REPRODUCTIVE BIOLOGY OF BORNEAN ENDEMIC
FISH
Hampala bimaculata
(Popta, 1905)
FX. WIDADI PADMARSARI SOETIGNYA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
i
STATEMENT LETTER
I hereby declare that dissertation entitled “Reproductive Biology of Bornean Endemic Fish Hampala bimaculata (Popta, 1905)” is original result of my own research
supervised by supervisory committee and has never been submitted in any form at any institution before. All information from other authors cited here are mentioned in the text and listed in the reference at the end part of the dissertation.
Bogor, September 2016
FX. Widadi Padmarsari Soetignya
iii
SUMMARY
FX. WIDADI PADMARSARI SOETIGNYA. Reproductive Biology of Bornean Endemic Fish Hampala bimaculata (Popta, 1905). Supervised by BAMBANG SURYOBROTO, MOHAMMMAD MUKHLIS KAMAL, and ARIEF BOEDIONO
Hampala bimaculata is an endemic species of Borneo. The fish is very sensitive to poor water quality, reason why inhabit mainly clear rivers or streams with running water with sandy to muddy bottoms. Although, H. bimaculata is a food fish, being also popular in sport fishing, little is known of the important aspects of its reproduction to assist with fisheries management planning. For the first time, this study described and determined the general reproductive biology of H. bimaculata with regards to gonads development, type of ovarian organization, spawning type, sex ratio, size at first maturity, spawning season, and fecundity. This study also estimated population and habitat preference of H. bimaculata based on fish population collected in Betung Kerihun National Park, West Kalimantan Province.
A total of 181 individuals specimens were collected from February to October 2013 and the collection continued from July to November 2014 using gill nets and anglings. The fish samples were caught in Embaloh (124’31.6”N -119’18.3”S and 11223’44”-11229’36.8”E) and Sibau (120’33.6”N -102’39.8”S and 11253’23”- 11315’08.1” E) watersheds in the Betung Kerihun National Park, West Kalimantan Province, Indonesia. The total length (TL) of the fish was taken on a fish measuring board to the nearest milimeter. The fish samples were weighed in terms of total weight (W) using digital balance. The fish samples were then dissected, sexed and the gonads were removed and weighed to an accuracy of 0.01 g.
Based on macroscopic and microscopic observations, four stages of testicular maturation were distinguished for H. bimaculata i.e. immature or resting, maturing, mature and spent. The ovaries development were classified into five stages: immature or resting, maturing, mature, ripe, and spawned-recovering. Histological analysis of the ovarian development indicated that H. bimaculata exhibits synchronous group, in that at least two groups (cluthches) of oocytes can be distinguished at the same time during the reproductive cycle. The presence of different oocytes development sizes in the spawned and recovering stage and polymodal distribution of oocyte diameter in ripe stage of this fish might indicate a batch spawner.
iv
prolonged spawning season, being restricted mainly from late July to October, but some spawning may also occur in early November. Fecundity of the fish was relatively high, which the relationships between fecundity and the parameters under study showed the fecundity is significantly correlated to the total length and total body weight.
The estimated H. bimaculata population in Sibau watershed was relatively abundant. Based on distribution of each maturity stage at the stations, the potential spawning sites of the fish were in the upper Menjakan river and the upper Apeyang river. The water velocity had positive effects, and while water depth had a negative effect on H. bimaculata based on analysis of linier model.
This study also exhibited that H. bimaculata have developed different reproductive strategies on various aspects of reproduction with H. macrolepidota, including size at first maturity, spawning type, spawning season and fecundity. These strategies were carried out as adaptation on environmental factors.
.
v
RINGKASAN
FX. WIDADI PADMARSARI SOETIGNYA. Biologi Reproduksi Ikan Endemik Borneo Hampala bimaculata (Popta, 1905). Dibimbing oleh BAMBANG SURYOBROTO, MOHAMMMAD MUKHLIS KAMAL, dan ARIEF BOEDIONO
Hampala bimaculata merupakan salah satu ikan endemik di Borneo. Ikan ini bernilai komersial sebagai ikan konsumsi dan belakangan populer untuk sport fishing. Ikan ini umumnya ditemukan di sungai berarus deras dengan kondisi perairan yang jernih dengan substrat beragam dari pasir sampai batu-batuan. Terbatasnya penyebaran dari populasi ikan ini menyebabkan sedikit informasi yang sudah dipelajari termasuk informasi tentang biologi reproduksinya. Kajian biologi reproduksi sangat bermanfaat dalam pengembangan budidaya dan manajemen konservasinya. Tujuan dari studi ini adalah mendiskripsikan dan menentukan aspek-aspek reproduksi dari H. bimaculata yang meliputi perkembangan gonad, tipe organisasi ovarium, tipe pemijahan, seks ratio, ukuran pertama kali matang kelamin, musim pemijahan, dan fekunditas. Studi ini juga mengestimasi besarnya populasi dan menentukan lokasi-lokasi yang berpotensi sebagai tempat pemijahan dari H bimaculata di perairan Taman Nasional Betung Kerihun.
Sebanyak 181 individu dikoleksi dari bulan Februari sampai Oktober 2013 dan dilanjutkan dari Juli sampai November 2014 dengan menggunakan jaring insang dan pancing. Sampel ikan diperoleh dari DAS Embaloh (124’31.6”N -119’18.3”S dan 11223’44”-11229’36.8”E) dan DAS Sibau (120’33.6”N -102’39.8”S dan 11253’23”- 11315’08.1” E) yang termasuk kawasan Taman Nasional Betung Kerihun National, Propinsi Kalimantan Barat, Indonesia. Semua sampel yang diperoleh diukur panjang total (mm), berat total, dan dibedah serta dilakukan pengukuran berat gonad dan berat hati. Panjang total (TL) ikan diukur dengan ketelitian 1 milimeter. Sampel ikan juga diukur berat total (W) dengan timbangan digital. Selanjutnya sampel ikan dibedah, ditentukan jenis kelamin dan diambil gonadnya untuk ditimbang dengan ketelitian sampai 0.01 g.
Berdasarkan analisis makroskopik dan mikroskopik, tahapan kematangan testis H. bimaculata dibedakan menjadi 4 tahap yaitu tidak masak (immature) atau masa istirahat (resting), permulaan masak (maturing), masak (mature) dan salin (spent), sementara tahapan kematangan ovarium diklasifikasikan dalam 5 tahap yaitu masak immature atau atau masa istirahat (resting), permulaan masak (maturing), masak (mature), bunting (ripe), dan mijah-salin ( spawned-recovering). Analisis histologi dari perkembangan ovarium mengindikasikan bahwa H. bimaculata termasuk synchronous group, sedikitnya terdapat dua grup oosit yang dapat ditemukan pada waktu yang sama selama musim reproduksi. Berdasarkan analisis histologi tingkat kematangan ovarium dan pola distribusi oosit pada tahap bunting (ripe), tipe pemijahannya adalah berpijah bertahap (batch spawner).
vi
pada populasi secara keseluruhan maupun pada populasi ikan yang sudah matang kelamin (tahap III dan IV). Ukuran pertama kali matang kelamin pada ikan H. bimaculata diperkirakan 375.7 mm untuk jantan dan 450.9 mm untuk betina. Analisis hubungan antara panjang dan berat ikan menunjukkan bahwa pola pertumbuhan ikan jantan adalah alometrik (b = 2.73) dan ikan betina isometrik (b=2.98). H. bimaculata di perairan Taman Nasional Betung Kerihun mempunyai musim pemijahannya berkepanjangan dan terutama berlangsung pada akhir bulan Juli sampai Oktober, tetapi sebagian kecil juga terjadi pada awal November. Fekunditasnya relatif tinggi. Hubungan antara fekunditas absolut dan fekunditas relatif secara signifikan berkorelasi terhadap panjang total dan berat total.
Populasi ikan H. bimaculata di perairan Sibau adalah relatif tinggi. Distribusi menurut stadia kematangan pada masing-masing stasiun menunjukkan lokasi pemijahan di perairan DAS Sibau adalah di bagian hulu dari Sungai Menjakan dan Sungai Apeyang. Hasil analisis model linier menunjukkan kelimpahan berkorelasi secara positif terhadap faktor kecepatan arus dan berkorelasi negatif terhadap kedalaman perairan.
Studi ini juga menunjukkan bahwa ikan H. bimaculata di perairan Betung Kerihun National Park telah mengembangkan strategi reproduksi yang berbeda dengan ikan H. macrolepidota dari berbagai aspek reproduksi yaitu ukuran pertama kali matang kelamin, tipe pemijahan, dan musim pemijahan. Strategi ini diduga dilakukan dalam upaya eksistensi dari faktor lingkungannya yang ekstrim di alam.
vii
Copyright © 2016. Bogor Agricultural University.
