Seminal carnitine and acetylcarnitine content and
carnitine acetyltransferase activity in
young Maremmano stallions
G. Stradaioli
a, L. Sylla
b, R. Zelli
b, A. Verini Supplizi
c,
P. Chiodi
d, A. Arduini
d, M. Monaci
b,∗aDepartment of Animal Production Science, University of Udine, via delle Scienze 208, 33100 Udine, Italy bDepartment of Pathology, Diagnostic and Veterinary Clinics,
University of Perugia, via S. Costanzo 4, 06126 Perugia, Italy
cSport Horse Research Center, University of Perugia, via S. Costanzo 4, 06126 Perugia, Italy dResearch and Development Department, Sigma tau s.p.d., 00040 Pomezia, Rome, Italy
Received 14 April 2000; received in revised form 7 August 2000; accepted 1 September 2000
Abstract
The reproductive characteristics and seminal carnitine and acetylcarnitine content as well as car-nitine acetyltransferase activity of young Maremmano stallions (n=25) are reported. The stallions were subjected to semen collection in November and January; in each trial two ejaculates were collected 1 h apart. The total motile morphologically normal spermatozoa (TMMNS) and the pro-gressively motile spermatozoa at collection and during storage at+4◦C were evaluated. Seminal l-carnitine (LC), acetylcarnitine (AC), pyruvate and lactate were measured using spectrophotomet-ric methods, whereas carnitine acetyltransferase activity was measured by radioenzymatic methods. Since there were no major significant differences in seminal and biochemical characteristics be-tween the November and January trials, data were also pooled for the first and second ejaculates. Significant differences (P < 0.001) were observed between the first and second ejaculates for sperm count (0.249±0.025 versus 0.133±0.014×109/ml), total number spermatozoa by ejacu-late (12.81±1.23 versus 6.36±0.77×109), progressively motile spermatozoa (48.6±3.0 versus
52.6±3.0%) and TMMNS (3.35±0.50 versus 2.02±0.37×109). In the raw semen the LC and AC were significantly higher in the first ejaculate than in the second (P <0.001), whereas, pyruvate and pyruvate/lactate ratio were higher in the second ejaculate (P <0.05). Seminal plasma AC and LC concentrations resulted higher in the first ejaculate (P <0.001). The pyruvate/lactate ratio was higher in the second ejaculate (P < 0.05). Both raw semen and seminal plasma LC and AC concentrations were positively correlated with spermatozoa concentration (P < 0.01); in raw semen AC was also correlated to TMMNS (P < 0.01). Lactate levels of raw semen was
∗Corresponding author. Tel.:+39-075-5857620; fax:+39-075-5857624. E-mail address: [email protected] (M. Monaci).
correlated to progressively motile spermatozoa after storage (P <0.01). In the second ejaculate, significant correlations were also observed among AC/LC ratio in raw semen and progressively motile spermatozoa after 48 and 72 h of refrigeration. Furthermore, AC levels were correlated to lactate concentration. The positive correlation between LC, AC and spermatozoa concentration, and between AC and TMMNS indicated carnitine as potential semen quality marker. Moreover, the correlation between AC/LC ratio and progressive spermatozoa motility after refrigeration, suggests that carnitine may contribute towards improving the maintenance of spermatozoa viability during in vitro storage. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Stallion; Seminal plasma; Semen; Carnitine; Acetylcarnitine
1. Introduction
To improve the impact of genetic selection plans in the equine industry, artificial insem-ination is employed, and stallions, utilized for this purpose, should have adequate semen quality and mating ability as a prerequisite for good reproductive performance (Amann, 1981; Jasko et al., 1991; Pickett, 1993). Therefore, they must be selected for their re-productive characteristics (Parlevliet and Colenbrander, 1999). Clinical examination of the reproductive system and laboratory assessment of seminal characteristics are currently used for breeding soundness evaluation of stallion (Jasko, 1992; Parlevliet et al., 1994).
