques were observed for each host.
Morphological Observation of Phage by Using Transmission Electron Microscope (TEM)
The morphology of phage FBa1 was examined by transmission electron microscope. Stock solution phage (5 µL) was dropped in to grid for 30 seconds, and then dried up with filter paper. Uranyl acetate 2% solution (5 µL) was also dropped in to grid for 1 minute, and then dried up with filter paper for 60 minutes. The grid placed in holder and left for perfect dry. Specimen was observed using transmission electron microscope (TEM JEOL JEM-1010 model) at 80 kV, and phages were examined at 20000x – 80000x magnification.
Analysis of Phage Proteins
The protein composition of phage FBa1 was examined by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Stock phage were mixed with buffer sample (4:1), and then boiled for 5 – 10 minutes. As much as 60 µL phage protein samples were loaded onto a SDS-PAGE gel (12% acrilamide) at 20 mA, 50 volt, for 3.5 h. Silver staining was used for visualization of the result.
RESULTS
Identification of Host Bacterium
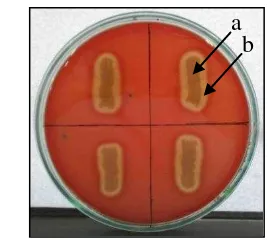
7 around the colonies, it was indicated that the isolate exhibited -haemolytic (Figure 2). Several biochemical tests on API 50CHB were used to identify species of the bacterial isolate (Table 1). The isolate was identified as B. pumilus with 99.9 % match in identity by API 50CHB. The antibiotic test of B. pumilus showed that the isolate was resistant to ampicillin and clindamycin (Table 2).
Figure 2 Bacillus pumilus isolate on blood agar (a); -haemolytic zone (b).
Table 1 Identification of host bacterium isolated from water in Cigudeg, Bogor Identification
test Results
API 50 CHB Positive : glycerol, L-arabinose, ribose, glucose, D-fructose, D-mannose, D-mannitol, amygdalin, arbutin, esculin
Table 2 Antibiotic susceptibility test results of Bacillus pumilus
Antibiotic
*Standards by CLSI (2012), S: sensitive, R: resistant a
8
Isolation and Purification of Phage
The phage isolate of B. pumilus was found in a water sample collected from Ciapus River in Bogor. Samples were collected from river water because of phage is naturally part of environmental ecosystem. Lytic activity were detected by plaques using double agar method. Only one sample that positive contained paghes specific to B. pumilus (Table 3). Treatment phages inoculate to B. pumilus
culture resulted in complete lysis of the bacterial cells. Plaques were formed after 3 h of incubation of agar plates. Differences in plaque morphology and sizes were observed. The lytic activity formed two types of plaque, haloing plaque and plaque without halo (Figure 3). Plaque diameter average was 0.5 to 2 mm after 18 h incubation at 37 oC.
Three phages were successfully isolated and they had different on plaque morphology (Table 4). The phages were named FBa1, FBa2, and FBa3. Plaques were purified by single-plaque isolations. Three isolates of phage were purified based on plaque morphology, FBa1, phage from large plaque with halo; FBa2, phage from medium plaque with halo; and FBa3, phage from small plaque without halo. Purified phage was diluted in SM buffer and stored at 4 oC. Purified phages were test on B. pumilus for three times. Plaques morphology were the same as plaques morphology of isolation step, however different in plaque size or diameter (Figure 4).
Table 3 Detection of phage in water samples Station Water sample (mL) Lytic phage
1 50 - medium plaque with halo (b); FBa3, small plaque without halo (c). Bar = 2 mm
Table 4 Phage isolates from Ciapus River, Bogor Phage
isolate Morphology of plaque Plaque size (mm) FBa1 Translucent plaque, presence of halo 2
9
Figure 4 Morphology of purified plaques: FBa1 (a); FBa2 (b); FBa3 (c).
Phage Quantification
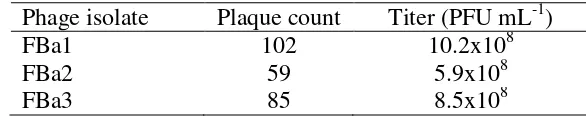
Natural phage number in water samples was too low to produce a quantifiable titer. Enrichment method was used to isolate phages and to produce quantifiable titer. Phage FBa1 had the highest titer, approximately 10.2x108 PFU mL-1 (Table 5).