All Rights Reserved
Prohibited to cite all or a part of this dissertation without referring to and mentioning the source. Citation permits to the purpose of education, research, scientific paper, report, or critism writing only; and it does not defame the name and honor of Bogor Agricultural University.
viii
ix
REPRODUCTIVE BIOLOGY OF BORNEAN ENDEMIC
FISH
Hampala bimaculata
(Popta, 1905)
FX. WIDADI PADMARSARI SOETIGNYA
Dissertation
submitted in partial fulfillment of the requirements for Doctoral Degree
In
Major of Animal Bioscience
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
x
Examiners in the closed examination: Prof Dr Ir Ridwan Affandi DEA
Prof Dr Endi Setyadi Kartamihardja MSc
Examiners in the open examination: Dr Ir Toni Ruchimat M.Sc
xi
Title : Reproductive Biology of Bornean Endemic Fish Hampala bimaculata (Popta,1905)
Name : FX. Widadi Padmarsari Soetignya Student ID : G362110031
Major : Animal Bioscience
Certified by: Supervisory Committee
Dr Drs Bambang Suryobroto Chair
Dr Ir Mohammad Mukhlis Kamal MSc Prof Drh Arief Boediono PhDPAVet(K)
Member Member
Acknowledged by
Chair of Animal Biosciences Dean of Graduate School
Dr Ir RR Dyah Perwitasari MSc Dr Ir Dahrul Syah MScAgr
xiii
FOREWORD
With the blessing of God Almighty, I am able to finish my dissertation. This paper is made to fulfill the requirement for doctoral degree at Bogor Agricultural University. The title of my paper is “Reproductive Biology of Bornean Endemic Fish Hampala bimaculata (Popta, 1905)”.
I would like to thank my advisors committee Dr Bambang Suryobroto, Dr Ir Mohammad Mukhlis Kamal MSc and Prof drh Arief Boediono PhD, PAVet (K) for the willingness to help, for their reviews of my dissertation and their helpful suggestions. I would like to thank my examiners Prof Dr Ir Ridwan Affandi DEA, Prof Dr Endi Setiadi Kartamiharja MSc and Dr. Ir Toni Ruchimat MSc; to the Chair of Animal Biosciences, Dr Ir RR Dyah Perwitasari MSc. I am indebted to Directorate General of Higher Education (DIKTI), Indonesia for the Graduate Scholarship (BPPS) and Management of Betung Kerihun National Park permitted me to research in Embaloh and Sibau Watersheds.
I thank you to Dean of Faculty of Agriculture at Tanjungpura University, for giving me the opportunity to finish my doctorate study. I would also like to thank those who helped me on the field and in laboratory work, Marcel Alvery Aldis, Mrs Retno, staff of Histopathology Laboratory, Faculty of Veterinary Medicine, Physiology and Pharmacology IPB , WWF Kalimantan Barat, Albertus Tjiu, Rachmat Hafiz Alkadri, Zulkifli and field team whose angling skills proved vital during collection of samples, and Sonja Steinacher for helping in field and correcting my article, Jesus Nunez for discussing gonad development.
I would like to thank the many undergraduate and graduate students for their support and friendship: Siti Ifadatin, Yuliadi Zamroni, Sri Catur R., Andi Darmawan, Maria Ulfah, Fitria Basalamah, Jusmaldi, Keliopas Krey, Eneng Nunuz, Yuni Cahya E, Novece Tantri, Mega and Astrid.
Finally, and most importantly, I express my gratitude to my parent, my sisters, the big family in Surabaya for their love, unfailing encouragement and support; dr Desak, dr Yan, and dr Yuda, who have taken care for the treatment, the best my friend, Mike who supported, and also helped me in writing articles.
May this paper is use full for all who study on Hampala bimaculata and reproductive biology.
Bogor, September 2016
xv
TABLE OF CONTENT
FOREWORD xiii
TABlE OF CONTENT xv
LIST OF TABLES xvi
LIST OF FIGURES xvii
LIST OF APPENDICES xviii
LIST OF ABBREVIATIONS xix
1 GENERAL INTRODUCTION 1
2 GONAD DEVELOPMENT OF Hampala bimaculata (Popta 1905)
4
Abstract 4
Introduction 4
Material and Methods 5
Results 6
Discussion 13
3 SEX RATIO, SPAWNING, AND FECUNDITY IN Hampala bimaculata (Popta, 1905) FROM BETUNG KERIHUN NATIONAL PARK
15
Abstract 15
Introduction 15
Material and Methods 16
Results 17
Discussion 23
Appendix 27
4 POPULATION AND POTENTIAL SPAWNING SITES OF Hampala bimaculata (Popta 1905) FROM BETUNG KERIHUN NATIONAL PARK
29
Abstract 29
Introduction 29
Material and Methods 30
Results 32
Discussion 35
Appendix 37
5 GENERAL DISCUSSION 41
6 CONCLUSIONS 44
REFERENCES 45
xvi
LIST OF TABLES
Table 2.1 Description of the testicular maturity stages of H. bimaculata
7 Table 2.2 Description of the ovarian maturity stages of H.
bimaculata
9 Table 3.1 Sex ratio of H. bimaculata samples during the study period 18 Table 3.2 Chi-square test for H. bimaculata sex ratio comparisons by
size class in Embaloh and Sibau Watersheds
18 Table 3.3 Ranges of total length, GSI and HSI for H. bimaculata
during sampling periods.
20 Table 3.4 Correlation coefficients r and regression equations for
relationships between absolute fecundity, relative fecundity and total body length, total body weight, gonade weight and GSI of H. bimaculata
23
Table 4.1 Summary of characteristics for each the station in sampling areas
31 Table 4.2 Chi-square for mature males and females H. bimaculata 32 Table 4.3 Catch rate of the fish caught by angling at each the three
sampling sites during sampling periods
32 Table 4.4 NPUE and relative abundance of the fish caught by gill
nets in sampling areas
xvii
LIST OF FIGURES
Figure 1.1 Geographic distribution of genus Hampala (Doi and Taki
1994) 2
Figure 1.2 Hampala in Betung Kerihun National Park A.Hampala
bimaculata B. H. macrolepidota 2 Figure 2.1 Study sampling area in Embaloh and Sibau Watersheds 6 Figure 2.2 Anatomy of the testicles of H. bimaculata 7 Figre 2.3 Histological apparance of the testicles of H. bimaculata 8
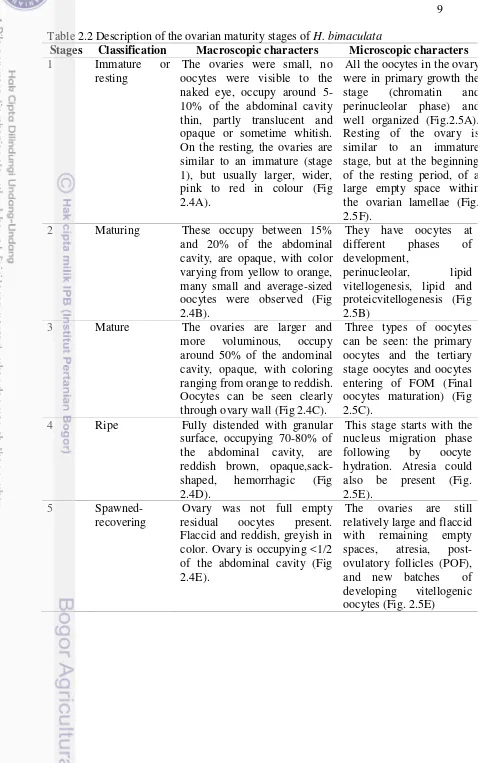
Figure 2.4 Anatomy of the ovaries in H. bimaculata 10
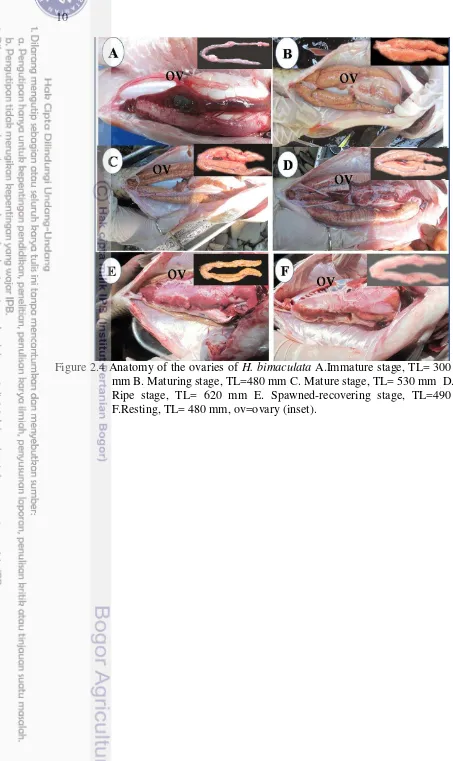
Figure 2.5 Histological apparance of the ovaries of Hampala
bimaculata. 11
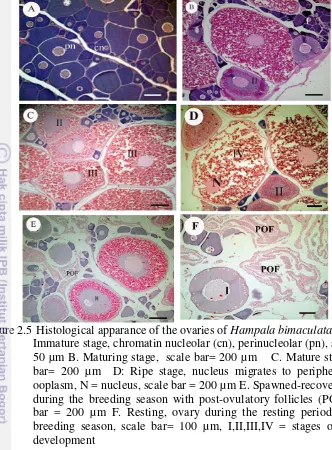
Figure 2.6 Histological apparance of the oocytes development stages of
Hampala bimaculata 12
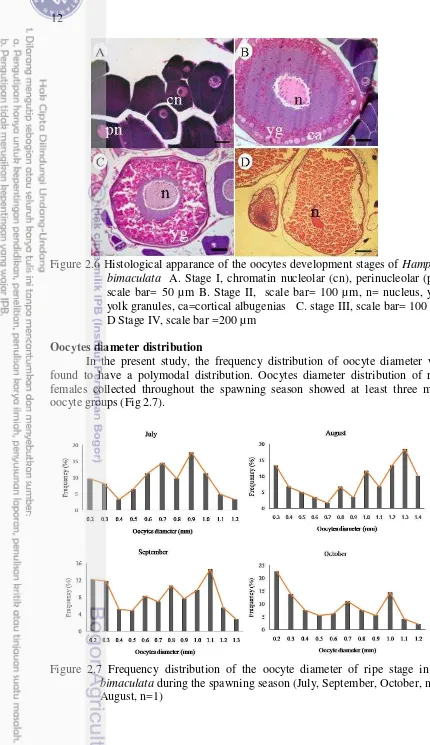
Figure 2.7 Frequency distribution of the oocyte diameter of ripe stage
in H. bimaculata during the spawning season 12
Figure 3.1 Size at first maturity in males and females H. bimaculata 19 Figure 3.2 Total length frequency distribution of each maturity stage for