Among the parameters which can affect semen quality (Magistrini et al., 1996) and sper-matozoa storage (Pickett, 1993; Bedford et al., 1995) seminal plasma constituents must be considered, which reflect changes in epididymis and accessory sexual gland secre-tions (Setchell et al., 1994). Stallion sexual gland markers include carnitine which has only been found in epididymal plasma (Magistrini et al., 1995a,b) and represents nearly all the carnitine available in seminal plasma, as observed in other mammals (Jeulin and Lewin, 1996). Carnitine is taken from the blood stream and then released in epididymal lumen by active epithelial pumps (Brooks, 1980), which are regulated by androgens in rat (Cooper et al., 1986a) and monkey (Cooper et al., 1986b). Carnitine is best known as a key compound in energy-producing processes since it modulates mitochondrial fatty acid oxidation. To accomplish this role, carnitine needs the concerted action of a discrete num-ber of membrane-bound, carnitine-dependent, long-chain acyltransferases, also known as carnitine palmitoyltransferases (CPTI and CPTII), and acyl-carnitine translocase (Bieber, 1988).
Spermatozoa increase their carnitine content and progressive motility during passage through the epididymis where carnitine is esterified within sperm cells in acetylcarnitine (Casillas, 1973). Acetylatedl-carnitine is the major form of acylcarnitine in mammal tissues (Bieber et al., 1982).
In this context, another important action of carnitine is to modulate the intramitochon-drial acetyl-CoA/free CoA ratio via carnitine acetyltransferase (CAT) (Uziel et al., 1988; Abdel-aleem et al., 1995), a mitochondrial enzyme able to catalyze the reversible transfer of the acetyl-unit from CoA to carnitine (Bieber et al., 1982).
thiolase, a reduction of acetyl-CoA by carnitine may relieve such an inhibitory effect (Wang et al., 1991; Abdel-aleem et al., 1995; Jeulin and Lewin, 1996). Both extra and intracellular acetylcarnitine provide readily available acetyl groups for spermatozoa motility (Milkowsky et al., 1976; Bruns and Casillas, 1990). In mature spermatozoa, high intracellularl-carnitine concentrations increase the utilization of pyruvate and lactate (Carter et al., 1980; Jones and Murdoch, 1996), thus holding the maximal “acetylation-state” of carnitine.
In human beings, seminall-carnitine content is correlated with spermatozoa count and progressive motility (Menchini-Fabris et al., 1984; Borman et al., 1989) and a reduction of the acetylcarnitine/l-carnitine ratio has been observed in asthenospermic patients (Golan et al., 1984; Bartellini et al., 1987). Moreover, significant reduction of seminal carnitine concentrations has been reported in azoospermic patients affected by bilateral agenesis of the vas deferens and epididymal obstruction (Menchini-Fabris et al., 1984; Casano et al., 1987), as well as during severe testicular failures (Lewin et al., 1981). Recently, a reduction of seminal plasma carnitine has been reported in infertile men (Zöpfgen et al., 2000). The positive correlation observed among seminal parameters and seminal carnitine concentra-tion allows proposing carnitine as a “good quality” semen marker (Menchini-Fabris et al., 1984).
Our preliminary observations on breeding stallions demonstrated a positive correlation among sperm quality parameters, such as spermatozoa count, motility, in vitro storage ability and seminal plasma carnitine activity (Stradaioli et al., 1995; Chiodi et al., 1997). In addition, a reduced seminal plasma acetylcarnitine content in two necrospermic infertile stallions has also been reported (Sighieri et al., 1991).
The aim of the study reported herein was to evaluate seminal carnitine and acetylcar-nitine content and caracetylcar-nitine acetyltransferase activity as markers of the semen quality and spermatozoa storage of young Maremmano stallions in breeding soundness examination.