Table 5 Phage quantification of FBa1, FBa2, and FBa3 Phage isolate Plaque count Titer (PFU mL-1)
FBa1 102 10.2x108
FBa2 59 5.9x108
FBa3 85 8.5x108
Host Range Determination
The host range of phages is defined by what bacterial cellscan lyse. The phages of FBa1, FBa2, and FBa3 were tested on exponential-phase cultures of other pathogenic bacteria i.e. Photobacterium damselae, Proteus mirabilis,
Salmonella sp., and EPEC K1.1. FBa1, FBa2, and FBa3 phage isolates were lytic
on Bacillus pumilus but they did not infect the other tested bacterial isolates (Table 6).
Table 6 Host range of phage FBa1, FBa2, FBa3 Phage
Isolate
Host bacteria
Bacillus pumilus
Photobacterium damselae
Proteus
mirabilis Salmonella sp.
EPEC K1.1
FBa1 + - - - -
FBa2 + - - - -
FBa3 + - - - -
10
Morphological Observation of Phage Using Transmission Electron Microscope (TEM)
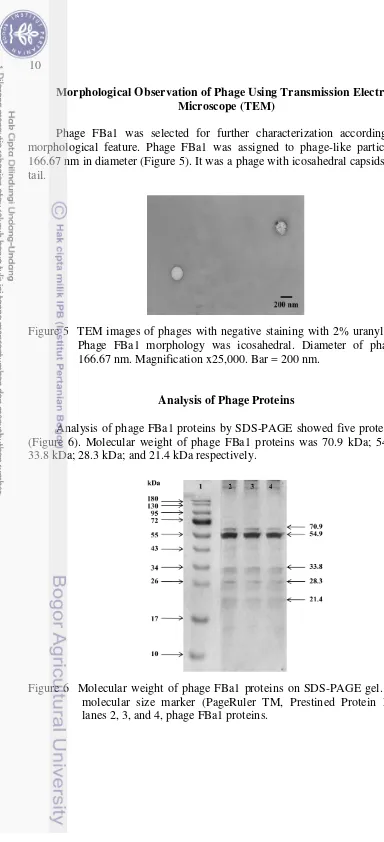
Phage FBa1 was selected for further characterization according to the morphological feature. Phage FBa1 was assigned to phage-like particles with 166.67 nm in diameter (Figure 5). It was a phage with icosahedral capsids without tail.
Figure 5 TEM images of phages with negative staining with 2% uranyl acetate. Phage FBa1 morphology was icosahedral. Diameter of phage was 166.67 nm. Magnification x25,000. Bar = 200 nm.
Analysis of Phage Proteins
Analysis of phage FBa1 proteins by SDS-PAGE showed five protein bands (Figure 6). Molecular weight of phage FBa1 proteins was 70.9 kDa; 54.9 kDa; 33.8 kDa; 28.3 kDa; and 21.4 kDa respectively.
11
DISCUSSIONS
Bacillus pumilus was isolated from water in Cigudeg, Bogor, West Java. It indicated that water environment in this location was contaminated with B. pumilus. There was no report about contamination of B. pumilus from water samples in Cigudeg, Bogor, previously. The B. pumilus isolate produced a toxin that lysed red blood cells in culture media. Hoult and Tuxford (1991) reported that
B. pumilus had haemolytic activity. Haemolysin toxin produced by B. pumilus
caused diarrhea in human. Haemolysin is a toxin having dermonecrotic and vascular permeability activities and causing fluid accumulation. It implicated in diarrheal illness.
Most studies on bacterial antibiotic resistance focused on pathogenic microorganisms. Very limited information on antimicrobial susceptibility profiles of B. pumilus was available. Ozkocaman (2006) reported that B. pumilus was resistant to penicillin and sensitive to ciprofloxacin. This study showed that B. pumilus isolate was resistant to ampicillin and clindamycin, but it was still sensitive to amoxicillin and ciprofloxacin. Resistant of antibiotic would be a problem. It could overcome by alternative solution of bacteriophage therapy.
Phage was isolated from water. Water sample was collected from river because phages can be found in the environments where their bacterial host inhabit. This study found phage isolate that can infect and kill B. pumilus through cell lysis. Phage progeny was released after host bacterial cell lysed. The progeny could diffuse in soft agar and then infect the surrounding cells. Lysis of the cells results a circular clear zone called a plaque. Interestingly, halos were formed around some of plaques. A secondary lysis zone around the centre of plaques was due to endolysins activity. Some bacterial cells were not yet fully lysed in secondary lysis zone. Plaque morphology (plaque haloing) in this results was similar to plaque of B. pumilus phage reported by Grilione and Carr (1960).