males and females H. bimaculata
19 Figure 3.3 Monthly changes in GSI mean of males and females H.
bimaculata
20 Figure 3.4 Monthly changes in HSI mean of males and females H.
bimaculata
21 Figure 3.5 Proportion of oocytes in the stages of primary growth,
cortical alveoli and vitellogenesis in Hampala. bimaculata in
different months of the ovarian cycle 21
Figure 3.6 Seasonal changes frequency of each the maturity stages of
both sexes in H. bimaculata 22
Figure 4.1 Sampling area in Sibau Watershed 31
Figure 4.2 Distribution of each maturity stage for population males and females H. bimaculat using angling method
xviii
LIST OF APPENDICES
Appendix 3.1 The length-weight relationship described with linear
function for male and female H. bimaculata 27 Appendix 3.2 Relationship between the absolute fecundity and body size
(total length and total weight) with linear function of H.
bimaculata 27
Appendix .3.3 Relationship between the relative fecundity and body size (total length and total weight) with linear function of H.
bimaculata 28
Appendix 3.4 Monthly rainfall (mm) data of Benua Martinus site (close to
sampling area) for 2013-2014 28
Appendix 4.1 Photos of the sampling sites 37
Appendix 4.2 Photos of fish species caught in Sibau watershed during sampling periods
xix
LIST OF ABBREVIATIONS
CPUE = catch per unit effort DO = dissolved oxygen Fa = absolute fecundity
Fr = relative fecundity
GSI = gonadosomatic index HSI = hepatosomatic index
IUCN = International Union for Conesrvation of Nature L = length
M = Menjakan river
NPUE = number per unit effort Ov = ovary
POF = post-ovulatory follicles Sg = spermatogonium
Sp = spermatid Sz = spermatozoid Ts = testicle
W= total body weight WG = gonad weight
1
1
GENERAL INTRODUCTION
Background
Reproductive biology is a study mainly involving the reproductive system and sex organs. Reproductive success is what enables a species to persist and is thus important to some populations. To maximize the likelihood of success, reproductive strategies differ greatly among fish species due to differences in environmental constrains and evolution history (Lowerre-Barbieri et al. 2011). Fishes display great diversity in reproductive strategies and associated traits (Helfman 1997) such as breeding system, spawning type, and spawning season. Most oviparous fishes lay many small eggs, typically less than 1 mm in diameter. Parental care varies greatly among fish species, while others display no parental care (Miller and Kendall 2009). The majority of fish species are considered iteroparous, spawning many times during their life span; while, few species spawn once and then die.
Several studies have described the reproductive biology of Cyprinids for example Garra ruffa (Abedi et al. 2011), Puntius denisonii (Solomon et al. 2011), Schizothorax o’connori (Ma et al. 2012), Labeobarbus batesii (Tiogue 2013). The review compiled information on such characteristics as maturity, fecundity, oocyte size, size at first maturity, and spawning habits. Several studies have also exhibited the reproductive biology for endemic fishes for example Rasbora tawarensis, an endemic to Lake Laut Tawar, Aceh, Indonesia (Muchlisin et al. 2010), Chela fasciata Silas,an endemic to the Western Ghats of Kerala (Divipala et al. 2013). However, the knowledge of reproductive biology haven’t been yet described to the Bornean endemic, Hampala bimaculata. On the other hand, few studies have investigated the reproductive biology of the widely spread form, H. macrolepidota (Abidin 1986, Zakaria et al. 2000, Uslichah and Syandri 2003, Intan et al. 2013). Observation on the spawning of H. macrolepidota in Zoo Negara Lake, Malaysia showed that it can spawn throughout the year; however, physicochemical and environmental factors determine the actual spawning time of this species. Spawning is protracted and coincides with the rainy season which extends from November to March the following year (Abidin 1986). Meanwhile, the main spawning periods of this fish in Kenyir Lake, Malaysia was protracted in February and the size at first sexually maturity was 160 mm (Zakaria et al. 2000).
2
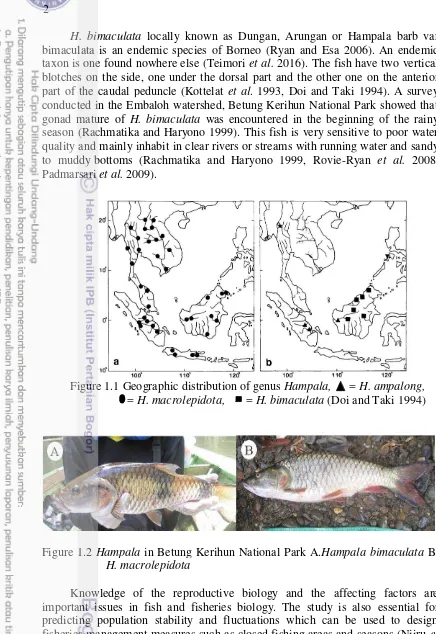
H. bimaculata locally known as Dungan, Arungan or Hampala barb var bimaculata is an endemic species of Borneo (Ryan and Esa 2006). An endemic taxon is one found nowhere else (Teimori et al. 2016). The fish have two vertical blotches on the side, one under the dorsal part and the other one on the anterior part of the caudal peduncle (Kottelat et al. 1993, Doi and Taki 1994). A survey conducted in the Embaloh watershed, Betung Kerihun National Park showed that gonad mature of H. bimaculata was encountered in the beginning of the rainy season (Rachmatika and Haryono 1999). This fish is very sensitive to poor water quality and mainly inhabit in clear rivers or streams with running water and sandy to muddy bottoms (Rachmatika and Haryono 1999, Rovie-Ryan et al. 2008, Padmarsari et al. 2009).