2. Materials and methods
2.1. Animals, sample collection and seminal analysis
The study was carried out over a 2-year period on Maremmano stallions (n=25), 42±5 months of age, which were assigned for physical soundness and pedigree to the 100-day performance test. The animals were maintained in standardized environmental conditions and training plans.
After a 2-month adaptation period, the stallions underwent breeding soundness evalua-tion. Physical examination of the reproductive tract, including transrectal ultrasound eval-uation of the accessory sexual glands (Toshiba Sonolayer, SAL32A, 5 MHz linear probe), was performed.
depending on the concentration, with non-fat dry skim milk-glucose extender (E–Z Mixin, Animal Reproduction System, Chino, CA, USA) at 37◦C. The spermatozoa concentration was measured with the Bürker haemocitometer, corrected for dilution by calculation. Suf-ficient 37◦C E–Z Mixin–amikacin (1000 units/ml) seminal extender was then added to a specific amount of semen to achieve a final spermatozoa concentration of 20×106/ml. The diluted semen was thoroughly mixed and then subdivided into three aliquots, which were then slowly cooled in a+4◦C refrigerator (Varner et al., 1988). After 24, 48 and 72 h, the samples were incubated for 30 min at 37◦C prior to spermatozoa progressive motility evaluation.
In addition, a smear of the native semen, stained with eosin–nigrosin (Blom, 1950), was prepared. The morphology and viability of 500 spermatozoa were assessed under bright-field illumination at 1000×(Optiphot 2, Nikon, Japan) according to the criteria outlined by Bielanski et al. (1982). The spermatozoa were clustered in five classes: normal, abnormal head shape, abnormal mid-piece and tail, curved mid-piece and tail, detached head and tail. Each sperm cell was placed in only one of the above classes; cells having more than one of the aforementioned morphologic features were classified according to the more proximal one. The percentage of morphologically normal, including cytoplasmic droplets and eccentric tail implant and abnormal live spermatozoa was calculated. The volume of the ejaculate was multiplied by the concentration (106spz/ml), the percentage of progressive motile spermatozoa and the percentage of morphologically normal live spermatozoa to obtain the total number of motile morphologically normal spermatozoa (Parlevliet et al., 1994).
Raw semen was centrifuged at 600×gfor 15 min and the 1 ml seminal plasma aliquots, after filtration through a 45mm disposable syringe filter (Durapore, Nalgene), were stored at−20◦C until analysis. Raw semen samples (1 ml) was also stored. An amount of 200
ml of 35% HClO4were added to duplicates of raw semen and seminal plasma for lactate and
pyruvate analysis.
Blood samples were collected by jugular venopuncture into a heparinized vacutainer before semen collection and plasma was stored at−20◦C until analysis.
2.2. Biochemical analysis
2.2.1. Freel-carnitine and acetylcarnitine
In order to measure free l-carnitine (LC) and acetylcarnitine (AC), 1 ml of 5% cold HClO4was added to 0.5 ml of blood plasma. The samples were centrifuged at 3000×gfor
10 min at+4◦C and analysis was carried out on the supernatant.
Absolute methanol (10 ml) were added to the 1 ml aliquot duplicates of raw semen and seminal plasma for LC and AC analysis and centrifuged; the methanol extracts were brought to dryness in a flow of N2and recovered with 1 ml bidistilled water. LC and AC
concen-trations were measured using spectrophotometric methods as indicated by Pearson et al. (1974).
2.2.2. Pyruvate and lactate
The perchlorised raw semen and seminal plasma samples were centrifuged at 5000×g
supernatant was analyzed by an automatic analyzer according to the spectrophotometric methods reported by Noll (1984) and Lamprecht and Heinz (1984).