Plaque morphology of phage FBa1 was a 2 mm diameter translucent plaque with halo, plaque of FBa2 was a 1 mm diameter translucent plaque with halo, and plaque of FBa3 was a 0.5 mm diameter translucent plaque without halo. The different of plaque size in this results may be caused by delays in adsorbtion. Delays in adsorbtion makes a lower adsorbtion rate and results a smaller plaque size (Abedon et al. 2001). Plaque size was also influenced by numerous factors, such as reducing the agar concentration, condition of incubation, and log phase cells of host bacteria (Clokie and Kropinski 2009). Reducing the agar concentration in the medium makes the phage diffuses easily. Condition of incubation must suitable for host bacteria to produces many phage progeny.
The differences of plaques morphology indicated the presence of different phages in the samples from where they were isolated. Phages were purified by single-plaque isolation. Three phage isolates were purified based on their plaque morphology. Plaques morphology were the same as plaques morphology of isolation step, hoever different in plaque size or diameter. Concentration of FBa1 phage was 10.2 x 108 PFU mL-1, FBa2 phage was 5.9x108 PFU mL-1, and FBa3 phage was 8.5x108 PFU mL-1.
12
generation time devided into three period, (1) diffusion of phage progeny to new host cells; (2) the phage eclipse period; (3) a period of progeny maturation (Abedon et al. 2001).The phage generation was controlled by the phage lysis time. The phage lysis time was defined by the infective phage particles released from the host bacteria. The longer lysis time results the larger burst size (Wang 2006). The FBa1 phage with larger burst size associated with longer lysis time. In the longer lysis time, FBa1 phage could continue to accumulate progeny virions before lysis. The larger burst size was formed the larger plaque size. Host cell growth in logarithmic phase was also effected the phage replication (Woody and Cliver 1995). In this study, log phase of host cell was used for optimum phage replication and produced large burst size.
This study was showed that phage FBa1, FBa2, and FBa3 had lytic activity to B. pumilus. It was not infect Photobacterium damselae, Proteus mirabilis,
Salmonella sp, and EPEC K1.1. FBa1, FBa2, and FBa3 phage isolates had a narrow host range. It was specific to B. pumilus. Specificity interaction of phage with bacterial cell is determined by specificity of adsorption, dependent on the structural of reseptors on bacterial cell surface (Braun and Hantke 1997). Phages bind to all possible bacterial cell surface receptors including pili (Roncero et al. 1990), flagella (Shade et al. 1967; Lovett 1972), capsule (Suthereland et al. 2004), teichoic acid (Raisanen et al. 2007), surface protein (Davison et al. 2005), and lipopolysaccharide (Lindberg et al. 1978).
Morphological observation of phage FBa1 using TEM negative staining. Uranyl acetate was used for negative staining in this study. Uranyl acetate is acidic, acts as afixative, inactives phages. Morphology of phage FBa1 showed an icosahedral capsid without tail of phage-like particles. The phage-like particles are phage without tail and were more abundant than tailed phage in environment (Ashelford et al. 2003). Phage FBa1 in this result were different from previously discovered of phiAGATE. Phage phiAGATE infecting B. pumilus by TEM analysis showed that virions phiAGATE had tail morphology and assumed that phage was a member of Myoviridae family (Barylski et al. 2014).
Phage FBa1 did not have a tail to attach Bacillus pumilus. Phage FBa1 may be have adsorption mechanism like phage infecting Pseudomonas syringae (Von Seggern et al. 1999). The virion is fixed to the distal part of pilus. The virion moves closer to cell surface and then binding to the baseplate of pilus. Pilus retraction brings the virion to contact with the host’s outer membrane, and crossing the outer membrane deposits the nucleocapsid in the periplasm. Phage-encoded lytic enzyme on the nucleocapsid surface digests the peptidoglycan layer,
and then brings the nucleocapsid into contact with the host’s plasma membrane. Electrochemical membrane potential, ATP molecules, enzymatic splitting of peptidoglycan layer may be important for penetration of genetic material phage inside the bacterial cell (Rakhuba et al. 2010).
13
cereus-infecting bacteriophage B4 (Son et al. 2012). And 21.4 kDa protein may correlated to endolysin of phage. Endolysin molecular weights of phage BtCS33 infecting Bacillus thuringiensis was 24 kDa and 11 kDa respectively (Yuan et al. 2012).