Figure 1.1 Geographic distribution of genus Hampala, = H. ampalong, = H. macrolepidota, = H. bimaculata (Doi and Taki 1994)
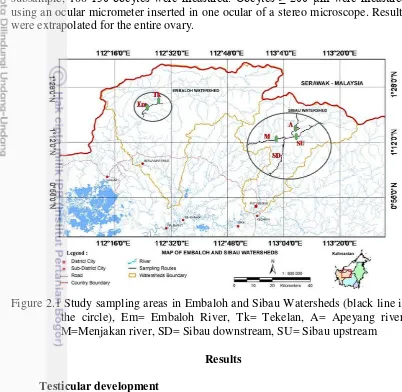
Figure 1.2 Hampala in Betung Kerihun National Park A.Hampala bimaculata B. H. macrolepidota
4
2
GONAD DEVELOPMENT OF
Hampala bimaculata
(Popta, 1905)
Abstract
Hampala bimaculata is an endemic species of Borneo, where it is a food fish, being popular sport fishing as well; however, little is known of the important aspects of its reproductive to assist with fisheries management planning. The objective of this study was to describe gonad development H. bimaculata macroscopically and microscopically. The fish samples were collected from February to October 2013 and the collection continued from July to November 2014 by using gill nets and anglings. Sampling sites in Betung Kerihun National Park included Embaloh and Sibau Watersheds. Analysis of macroscopic and histological observation showed that the testicles were classified into four stages: immature, maturing, mature and spent. The ovaries were classified into five stages: immature or resting, maturing, mature, ripe, and spawned-recovering. Histological observation revealed that the fish is an iteroparous with a group-synchronous of ovarian development. The presence of different oocytes development sizes in the spawned-recovering stage and the polymodal distribution of oocyte diameter in the ripe stage might indicate H. bimaculata being a batch spawner.
Keywords: reproductive biology; group synchronous; batch spawner Introduction
5 Domagała, 2003). Nunez and Duponchelle (2009) introduced male maturity stages into four stages.
Little information exists on the reproductive biology of the genus Hampala, especially concerning the gonad development. Abidin (1986), Uslichah and Syandri (2003), studied the reproduction of H. macrolepidota including gonad development and spawning. Meanwhile, studies on gonads development of H. bimaculata have not been yet carried out. Therefore, the objective of the study was to describe the gonad development, investigating macro and microscopic characteristics to determine gonad maturity stages of H. bimaculata. We also determined spawning type by using histological observation and distribution of oocytes at ripe stage. This gonad development investigation is conducted for the first time on this species in Betung Kerihun National Park.
Material and Methods
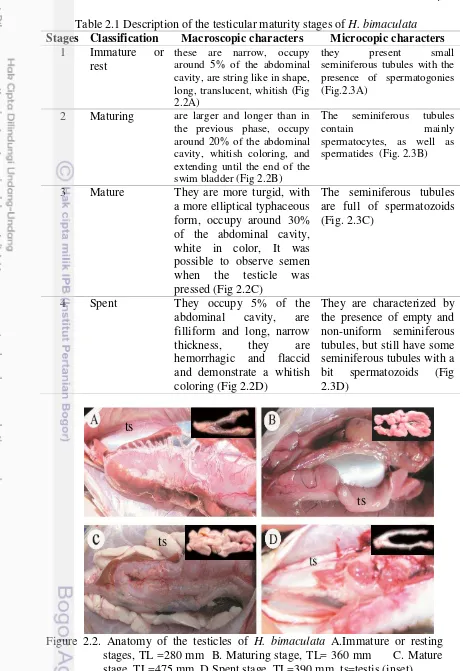
The fish specimens were collected from Embaloh (124’31.6”N -119’18.3”S and 11223’44”-11229’36.8”E) and Sibau (120’33.6”N -102’39.8”S and 11253’23”- 11315’08.1” E) watersheds in Betung Kerihun National Park, West Kalimantan Province. Embaloh Watershed consists of rivers and tributaries, which Embaloh River flows south as long as ±130 km. In this study, there are two stations in Embaloh watershed i.e Embaloh river (Em), and Tekelan river (Tk). Meanwhile, Sibau Watershed consists of four stations i.e. Sibau upstream (SU), Apeyang river (A), Menjakan river (M), and Sibau downstream (SD). Both Embaloh and Sibau Watersheds connect to Kapuas River (Fig 2.1).
Fish samplings were carried out from February to October 2013 and the collection continued from July to November 2014 using gill nets and anglings. Gill nets used in each river consisted of three units (1 and 1.5 inch mesh size each). The gill nets were positioned horizontally to the mouth of the river at 18.00 and left in place until 6.00 the following morning (a total of 12 hours). Sampling by using four anglings were done along up to downstream of from 8.00 to 12.00 and continued from 13.00 to 16.00 (a total ~6 hours). The fish samples were measured in terms of both total length (TL) to nearest 1 mm, and body weight (W) was weighed to the nearest gram using digital balance. Gonads were weighed to an accuracy of 0.01 g and subsequently, the fish was dissected to determine sex of fish and stage of maturity.
6
The spawning type was determined by histological observation of the ovaries and frequency of oocytes diameter. To determine the frequency distribution of oocyte diameters in H. bimaculata, is randomly distributed in the ovary, three sub-samples from ripe stage ovaries were taken from three sites of the right lobe of the ovary (anterior, middle and posterior ) (Almatar et al. 2004). For subsample, 100-150 oocytes were measured. Oocytes ≥ 200 µm were measured using an ocular micrometer inserted in one ocular of a stereo microscope. Results were extrapolated for the entire ovary.
Figure 2.1 Study sampling areas in Embaloh and Sibau Watersheds (black line in the circle), Em= Embaloh River, Tk= Tekelan, A= Apeyang river; M=Menjakan river, SD= Sibau downstream, SU= Sibau upstream
Results
Testicular development
The observations in this study revealed that, the testes of H. bimaculata are paired organs, elongated structure, rather equal in size, but occasionally one is slightly shorter than the other. The testes are located lying dorsally in the abdominal cavity. Each of testes consists of numerous of thin-wall lobules and spermatogenesis occurs in nests within the lobule. During the breeding season, the lobules of mature male fish become greatly distended with spermatozoa.
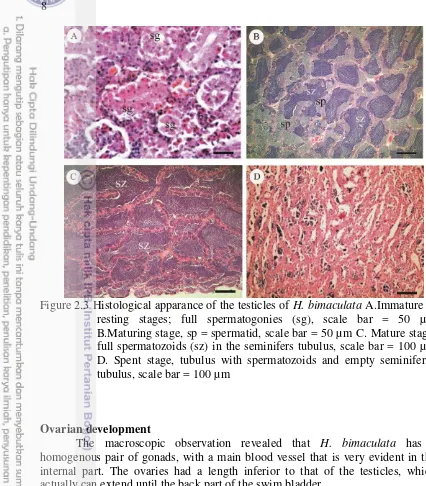
7 Table 2.1 Description of the testicular maturity stages of H. bimaculata Stages Classification Macroscopic characters Microcopic characters
1 Immature or rest
these are narrow, occupy around 5% of the abdominal cavity, are string like in shape, long, translucent, whitish (Fig 2.2A)
they present small seminiferous tubules with the presence of spermatogonies (Fig.2.3A)
2 Maturing are larger and longer than in the previous phase, occupy around 20% of the abdominal cavity, whitish coloring, and extending until the end of the swim bladder (Fig 2.2B)
The seminiferous tubules
contain mainly
spermatocytes, as well as spermatides (Fig. 2.3B)
3 Mature They are more turgid, with a more elliptical typhaceous form, occupy around 30% of the abdominal cavity, white in color, It was possible to observe semen when the testicle was pressed (Fig 2.2C)
The seminiferous tubules are full of spermatozoids (Fig. 2.3C)
4 Spent They occupy 5% of the abdominal cavity, are filliform and long, narrow thickness, they are hemorrhagic and flaccid and demonstrate a whitish coloring (Fig 2.2D)
They are characterized by the presence of empty and non-uniform seminiferous tubules, but still have some seminiferous tubules with a bit spermatozoids (Fig 2.3D)
t
s
ts
ts
8
[image:30.595.45.471.59.545.2]
Figure 2.3 Histological apparance of the testicles of H. bimaculata A.Immature or resting stages; full spermatogonies (sg), scale bar = 50 µm B.Maturing stage, sp = spermatid, scale bar = 50 µm C. Mature stage, full spermatozoids (sz) in the seminifers tubulus, scale bar = 100 µm D. Spent stage, tubulus with spermatozoids and empty seminiferus tubulus, scale bar = 100 µm
Ovarian development
The macroscopic observation revealed that H. bimaculata has a homogenous pair of gonads, with a main blood vessel that is very evident in the internal part. The ovaries had a length inferior to that of the testicles, which actually can extend until the back part of the swim bladder.
9 Table 2.2 Description of the ovarian maturity stages of H. bimaculata
Stages Classification Macroscopic characters Microscopic characters
1 Immature or
resting
The ovaries were small, no oocytes were visible to the naked eye, occupy around 5-10% of the abdominal cavity thin, partly translucent and opaque or sometime whitish. On the resting, the ovaries are similar to an immature (stage 1), but usually larger, wider, pink to red in colour (Fig 2.4A).