2.2.3. Carnitine acetyltransferase activity
Carnitine acetyltransferase activity in raw semen was measured radioenzymatically at 37◦C as previously described by Chiodi et al. (1994). The medium (pH 7.4), which contained 2 mg/ml of raw semen protein, was composed as follows: 0.25 mM of EDTA (Sigma, St. Louis, MO, USA), 100 mM of Hepes (Sigma, St. Louis, MO, USA), 0.08% (w/v) Triton X-100 (Sigma, St. Louis, MO, USA), 1mg/ml of antimycin A (Sigma, St. Louis, MO, USA), 1mg/ml of Rotenone (Sigma, St. Louis, MO, USA), 0.5 mM (acetyl-1-14C-) Coenzyme A (0.6 Ci/mol) (Amersham Pharmacia Biotech, Buckinghamshire, UK) and 12 mM LC (Sigma tau s.p.a., Pomezia, Rome, Italy). Incubation was carried out with 0.3 ml of the aforementioned medium for 2 min before and 2 min after addition of LC. The reaction was stopped with the addition, under stirring, of 0.3 ml of 2×8 Dowex resin (Fluka Chemie A.G., Switzerland) diluted 1:1 (w/v). Following the addition of the resin the samples were placed in an ice bath for 5 min, shaken up three times and then centrifuged at 3000×gfor 10 min. The incorporation of (acetyl-1-14C-) in AC was evaluated on a 0.3 ml aliquot in a scintillation vial and radioactivity was determined by liquid scintillation counting.
2.3. Statistical analysis
Statistical analysis of seminal and biochemical data was performed using a repeated mea-sure design. The first ejaculate versus the second one, and the interaction between semen collection trials (November versus January) were considered as main factors; differences between means were compared with the LSD procedure (SPSS, 1997). Coefficient of cor-relation was performed with a two tails Pearson model (SPSS, 1997).
3. Results
No congenital or acquired abnormalities of the genital tract were detected in the animals. Two stallions were excluded from the performance test before the January semen collection trial, due to muscle skeletal pathology. Since there were no major significant differences in seminal and biochemical characteristics between the November and January trials, data were also pooled for the first and second ejaculates, irrespective of the trial.
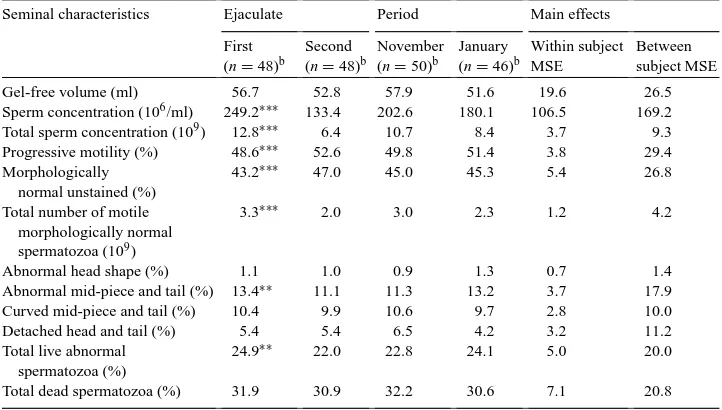
Table 1
Seminal characteristics (LS means) of Maremmano stallions (n=25) in the first and second ejaculates and during the two experimental periods (MSE: mean square error)a
Seminal characteristics Ejaculate Period Main effects
First (n=48)b
Second (n=48)b
November (n=50)b
January (n=46)b
Within subject MSE
Between subject MSE
Gel-free volume (ml) 56.7 52.8 57.9 51.6 19.6 26.5
Sperm concentration (106/ml) 249.2∗∗∗ 133.4 202.6 180.1 106.5 169.2 Total sperm concentration (109) 12.8∗∗∗ 6.4 10.7 8.4 3.7 9.3 Progressive motility (%) 48.6∗∗∗ 52.6 49.8 51.4 3.8 29.4 Morphologically
normal unstained (%)
43.2∗∗∗ 47.0 45.0 45.3 5.4 26.8
Total number of motile morphologically normal spermatozoa (109)
3.3∗∗∗ 2.0 3.0 2.3 1.2 4.2
Abnormal head shape (%) 1.1 1.0 0.9 1.3 0.7 1.4
Abnormal mid-piece and tail (%) 13.4∗∗ 11.1 11.3 13.2 3.7 17.9 Curved mid-piece and tail (%) 10.4 9.9 10.6 9.7 2.8 10.0
Detached head and tail (%) 5.4 5.4 6.5 4.2 3.2 11.2
Total live abnormal spermatozoa (%)
24.9∗∗ 22.0 22.8 24.1 5.0 20.0
Total dead spermatozoa (%) 31.9 30.9 32.2 30.6 7.1 20.8
aEffect for period was never significant. bNumber of ejaculates.