Lytic phage was produced holin and endolysin to degradate bacterial cell wall (Hanlon 2007). The combinated action of the holin-lysin proteins was important in releasing of new phage particles from an infected cell (Daniel et al. 2007). Holin forms a hole in the cell membrane, and endolysin passes through the hole and destroys the peptidoglycan structure. Two gene products were determined to be the possible lysin, an N-acetylmuramoyl-L-alanine amidases and/or L-alanoyl-D-glutamate peptidase. The endolysin have two domains connected by a short linker: the N-terminal catalytic domain is responsible for cell lytic activity and the C-terminal cell wall binding domain that recognizes and binds a specific substrate in the cell wall of target bacteria (Fischetti 2008).
CONCLUSIONS
Phage FBa1, FBa2, FBa3 were isolated from Ciapus River in Bogor, West Java. They were capable to lyse bacterial cells and specific for Bacilllus pumilus. The phage FBa1 was typical icosahedral capsid phage-like particles with 166.67 nm in diameter. Molecular weight of phage FBa1 proteins was 70.9 kDa; 54.9 kDa; 33.8 kDa; 28.3 kDa; and 21.4 kDa respectively. Phage FBa1, FBa2, and FBa3 had potency for biocontrol of Bacilllus pumilus in the environment and to be applied in food production process.
REFERENCES
Abedon ST, Herschler TD, Stopar D. 2001. Bacteriophage latent-period evolution as a response to resource availability. Appl Environ Microbiol. 67(9):4233-4241.doi:10.1128/AEM.67.9.4233-4241.2001.
Agata N, Ohta M, Mori M, Isobe M. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 129(1):17-20.doi:10.1111/j.1574-6968.1995.tb07550.x.
Ashelford KE, Day MJ, Fry JC. 2003. Elevated abundance of bacteriophage infecting bacteria in soil. Appl Environ Microbiol. 69:285-289.doi:10.1128/AEM.69.1.285–289.2003
Barylski J, Nowicki G, Gozdzicka-Jozefiak A. 2014. The discovery of phiAGATE, a novel phage infecting Bacillus pumilus, leads to new insights into the phylogeny of the subfamily Spounavirinae. PLOS ONE. 9(1):86632-86645.doi:10.1371/journal.pone.0086632.
Braun V and Hantke K. 1977. Bacterial receptors for phages and colicins as constituents of specific transport systems. Microbial Interactions. Receptor
14
Budiarti S. 2011. Antibiotic Resistance Escherichia coli isolated from faecal of healthy human. J Int Environ Appl Sci. 6(3):359-364.
Budiarti S, Hidayati R, Rusmana I. 2011. Infectivity of lytic phage to Enteropathogenic Escherichia coli from diarrheal patients in Indonesia. J US-China Med Sci. 8:273-282.
Callaway TR, Edrington TS, Brabban AD, Anderson RC, Rossman ML, Engler MJ, Carr MA, Genovese KJ, Keen JE, Looper ML, Kutter EM, Nisbet DJ. 2008. Bacteriophage isolated from feedlot cattle can reduce Escherichia coli
O157:H7 populations in ruminant gastrointestinal tracts. Foodborne Pathogens and Diseases. 5(2):183-191.doi:10.1089=fpd.2007.0057.
Carey-Smith GV, Billington C, Cornelius AJ, Hudson A, Heinemann JA. 2006. Isolation and characterization of bacteriophages infecting Salmonella spp. J
FEMSLE. 217:1-5.doi:10.1111/j.1574-6968.2006.00217.x.
Clokie MRJ, Kropinski AM. 2009. Bacteriophages Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions. New York (US): Humana Press.
[CLSI] Clinical and Laboratory Standards Institute. 2012. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement. Pennsylvania (US): CLSI.
Daniel A, Bonnen PE, Fischetti VA. 2007. First complete genome sequence of two Staphylococcus epidermidis bacteriophages. J Bacteriol. 189:2086-2100.doi:10.1128/JB.01637-06.
Davison S, Couture-Tosi E, Candela T, Mock M, Fouet A. 2005. Identification of the Bacillus anthracis phage receptor. J Bacteriol. 187(19):6742-6749.doi:10.1128/JB.187.19.6742-6749.2005.
Duckworth DH. 1976. Who discoveres bacteriophage?. Bacteriol Rev. 40(4):793-802.
Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 11:393-400.doi:10.1016/j.mib.2008.09.012.
Bacillus anthracis features unusual genome characteristics. PLOS ONE. 9(1):85972-85980.doi: 10.1371/journal.pone.0085972.
Ghosh AR, Nair GB, Dutta P, Pal SC Sen D. 1991. Acute diarrhoeal disease in infants aged below six months in hospital in Calcuta, India: an aetiological study. Trans R Soc Trop Med Hyg. 85(6):796-798.doi:10.1016/0035-9203(91)90459-C.
Giuliano M, Barza M, Jacobus NV, Gorbach SL. 1987. Effect of broad spectrum parenteral antibiotics on compotition of intestinal microflora in humans.
Antimicrobial Agents and Chemotherapy. 31:202-206.
Goode DH, Allen VM, Barrow PA. 2003. Reduction of experimental Salmonella
15 Grilione PL and Carr JH. 1960. Isolation and study of a bacteriophage for Bacillus
pumilus. J Bacteriol. 80:47-50.
Hanlon GW. 2007. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents. 30:118-128.doi:10.1016/j.ijantimicag.2007.04.006. Tavares P. 2011. Bacteriophage infection in rod-shaped gram-positive bacteria: evidence for preferential polar route for phage SPP1 entry in
Bacillus subtilis. J Bacteriol. 193:4893-4903.doi:10.1128/JB.05104-11. [Kemenkes RI] Kementerian Kesehatan Republik Indonesia. 2012. Profil data
kesehatan Indonesia tahun 2011. Jakarta (ID): Kemenkes.
Kubler-Kielb J, Coxon B, Schneerson R. 2004. Chemical structure, conjugation, and cross-reactivity of Bacillus pumilus Sh18 cell wall polysaccharide. J Bacteriol. 186(20):6891-6901.doi:10.1128/JB.186.20.6891–6901.2004. Lee WJ, Billington C, Hudson JA, Heinemann JA. 2011. Isolation and
characterization of phages infecting Bacillus cereus. Lett Appl Microbiol. 52:456-464.doi:10.1111/j.1472-765X.2011.03023.x.
Leverentz B, Conway WS, Alavidze Z, Janisiewicz WJ, Fuchs Y, Camp MJ, Chighladze E, Sulakvelidze A. 2001. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J Food Protection. 64:1116-1121.
Levin BR and Bull JJ. 2004. Population and evolutionary dynamics of phage therapy. Microbiology: Nature Reviews. 2:166-173.
Lindberg AA, Wollin R, Gemski P, Wohlhieter JA. 1978. Interaction between bacteriophage Sf6 and Shigella flexneri. J Virol. 27:38-44.
Logan NA. 2011. Bacillus and relatives in foodborne illness. J Appl Microbiol. 112:417-429.doi:10.1111/j.1365-2672.2011.05204.x.
Lovett PS. 1972. PBP1: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 47:743-752.
Martínez B, Obeso JM, Rodríguez A, García P. 2008. Nisin-bacteriophage crossresistance in Staphylococcus aureus. Int J Food Microbiol. 122(3):253-258.doi:10.1016/j.ijfoodmicro.2008.01.011.
Matarante A, Baruzzi F, Cocconcelli PS, Morea M. 2004. Genotyping and toxigenic potencial of Bacillus subtilis and Bacillus pumilus strains occurring in industrial and artisanal cured sausages. Appl Environ Microbiol. 70(9): 5168-5176.doi:10.1128/AEM.70.9.5168–5176.2004.
Normande BE. 2013. Bakteri enteropatogen pada penderita diare dan kondisi higiene sanitasi lingkungan inang di Kecamatan Cigudeg Kabupaten Bogor [skripsi]. Bogor (ID): Institut Pertanian Bogor.
16
Parvathi A, Krishna K, Jose J, Joseph N, Nair S. 2009. Biochemical and molecular characterization of Bacillus pumilus isolated from coastal environment in Cochin, India. Braz J Microbiol. 40:269-275.
Raisanen L, Draing C, Pfitzenmaier M, Schubert K, Jaakonsaari T, von Aulock S, Hartung T, Alatossava T. 2007. Molecular interactin between lipoteichoic acids and Lactobacillus delbrueckii phages depends on D-alanyl and α -glucose substitution of poly(glycerophosphate) backbones. J Bacteriol. 189:4135-4140.doi:10.1128/JB.00078-07.