All the oocytes in the ovary were in primary growth the stage (chromatin and perinucleolar phase) and well organized (Fig.2.5A). Resting of the ovary is similar to an immature stage, but at the beginning of the resting period, of a large empty space within the ovarian lamellae (Fig. 2.5F).
2 Maturing These occupy between 15% and 20% of the abdominal cavity, are opaque, with color varying from yellow to orange, many small and average-sized oocytes were observed (Fig 2.4B).
They have oocytes at different phases of development,
perinucleolar, lipid vitellogenesis, lipid and proteicvitellogenesis (Fig 2.5B)
3 Mature The ovaries are larger and more voluminous, occupy around 50% of the andominal cavity, opaque, with coloring ranging from orange to reddish. Oocytes can be seen clearly through ovary wall (Fig 2.4C).
Three types of oocytes can be seen: the primary oocytes and the tertiary stage oocytes and oocytes entering of FOM (Final oocytes maturation) (Fig 2.5C).
4 Ripe Fully distended with granular surface, occupying 70-80% of the abdominal cavity, are reddish brown, opaque,sack-shaped, hemorrhagic (Fig 2.4D).
This stage starts with the nucleus migration phase following by oocyte hydration. Atresia could also be present (Fig. 2.5E).
5 Spawned-
recovering
Ovary was not full empty residual oocytes present. Flaccid and reddish, greyish in color. Ovary is occupying <1/2 of the abdominal cavity (Fig 2.4E).
The ovaries are still relatively large and flaccid with remaining empty spaces, atresia, post-ovulatory follicles (POF), and new batches of developing vitellogenic oocytes (Fig. 2.5E)
10
11
Figure 2.5Histological apparance of the ovaries of Hampala bimaculata A. Immature stage, chromatin nucleolar (cn), perinucleolar (pn), scale bar= 50 µm B. Maturing stage, scale bar= 200 µm C. Mature stage, scale bar= 200 µm D: Ripe stage, nucleus migrates to periphery of the ooplasm, N = nucleus, scale bar = 200 µm E. Spawned-recovering stage during the breeding season with post-ovulatory follicles (POF), scale bar = 200 µm F. Resting, ovary during the resting period after the breeding season, scale bar= 100 µm, I,II,III,IV = stages of oocytes development
12
Figure 2.6Histological apparance of the oocytes development stages of Hampala bimaculata A. Stage I, chromatin nucleolar (cn), perinucleolar (pn), scale bar= 50 µm B. Stage II, scale bar= 100 µm, n= nucleus, yg= yolk granules, ca=cortical albugenias C. stage III, scale bar= 100 µm D Stage IV, scale bar =200 µm
Oocytes diameter distribution
In the present study, the frequency distribution of oocyte diameter was found to have a polymodal distribution. Oocytes diameter distribution of ripe females collected throughout the spawning season showed at least three main oocyte groups (Fig 2.7).
[image:34.595.80.470.375.820.2]13 Discussion
Identification and classification of macroscopic maturity stages plays a key role in the assessment of fishery resources. For many species, it is not possible to distinguish immature and resting macroscopically (Saborido-Rey and Junquera 1998, Ferreri et al. 2009). Likewise, it was observed for H. bimaculata. In both stages the oocytes are not visibleand only by histological analyses it is possible to identify each stage accurately. This misclassification has an impact on the estimation of the mature proportion of the stock because resting stage have already contributed to the spawning biomass of that year and are macroscopically considered immature. The maturity stages are used to determination of spawning activity.
Histological analysis of ovarian development indicated that H. bimaculata exhibits synchronous group (Figs 2.5C, 2.5D), in that at least two group (cluthches) of oocytes can be distinguished at the same time during the reproductive cycle (Blazer 2002, Albieri et al. 2010). At the ripe stage, the major part of the ovary was occupied by stage IV oocytes that comprise a synchronous population of larger oocytes, defined as a clutch. However, a large number of previtellogenic and vitellogenic oocytes (stages I, II and III) were also detected among the mature oocytes. After spawning, the ovaries of H. bimaculata contained primary growth, cortical alveoli and vitellogenesis stages (Fig. 2.5E).
The spawning type is the way how females release mature oocytes in a reproductive period. Histological observations of the spawned-recovering stage (Fig 2.5E) showed that, the post-ovulatory follicles occurred together with a heterogenous population of secondary developing oocytes in the ovaries of the reproductively active females. This strongly suggests that H. bimaculata spawns multiple times in a season. According to Honji et al. (2006), Chen and Tzeng (2009), Nunez and Duponchelle (2009), the presence of development oocytes of different sizes in spawned stage characterizes the beginning of one or more new spawning cycles until the end of the breeding season which differs at multiple spawners from single spawners. During the breeding season the polymodal distribution of oocyte diameter was found in ripe stage, this may validate H. bimaculata being a batch spawner. Thus, the ovarian cycle in H. bimaculata can described as below: the ovarian cycle can start either with immature females, which enter the cycle for the first time by reaching sexual maturity, or with adult resting females, which restart the cycle by entering the developing stage at the beginning of each spawning season. After spawning, the ovaries of spawned stage contain new batches of developing vitellogenic oocytes and they will evolve directly into the maturing stage to initiate a new spawning cycle. If spawning is completed (may two or more spawning events), their ovaries will evolve into resting stage until the next breeding season.
14
reproductive strategy include reduction in larval crowding and decreased impact of predation and unfavorable environmental conditions on eggs and larvae (McEvoy and McEvoy 1992). We suggested that batch spawning of H. bimaculata should be an adaptive strategy to the habitat conditions, where its habitat in running waters has the environmental factors are quite dynamics and may also related to the abundance of zooplankton as main food for juvenils of H. bimaculata.
15
3
SEX RATIO, SPAWNING AND FECUNDITY IN
HAMPALA
BIMACULATA
(POPTA, 1905) FROM BETUNG KERIHUN
NATIONAL PARK
Abstract
The sex ratio, spawning and fecundity in Hampala bimaculata, an endemic Cyprinid of Borneo were studied by examining 181 individuals collected monthly between February and October 2013 and continued bimonthly between July and November 2014 using gill nets and anglings in Betung Kerihun National Park, West Kalimantan Province. The overall sex ratio was 1.00:2.23 males to females shows significant deviation from the expected 1: 1 (p<0.05). Females seem to be dominant compared with males. The size at first maturity of males were estimated to be 375.7 mm and 450.9 mm in TL for females. Based on the three methods, H. bimaculata in the waters of Betung Kerihun National Park has a prolonged spawning season, being restricted mainly from August to October, some spawning may also occur in late July and early November. The relationships between both in absolute as well as in relative fecundity and the parameters under study showed the fecundity significantly correlated to total length and total body weight. This study presents new original data on basic traits of the natural history of H. bimaculata. The observed results can be used to assist the management strategies and conservation of the species and its habitat.
Key words: the gonadosomatic index, spawning, maturity stages, sex ratio Introduction
Fishes exhibit great diversity in reproductive strategies and associated traits (Helfman 1997) such as breeding system, spawning type, and spawning season. The spawning strategy adopted by a fish species is the combination of a range of adaptive traits that tends to maximize the number of offspring production, where, such adaptive traits include the frequency and timing of spawning (Nunn et al. 2007). The spawning season in fishes is influenced by various factors (Khaironizam and Zakaria-Ismail 2013), which, the condition of the gonad is an indicator of the spawning season. Histological investigation of ovaries and determination of proportions of oocytes in different stages of development is an appropiate method for the description of spawning characteristics of fish species (Lefler et al. 2003).
16
fecundity may vary as a result of different adaptations to environmental habitats (Whittames et al. 1995), even whitin stock, fecundity is known to vary annually (Kjesbu et al. 1998). It has been showed to be proportional to fish size and condition (Murua et al. 2003). Larger fish have more high fecundity, both in absolute as well as in relative fecundity. For a given size, females in better condition display higher fecundity (Kjesbu et al. 1991).
Many studies of spawning and fecundity in Cyprinids have been reported by Abidin and Kok Jee (1984), Ali and Kadir (1996), Alam and Pathak (2010), Abedi et al. (2011), Ma et al. (2012), Domagala et al. (2015); however, it has not been documented yet in H. bimaculata. To estimate fecundity of population is required data on sex ratio, therefore, the main objectives of the present study were to describe and determine sex ratio, spawning and fecundity of H. bimaculata from the waters of Betung Kerihun National Park.