∗∗P<0.01;∗∗∗P<0.001.
Biochemical analysis data, pyruvate/lactate and AC/LC ratios in raw semen are reported in Table 2. In the raw semen the LC and AC were significantly higher in the first ejaculate than in the second (P <0.001), whereas, pyruvate and pyruvate/lactate ratios were higher in the second ejaculate (P <0.05).
Table 2
Free carnitine, acetylcarnitine, pyruvate and lactate levels, pyruvate/lactate, acetylcarnitine/carnitine ratio and CAT activity in raw semen (LS means) of Maremmano stallions (n=25) in the first and second ejaculates and during the two experimental periods (MSE: mean square error)a
Ejaculate Period Main effects
Carnitine (nmol/ml) 1067.0∗∗∗ 559.5 676.0 950.6 323.8 515.4 Acetylcarnitine (nmol/ml) 139.0∗∗∗ 78.6 98.2 119.5 51.1 76.7 Acetylcarnitine/carnitine 0.165 0.162 0.191 0.136 0.071 0.100 Carnitine (nmol/106
spermatozoa)
5.244 5.255 4.289 6.210 2.557 5.035
Acetylcarnitine (nmol/106
spermatozoa)
0.753 0.751 0.656 0.848 0.318 0.512
Pyruvate (nmol/ml) 0.076∗ 0.161 0.090 0.147 0.102 0.081
Lactate (nmol/ml) 2.396 3.285 2.919 2.762 2.087 2.152
Pyruvate/lactate 0.031∗ 0.051 0.041 0.041 0.020 0.029 CATc(nmol/min/106
spermatozoa)
0.431 0.506 0.402 0.535 0.217 0.270
aEffect for period was never significant. bNumber of ejaculates.
cCarnitine acetyltransferase.
∗P
<0.05;∗∗∗P
<0.001.
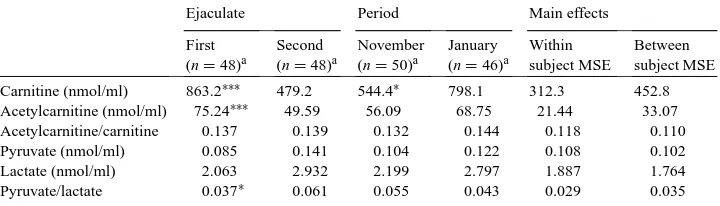
Seminal plasma AC and LC concentrations (Table 3) resulted significantly higher in the first ejaculate (P <0.001); moreover, the ejaculate obtained in the January trial presented higher LC values than the November trial (P <0.05). The pyruvate/lactate ratio was higher in the second ejaculate (P <0.05).