Rakhuba DV, Kolomiets EI, Szwajcer Dey E, Novik GI. 2010. Bacteriophage reseptors, mechanisms of phage adsorbtion and penetration into host cell.
Pol J Microbiol. 59:145-155.
Roncero C, Darzins A, Casadaban MJ. 1990. Pseudomonas aeruginosa
transposable bacteriophages D3112 and B3 require pili and surface growth for adsorbtion. J Bacteriol. 172:1899-1904.
Sartika D, Budiarti S, Sudarwanto M. 2012. Phage FR38 treatment on sprague dawley rat inferred from blood parameters and organ systems. Hayati J Biosci. 19(3):131-136.doi:10.4308/hjb.19.3.131.
Shade SZ, Adler J, Ris H. 1967. How bacteriophage χ attack motile bacteria. J Virol. 1:599-609.
Sheppard JD, Jumarie C, Cooper DG, Leprade R. 1991. Ionic channels induced by surfactin in planar lipid bilayer membranes. Biochimia et Biophysica Acta. 1064:13-23.
Sillankorva SM, Oliveira H, Azeredo J. 2012. Bacteriophages and their role in food safety. Int J Microbiol. 2012:1-13.
Snyder L and Champness W. 2007. Molecular genetics of bacteria -3rd ed. Washington (US): ASM Press.
Son B, Yun J, Lim J-A, Shin H, Heu S, Ryu S. 2012. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 12:33-41.
Sonenshein AL. 2000. Control of sporulation initiation in Bacillus subtilis. Curr Opinion Microbiol. 3:561-566.
Soni KA and Nannapaneni R. 2010. Removal of Listeria monocytogenes biofilms with bacteriophage P100. J Food Protection. 73(8):1519-1524.
Spricigo DA, Bardina C, Cortes P, Llagostera M. 2013. Use of bacteriophage cocktail to control Salmonella in food and the food industry. Int J Food Microbiol. 165:169-174.doi:10.1016/j.ijfoodmicro.2013.05.009.
Suominen I, Andersson MA, Andersson MC, Hallaksela AM, Kampfer P, Rainey FA, Salkinoja-Salonen M. 2001. Toxic Bacillus pumilus from indoor air, recycled paper pulp, Norway spruce, food poisoning outbreaks and clinical samples. Syst Appl Microbiol. 24:267-276.doi:10.1078/0723-2020-00025. Suthereland IW, Hughes KA, Skillman LC, Tait K. 2004. The interaction of
phage and biofilms. FEMS Microbiol Lett. 232:1-6.doi:10.1016/S0378-1097(04)00041-2.
17 Von Seggern DJ, Chiu CY, Fleck SK, Stewart PL, Nemerow GR. 1999. A
helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J Virol. 73:1601–1608.
Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB. 2009. Bergey's Manual of Systematic Bacteriology: Volume 3: The Firmicutes. New York (US):Springer Science & Business Media. Wang I-N. 2006. Lysis timing and bacteriophage fitness. Genetics. 172:17-26.doi:
10.1534/genetics.105.045922.
Woody MA and Cliver DO. 1995. Effects of temperature and host cell growth phase on replication of F-specific RNA coliphage Q . Appl Environ Microbiol. 61(4):1520-1526.
Yokohama K, Ito M, Agata N, Isobe M, Shibayama K, Horii T, Ohta M. 1999. Pathological effect of synthetic cereulide, an emetic toxin of Bacillus cereus, is reversible in mice. FEMS Immunol Med Microbiol. 24(1):115-120.doi:10.1111/j.1574-695X.1999.tb01272.x.
Young R. 2002. Bacteriophage holins: deadly diversity. J Mol Microbiol Biotechnol. 4(1):21-36.
Yuan Y, Peng Q, Gao M. 2012. Characteristics of a broad lytic spectrum endolysin from phage BtCS33 of Bacillus thuringiensis. BMC Microbiol. 12:297-305.
18
AUTOBIOGRAPHY
The author was born on March 26th 1987 in Grobogan, Central Java. The author completed her senior high school in 2005 from SMA N 1 Purwodadi, Grobogan, Central Java. She later joined Biology Education Study Programe, Faculty Mathematics and Natural Science in Yogyakarta State University and graduated in 2009.
In July 2010 until June 2011, the author work in SMA Insan Madani, Meukek, South Aceh as a teacher. After that, she continued work as a teacher in SMA Averos, Sorong, West Papua in July 2011 until June 2012.