Material and methods Material and methods
A total of 181 idividuals of H. bimaculata were collected using gill nets and anglings from Embaloh (124’31.6”N-119’18.3”S and 11223’44” -11229’36.8”E) and Sibau (120’33.6”N-102’39.8”S and 11253’23”- 11315’08.1” E) watersheds in Betung Kerihun National Park. The total length (TL) of the fish was taken on a fish measuring board to the nearest milimeter. The fish samples were weighed in term of total body weight (W) using digital balance. The fish samples were then dissected, sexed and the gonads were removed and weighed to an accuracy of 0.01 g. The spawning season was determined through the analysis of the relative frequencies of gonadal maturation stages, GSI calculations (Weng et al. 2005) and proportion of oocytes in the stages of primary growth, cortical alveoli and vitellogenesis in samples (Lefler et al. 2008).
Oocytes in the stages of primary growth, cortical alveoli and vitellogenesis were counted on histological sections using a light microscope. Ovarian samples collected at different times were compared. The proportions of oocytes in the three stages of development were analyzed as well as the change in the distribution of this proportion with the advance in the ovarian cycle (Lefler et al. 2008).
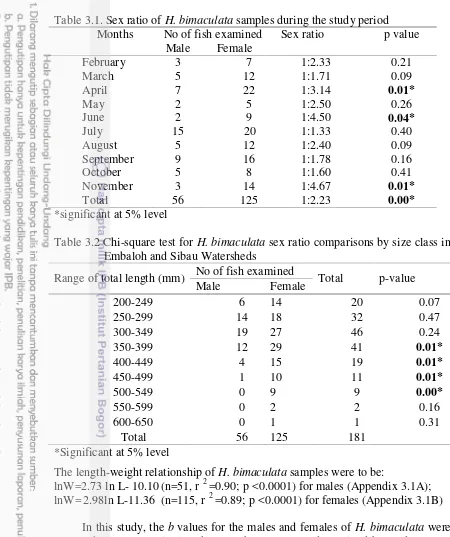
Fishes with gonad stages 3 and 4 were considered sexually mature (Kendall and Gray 2009). To estimate the size at first maturity, the total length was plotted against the frequency percentage at mature individuals during the spawning season and then the length at which 50% of the total individual number was consider as the size at first maturity (Shallof and Salama 2008).
The gonadosomatic index (GSI) was estimated as quaotient between the gonad weight and total body weight. GSI= 100WG W-1 where WG was the
gonad weight and W, the total body weight of the fish (Kendall and Gray 2009, Albierri et al. 2010, Hliwa et al. 2011). The hepatosomatic index (HSI) was also analyzed using following formula Thulashiva and Sivanshantini (2013). HSI = 100WL W–1 where: WL is the weight of liver and W is the total body weight of
the fish.
17 ovaries were taken and weighed (Weng et al. 2005). The total number of ripe eggs in the ovary is estimated by multiplying the number of ripe eggs in the sample by the ratio of the ovary weight to the sample weight, F = (ovary weight x number of eggs in the sub-sample) / sub-sample weight. The relative fecundity (Fr) was expressed by dividing the absolute fecundity (Fa) by the fish body weight. The result was the number of eggs per gram of body weight (Yeldan and Avsar 2000, Murua et al. 2003, Hossain et al. 2012).
The sex ratio was expressed as (the number of males)/(number of both sexes combined). The sex ratio was analyzed by Chi-square test, in order to verify whether the proportion of males and females differed from the expected ratio 1:1 (Lanes et al. 2012). The length-weight relationship was calculated using the expression: W= aLb, where the Wis the body weight in g and L is the total length. Parameter a and b were estimated by linier regression analysis based on natural logarithms: ln(W)= ln (a)+b ln (L) (Hossain et al. 2012). Values of the exponent b provide information on fish growth. When b =3 increase in weight is isometric and the value of b is other than 3, weight increase is allometric, (positive allometric if b>3, negative allometric if b<3). The null hypothesis is of the isometric growth (Ho, b=3) was tested by t-test, using statistic; ts =(b-3)×Sb-1., where Sb is the standard error of the slope, for =0.05 for testing significant differences among slope (b) between two regressions for the same species (Morey et al. 2003, Sangun et al. 2007). Linier regression analysis was used to determination relationship between fecundity and body size (total length, total body weight) (Hossain et al. 2012). The GSI and HSI data were analyzed by Kruskal-Wallis to determine significance of each data treatment. The all analysis of statistics were prepared using R program.
Results
Sex ratio
18
Table 3.1. Sex ratio of H. bimaculata samples during the study period Months No of fish examined Sex ratio p value
Male Female
February 3 7 1:2.33 0.21
March 5 12 1:1.71 0.09
April 7 22 1:3.14 0.01*
May 2 5 1:2.50 0.26
June 2 9 1:4.50 0.04*
July 15 20 1:1.33 0.40
August 5 12 1:2.40 0.09
September 9 16 1:1.78 0.16
October 5 8 1:1.60 0.41
November 3 14 1:4.67 0.01*
Total 56 125 1:2.23 0.00*
*significant at 5% level
Table 3.2 Chi-square test for H. bimaculata sex ratio comparisons by size class in Embaloh and Sibau Watersheds
Range of total length (mm) No of fish examined Total p-value
Male Female
200-249 6 14 20 0.07
250-299 14 18 32 0.47
300-349 19 27 46 0.24
350-399 12 29 41 0.01*
400-449 4 15 19 0.01*
450-499 1 10 11 0.01*
500-549 0 9 9 0.00*
550-599 0 2 2 0.16
600-650 0 1 1 0.31
Total 56 125 181
*Significant at 5% level
The length-weight relationship of H. bimaculata samples were to be:
lnW=2.73 ln L- 10.10(n=51, r 2 =0.90; p <0.0001) for males (Appendix 3.1A); lnW=2.98ln L-11.36 (n=115, r 2 =0.89; p <0.0001) for females (Appendix 3.1B)
19
Figure 3.1 Size at first maturity in males and females H. bimaculata
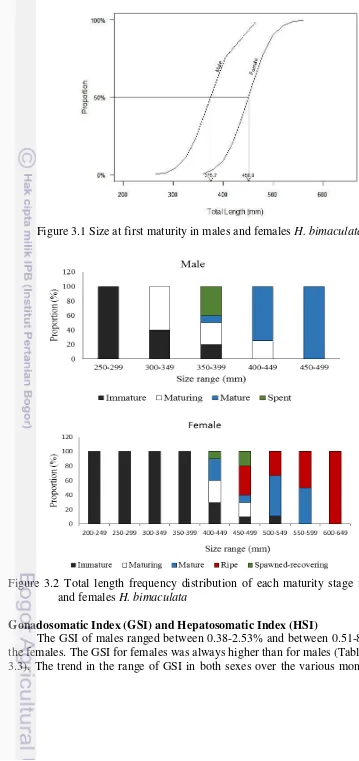
Figure 3.2 Total length frequency distribution of each maturity stage for males and females H. bimaculata
Gonadosomatic Index (GSI) and Hepatosomatic Index (HSI)
20
study period substantially confirms the result that June is the months when the fish start to breed. The trend in the mean of GSI over the various month during study period substantially confirms the result that June is the month when the fish start to breed. The GSI increased gradually and reached higher values during July to October. We observed a decreasing rapidly trend in the GSI value from November and steadily until May. Female GSIs which peaked from July and September were significantly different from the other months in pre-spawning season (February, March, April) (p<0.05). Male GSIs peaked in July and August were also significantly different from the other months in pre-spawning season (February, March, April, May) (p<0.05).
In this study, the HSI of males ranged 0.48-1.70 and 0.43-1.74 for females. The trend in the males HSI were low during July and October, meanwhile the trend of HSI females was low in July, September and October. It was opposite with the trend of GSI. Male HSIs in July and October were significantly different from April and May (p<0.05). Female HSI in July, September and October were significantly different from March (Table 3.3, Fig. 3.4).
Table 3.3 Ranges of total length, GSI and HSI for H. bimaculata during sampling periods
Months Range total length (mm)
GSI HSI
Male Female Male Female Male Female
February 220-300 230-390 0.55-0.60c 0.57-0.74c 0.82-1.00ab 1.05-1.37ab March 205-280 225-410 0.38-0.53c 0.51-0.80c 0.82-1.00ab 1.05-1.47a April 210-320 220-460 0.53-0.67c 0.54-0.88c 0.82-1.07a 0.85-1.46abc May 300 225-390 0.70-0.75c 0.58-0.83bc 1.04-1.07a 1.06-1.19abc June 310-320 270-430 0.77-0.85bc 0.77-1.45ab 0.77-0.85ab 0.77-1.38bc July 280-430 280-530 0.60-2.53a 0.64-7.22a 0.48-1.70b 0.52-1.42c August 300-410 330-570 0.89-2.50ab 0.80-5.15a 0.81-1.15ab 0.63-1.28bc September 260-390 280-620 0.67-1.89a 0.63-8.03a 0.81-1.06ab 0.43-1.74c October 250-475 300-480 0.63-2.22ab 0.75-3.02a 0.68-0.99b 0.70-1.50c November 260-390 250-490 0.71-1.14ab 0.73±1.04ab 0.91-0.93ab 0.69-1.09bc
Total
[image:42.595.101.453.564.737.2]*Mean of GSI and HSI in the same followed by a different superscript indicates significantly different (P < 0.05).