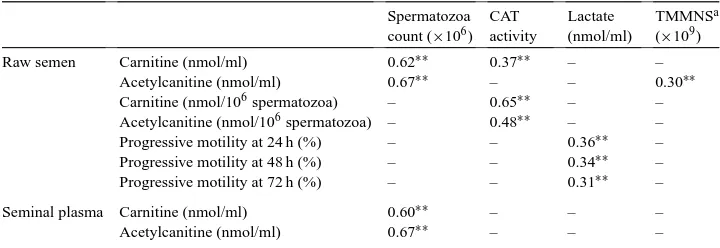
Significant correlation coefficients among seminal and biochemical characteristics of Maremmano stallions are reported in Table 4. Both raw semen and seminal plasma LC and
Table 3
Free carnitine, acetylcarnitine, pyruvate and lactate levels, pyruvate/lactate, acetylcarnitine/carnitine ratio in sem-inal plasma (LS means) of Maremmano stallions (n=25) in the first and second ejaculates and during the two experimental periods (MSE: mean square error)
Ejaculate Period Main effects
Carnitine (nmol/ml) 863.2∗∗∗ 479.2 544.4∗ 798.1 312.3 452.8 Acetylcarnitine (nmol/ml) 75.24∗∗∗ 49.59 56.09 68.75 21.44 33.07 Acetylcarnitine/carnitine 0.137 0.139 0.132 0.144 0.118 0.110
Pyruvate (nmol/ml) 0.085 0.141 0.104 0.122 0.108 0.102
Lactate (nmol/ml) 2.063 2.932 2.199 2.797 1.887 1.764
Pyruvate/lactate 0.037∗ 0.061 0.055 0.043 0.029 0.035
aNumber of ejaculates.
∗P
<0.05;∗∗∗P
Table 4
Significant correlation coefficients among seminal and biochemical characteristics of Maremmano stallions (n =96 ejaculates)
Spermatozoa count (×106)
CAT activity
Lactate (nmol/ml)
TMMNSa
(×109)
Raw semen Carnitine (nmol/ml) 0.62∗∗ 0.37∗∗ – –
Acetylcanitine (nmol/ml) 0.67∗∗ – – 0.30∗∗
Carnitine (nmol/106spermatozoa) – 0.65∗∗ – – Acetylcanitine (nmol/106spermatozoa) – 0.48∗∗ – – Progressive motility at 24 h (%) – – 0.36∗∗ – Progressive motility at 48 h (%) – – 0.34∗∗ – Progressive motility at 72 h (%) – – 0.31∗∗ –
Seminal plasma Carnitine (nmol/ml) 0.60∗∗ – – –
Acetylcanitine (nmol/ml) 0.67∗∗ – – –
aTotal number of motile morphologically normal spermatozoa.
∗∗P<0.01.
AC concentrations were positively correlated with spermatozoa concentration (P <0.01). In raw semen AC was also correlated to the total number of motile morphologically nor-mal spermatozoa (P <0.01), while carnitine acetyltransferase activity was correlated to LC and AC. Lactate levels of raw semen were correlated to progressively motile sperma-tozoa after storage at+4◦C (P < 0.01). In the second ejaculate, significant correlations were also observed among AC/LC ratio in raw semen and progressively motile sperma-tozoa after 48 and 72 h of refrigeration (r =0.47;P < 0.01 andr = 0.45;P < 0.05, respectively). Furthermore, AC levels were correlated to lactate concentration (r =0.57;
P <0.01).
Blood plasma AC and LC concentrations did not differ significantly among semen col-lection trials (data not shown). Blood LC levels were three-fold higher than those of AC (18.25±1.02 versus 5.90±0.35 nmol/ml, respectively).
4. Discussion
Stallion reproductive characteristics are affected by age and breed (Dowsett and Pattie, 1982; Dowsett and Pattie, 1987; Pickett et al., 1989; Dowsett and Knott, 1996). In the study reported herein, the young Maremmano stallions were maintained in standardized environmental conditions during the 100-day performance test, allowing a more reliable statistical evaluation of their reproductive characteristics. We have included the semen collection period effects in the statistical analysis, although no significant differences were observed between seminal characteristics in the November and January trials.
The gel free volume of the ejaculates was similar to those reported for Quarter Horses (Pickett et al., 1976) and smaller than those of Dutch Warmblood stallions (Parlevliet et al., 1994).
Spermatozoa concentration and the total number of spermatozoa in the first ejaculate were about two-fold the second one. These findings are in agreement with data reported by Pickett et al. (1976) and by Parlevliet et al. (1994), both for differences between ejaculates and value per se, thus suggesting that Maremmano stallions do not differ from other breeds with regard to sperm output.