21
Figure 3.4 Monthly changes in HSI mean of males and females H. bimaculata (blue line= male, red line= female)
Proportion of oocytes stages
[image:43.595.105.494.42.842.2]Percentages of oocytes in different stages of development were compared in different periods of the ovarian cycle (Fig. 3.5). Between November and May only oocytes in the stage of primary growth were present in the ovaries. In the samples collected in June, the number of oocytes in the stage of primary growth was much higher than that of oocytes in the stages of cortical alveoli and vitellogenesis. In August, after first spawning the number of oocytes in the stages of primary growth and vitellogenesis was almost equal while cells in the stage of cortical alveoli were present only in small numbers. During July, September and October, the oocytes in the stage of vitellogenesis were in majority and the number of cells in the stage of cortical alveoli was relatively low.
[image:43.595.132.483.88.296.2]22
Changes in frequency maturity
[image:44.595.40.471.75.741.2]In this study, proportions of mature (mature for male, mature and ripe stages for female) were 10.71% (6 of 56 individuals) for males and 15.2% (19 of 125 individuals) for females (Fig. 3.6) during the breeding season between July to October. The immature or resting stage was encountered throughout the year. In June, the maturing stage was first observed. Percentage of mature increased during July to October. In August, spawned-recovering stage for female was first observed which extended until October. In September, spent stage of male was first observed which extended until early November. In November, all mature females have spawned completely, which they have evolved into resting stage.
Figure 3.6 Seasonal changes frequency of each the maturity stages of both sexes in H. bimaculata
Spawning season
23 and the relative frequency of different maturity stages (Fig. 3.6). The GSI appears that the fish go into a quiescent phase during the months November to May. In this period, both sexes have no mature, lower GSI and only oocytes in the stage of primary growth were found. Reproductive activity was restored in June, when the cortical alveoli stage and the maturing stage of fish were first observed. An increase in the GSI value in July indicates that is the intensive cell growth process start before spawning. The maximum values of the GSI is a marked increase in GSI values from July to October coinciding with those of high percentage of matured individuals and oocytes in the stage of vitellogenesis dominate in the ovaries that is indicated of a breeding season from July to October. First spawning activity occurred in August during first spawned stage of female. Thus, these three methods showed that H.bimaculata in the waters of Betung Kerihun National Park has a prolonged spawning season, being restricted mainly from August to October, some spawnings may also occur in late July and early November.
Fecundity
The absolute fecundity varied from a minimum of 30.640 to a maximum of 168.530 with a mean of 66.400 eggs, corresponding to fish total length from 420 to 620 mm. The relative fecundity, expressed as eggs g-1 of body weight of the female, displayed an interval, from a minimum of 25.53 eggs g-1 to a maximum of 94.94 eggs g-1. The relationships between absolute fecundity and the parameters under study showed that absolute fecundity significantly correlated to total length (r2 =0.68, p<0.0001) and total body weight (r2 =0.76, p<0.0001), (Appendices 3.2A and B). As well, analysis of regression showed that there were a significant relationship between relative fecundity (Fr) and total body length (r2 =0.47,
p<0.0001) and total weight (r2 =0.34, p<0.05) (Appendices 3.3 A and B).
Table 3.4 Correlation coefficients r and regression equations for relationships between absolute fecundity, relative fecundity and total body length and total body weight of H. bimaculata (n=15 individuals)
Relations Linear regression r2 p- value
Fa- L lnFa=4,03lnL-14,05 0,68*** <0.0001
Fa-W lnFa=1,68lnW-1,44 0,76*** <0.0001
Fr-L lnFr=2.02lnL-8.98 0.47*** <0.0001
Fr-W lnFr=0.68lnW-1.45 0.34* <0.05
Discussion
In this study, the overall sex ratio was 1:2.23 males to females, which shows significant deviation from the expected 1: 1 (p<0.05). This ratio is similar to that observed in other Cyprinids: Labeo cylindricus (1:1.63) (Weyl and Booth 1999); Gymnocephalus cernuus L. (1:5.63) (Lorenzoni et al. 2009), Labeobarbus batesii (1: 1.42) (Tiogue et al. 2013). However, this is found in different numbers with population of H. macrolepidota with being not significantly different from the expected ratio 1:1 (Abidin 1986).
24
account that some factor is modifying the equilibrium. The sex ratio may be influenced by a number of factors including a sex difference in longevity, a sex difference in growth, and the sampling methodology (Liao and Chang 2011, Ma et al. 2012). The sex ratio might also be affected by differential fishing factors related to seasons and schooling in feeding and spawning grounds. Skewed ratios may also occur as a result of the differences in natural and fishing mortality between sexes (Guopong et al. 2008) and food availability.
In this study, there is a significant difference in sex ratio in size structure which departed from (1:1) for fish measuring between 350 and 549 mm TL. In this case, that may be related to growth rate. Differentiation in growth rate between sexes can cause an unbalanced proportion since the sex presenting faster growth rate will go through the most vulnerable smaller size phase quickly and, therefore, diminish the predation proportion (Vicentini and Araujo 2003). On the other hand, the sex with slower growth rate will be more likely to undergo predation, with its abundance decreased disproportionately in next development phases.
We also observed a significantly length-weight relationship in H. bimaculata, the b values for the males and females of H. bimaculata. These values characterize negative allometric growth for male and the b value of female H. bimaculata was close to 3 (b ~ 2.98), indicative of isometric growth. Bagenal and Tesch (1978) suggested that the b coefficient may vary among congeneric species and stocks of the same species. The value of ‘b’ differs not only between species, but sometimes also between the stock of the same species due to sex, maturity, seasons and even time of day because of changes in stomach fullness.
Females had higher values for b. The greatest slope found in females suggested a higher growth rate, and it was probably related to the greater weight of the gonads of females compared to males. This was agree with few research: Gymnocephalus cernuus (Lorenzoni et al. 2009), Trachinotus marginatus (Lemos et al 2011), Rasbora rubrodorsalis (Morioka et al. 2014). This difference may be explained by the more rapid development of the female gonads relative to the increase in the length or weight of the fish, with marked changes in the body shape of the females occurring throughout the sexual cycle (Dickerson et al. 2002). In addition, this could be attributed to differences in metabolism between females and males, such as differences in oxygen consumption, differences in the level of surplus energy between reproduction and somatic growth, and differential food ingestion (Rijnsdorp and Ibelings 1989).
25 Various factors, both intrinsic and extrinsic, have been shown to regulate gonadal development and spawning in fish. The intrinsic factor is the internal gonadal rhythm (hormonal), while extrinsic factors are the external environmental factors such as changes in water quality characteristics, inter specific interactions and the occurrence of suitable spawning sites which will determine the actual time of breeding (Abidin 1986, Khaironizam and Zakaria-Ismail 2013). Many studies have reported high correlation of rainy season with spawning peaks of tropical fishes associated with flooding of rivers and lakes (Abidin 1986, Ali and Kadir 1996, Muchlisin et al. 2010). In this study, rainfall was not apparently the most important external factor regulating breeding in H. bimaculata. During spawning season (July to October), rainfall seemed fluctuation (Appendix 3.4). However, we suggested restored breeding activity in June was likely triggered by high rainfall in May. In addition, an opposite trend between the GSI and HSI (Table 3.3) also suggested that the required energy for gametogenesis of H. bimaculata may be derived from their liver. This is similar to Schizothorax o’connori (Ma et al. 2012).
The choice of a particular season in fishes for breeding is influenced by various factors. Among these are changes in water quality characteristics, inter specific interactions and the occurrence of suitable spawning sites (Khaironizam and Zakaria-Ismail 2013). The breeding season of most fishes in flooding rivers coincides with annual flood. This flood adaptive spawning of fish suggested to extended breeding habitat, increase food availability and avoid crowding and predation pressures. Our observation showed that the fish migrated from the main river to the tributaries or from upstream to downstream where it bred in the flooded waters.
The fecundity results, provided by us, represent the first such data on H bimaculata. No report on the fecundity of H. bimaculata from other places was available for comparison with that of the present study. The absolute fecundity of H. bimaculata can be considered to be high compared with other species, H. macrolepidota which it was 7132 to 62031 with a mean of 29445 eggs with total lengths ranging from 205 to 375 mm (Abidin 1986). This result was probably related to the size of the fish sampled from the study areas, which H. bimaculata mature samples (420 to 620 mm) were caught with larger in size than H. macrolepidota. Fecundity potential is strongly influenced by female size (Beacham and Murray 1993), trade-off between egg size and egg number (Rideout et al. 2000), reproductive strategy and spawning pattern of the species (Lambert 2008).
26
27 Appendix
Appendix 3.1A The length-weight relationship of male H. bimaculata described with linear function (ln W = 2.73 ln L- 10.10; n=51, r 2 =0.90; p <0.0001)
3.1B The length-weight relationship of female H. bimaculata described with linear function (lnW= 2.98ln L-11.36; n=115, r 2 =0.89; p <0.0001)
A B
Appendix 3.2 A The absolute fecundity-total length relations of H. bimaculata described with linear function (lnFa= 4.03ln TL-14.05, r2= 0.68 p<0.001)
3.2 B. The absolute fecundity-total weight relations of H. bimaculata described with linear function (lnFa=1.68lnW-1.44, r2= 0.76 p<0.001)
A B
28
Appendix 3.3A The relative fecundity-total length relations of H. bimaculata described with linear function (lnFr=2.02lnL-8.984, r2= 0.47 p<0.001)
3.3B The relative fecundity-total weight relations of H. bimaculata described with linear function (lnFr=0.68lnW-1.45, r2= 0.34 p<0.05)
A B
Appendix 3.4 Monthly rainfall (mm) data of Benua Martinus site (close to sampling area) for 2013-2014 (Source: Badan Meteorologi, Klimatologi dan Geofisika Stasiun Klimatologi Siantan Pontianak)
Years Months
30
4
POPULATION AND POTENTIAL SPAWNING SITES OF
Hampala bimaculata
IN BETUNG KERIHUN NATIONAL PARK
Abstract
Understanding spawning site of the fish is an important consideration for the management of fish populations. Fish and environmetal variables data were undertaken in the Sibau Watershed, Betung Kerihun National Park in monthly from July 2013 to October 2013 and continued in bimonthly from July to November 2014. A total 107 individuals of H. bimaculata were caught by anglings (76 individual) and gill nets (31 individuals) during sampling periods. The population consists of 37 males and 70 females ranging between 200 to 620 mm. The sex ratio for mature both sexes was 1.00: 2.37 (M:F). Relative abundance by angling method showed that population H. bimaculata was more abundant than other fish population. Specifically, the highest abundance was encountered in Apeyang river followed by Sibau upstream, and Menjakan river. By contrary, low abundance of the fish was encountered at Sibau downstream, where the station was dominated by tinfoil barb, Barbonymus schwanenfeldii. The potential spawning sites of the fish were in the upper Menjakan river and in the upper Apeyang river. Analysis of linier model showed that abundance of H. bimaculata is significantly positively correlated with water velocity, and negatively correlated with water depth.
Key words: NPUE, abundance, environmental factor, spawning site
Introduction
Fish spawning takes place in spatially limited areas with attributes that favour reproductive success through higher egg survival (Bellier et al. 2007 and Planque et al. 2004). There are several ways of reproduction at fish species. Broadcast spawning, which involves shedding the eggs and sperm into the water column, is one of the more frequent strategies (Balon, 1984). The presence of eggs and larvae of spawners can be indicative of spawning sites, although it should be noted that later larval stages may have been adverted away from the spawning site (Elli et al. 2012, Abu El-Regal 2013). Mature fish with running eggs or sperm can also be indicative of spawning sites (Elli et al. 2012). In addition, spawning can indeed be affected by, and reflect, the adult stock depletion, habitat disturbance, climate change and other processes (Begg and Marteinsdottir 2002, Rijnsdorp et al. 2009). Many studies on the spawning habitats have been also performed for fish species. Marteinsdottir et al. (2000) found that egg production, based on abundance, size, and age composition of spawning cod, might vary extensively among different spawning areas. Meanwhile, choices of the geographical location of spawning might influence the offspring survival (Cushing 1990, Hutchings and Myers 1993).
31 8 endemic species to Borneo. One of the endemic fish is H. bimaculata. Preliminary observations indicate that these fish are sharing habitat with high economic fish Tor, but the environmental factors that specifically controls it have not been studied yet including potential spawning habitats of the fish. Information about H.bimaculata population in Betung Kerihun NP is scarce. An understanding of the distribution of fish spawning and other ecologically important fish habitats is essential for better fishery management. Quantitative data on population size and environmental factors that controls it, haven’t been studied yet either. Therefore, the objectives of this study were to estimate the population and identify the potential spawning sites of H. bimaculata based on distribution of mature fish with running eggs or sperm. We also determined the environmental variables that affect habitat used by the fish in Sibau Watershed.
Material and Methods
The fish specimens were collected from Sibau Watershed (120’33.6”N -102’39.8”S and 11253’23”- 11315’08.1” E) watershed during spawning season which is between July to October 2013 and continued between July to November 2014 (Fig. 4.1). Sampling method was done by using purposive sampling based on preliminary observation of habitat preference of the fish. Samples were taken by using angling and gillnet. Sampling area for angling method included Sibau up stream, Apeyang River, Menjakan River and Sibau downstream. Exception in Sibau downstream, each the river consists of two stations, thus the total stations were seven stations for sampling sites (Table 4.1). While sampling area for gillnet method included Sibau upstream, Apeyang and Menjakan rivers. Gill nets used in each river consisted of three units (1 and 1.5 inch mesh size each), covering an area of ~150 m2. The gill nets were positioned horizontally to the mouth of the river at 18.00 and left in place until 6.00 the following morning (a total of 12 hours). Sampling by 4 anglings were done along up to downstream from 8.00 to 12.00 and continued from 13.00 to 16.00 (a total ~6 hours). Immediately photographs were taken prior to preservation. Fishes collected were identified by using Kottelat et al (1993), counted and fixed in 10% formalin. After 48 hours, the fishes were transferred to 70% alcohol.
32
[image:54.595.38.458.73.583.2]Figure 4.1 Sampling area in Sibau Watershed; M=Menjakan river; A= Apeyang river; SD= Sibau downstream; SU= Sibau upstream
Table 4.1 Summary of characteristics for each the station in sampling areas
Characteristics Sibau upstream (SU)
Apeyang river (A)
Menjakan river (M) Sibau downstream (SD) Riparian vegatation Dipterocarpacea shrubs Dipterocarpacea shrubs Dipterocarpacea shrubs Farming, shrubs, Dipterocarpacea Depth range
(cm) SU1=70-125 SU2=100-175 A1= 40-70 A2=60-90 M1=40-80 M2=70-100 150-240
Subtrate type SU1= rubble, cobble, boulder SU2= sand, rubble, boulder
A1= rubble, cobble, bolder, woods
A2= sand,
gravel, boulder
M1= rubble, cobble, boulder, woods
M2 = sand, gravel, boulder
Sand, gravel, boulder
Habitat type SU1=Riffle, rapid SU2=riffle
A1=pool, riffle, rapid
A2=riffle
pool, riffle, rapid
M2= pool, riffle
Run, riffle
Percentage of canopy* SU1=60-70% SU2=40-60% A1= >70% A2=60-70% M1= >70% M2= 40-70% <40%
*Source: Rachmatika and Haryono (1999)
33 RESULT
Population of H. bimaculata
A total 107 individuals (juvenils and adults) of H. bimaculata caught by anglings (76 individual) and gill nets (31 individuals) during sampling periods. Population consists of 37 males and 70 females ranged 200 to 620 mm. Calculation sex ratio for mature males and females which reproductive females dominant to reproductive males (1.00:2.37) (Table 4.2).
Table 4.2 Chi-square for mature males and females H. bimaculata
Months No of fish examined Total p-value
Male Female
July 2 8 10 0.058
August 1 2 3 0.564
September 3 6 9 0.317
October 2 3 5 0.655
Total
Sex ratio (M/F)
8 19 27
1.00:2.37
0.034*
Calculations of abundance based on catch rate by anglings method showed that populations H. bimaculata were higher than other fish which the highest population was upper Apeyang River (A1) followed upper Menjakan river. In contrary, low abundance of the fish was encountered at Sibau down stream with catch rate 0.18, where the station was dominated by tinfoil barb, Barbonymus schwanenfeldii (Table 4.3).
Table 4.3 Catch rate of the