The percentage of progressively motile and of morphologically normal live spermatozoa increased in the second ejaculate, although they were lower than data reported in other breeds. The total number of motile morphologically normal spermatozoa was less than half of the value reported in maiden Dutch Warmblood stallions (Parlevliet et al., 1994).
The number of subjects which were evaluated in our study was not sufficient to make an exhaustive analysis of the variance; nevertheless, among the characteristics considered, the major dispersion of the data were linked to the differences between subjects, as depicted by the higher between subject mean square error.
Blood free carnitine content was similar to that previously reported in young thoroughbred horses both at stud and during training (Foster et al., 1989).
The seminal plasma LC levels presented herein are in agreement with previous reports using nuclear magnetic resonance analysis (Magistrini et al., 1995a,b) and high pressure liquid chromatography (Stradaioli et al., 1995).
In human beings, seminal plasma LC and AC levels ranged from 200 to 1300 nmol/ml and from 60 to 280 nmol/ml, respectively (Menchini-Fabris et al., 1984; Setchell et al., 1994), which do not differ greatly from our results in Maremmano stallions. In the ram, LC levels resulted highly correlated with sperm concentration and seminal plasma contains five-fold more LC and 40-fold more AC than in the stallion (Brooks, 1979). The high carnitine levels of ram seminal plasma could be due in part to the differences in ejaculate volumes and sperm density between these species. Nevertheless, this phenomenon is also related to specie differences; indeed, in the rat epididymal plasma LC concentration is 60 mM (Bremer, 1983), while in the ram and in the stallion is 19 and 11 mM, respectively (Jones, 1978). In bovine frozen semen, diluted with egg yolk citrate and glycerol, LC content ranged from 110 to 230 nmol/ml (Carter et al., 1980), which was lower than in the Maremmano stallion.
To our knowledge, this is the first report on LC and AC content in stallion raw semen and seminal plasma evaluated in two successive ejaculates. LC and AC levels in the first ejaculate resulted about two-fold the second one, both in raw semen and seminal plasma. These results are related to the differences in spermatozoa concentration between the two ejaculates, as demonstrated by the strong correlation between LC and AC. Indeed, both the ejaculates resulted identical when LC and AC are expressed as nmol/106spermatozoa. Similarly, French researchers observed that in fractionated semen collection carnitine levels increase with spermatozoa concentration, thus allowing us to propose carnitine as a marker of epididymal functionality (Magistrini et al., 1998).
acetylation levels of carnitine were higher in sperm cells than in seminal plasma (Brooks, 1979). These metabolites are in equilibrium within the sperm cell due to carnitine acetyl-transferase activity, as evident by direct correlation shown in Table 4, which maintains the correct acetyl-CoA/free CoA ratio. The correct ratio acetyl-CoA to free CoA is fundamental in order to maintain the correct functionality of the Kreb’s cycle and, therefore, a sufficient availability of ATP necessary for spermatozoa motility. Intracellular LC accumulated by spermatozoa might perform a buffering role, trapping excess mitochondrial acetyl-CoA as AC, and this system would protect the activity of pyruvate dehydrogenase, and other key en-zymes for mitochondrial respiration, which are inhibited by excess acetyl-CoA (Uziel et al., 1988; Abdel-aleem et al., 1995; Jeulin and Lewin, 1996). Moreover, in mammalian sperma-tozoa, AC may replace the energy storage function of high-energy phosphate compounds (Smith et al., 1985).
Pyruvate, lactate and pyruvate/lactate ratios were always higher in the second ejaculate. One can speculate that this could be related to the higher content on both unstained and motile cells in the second ejaculate, as lactate and pyruvate are an intermediate of the glycolytic pathway of live metabolizing spermatozoa. Findings by Leone et al. (1989) that sperm concentration and spermatozoa motility scores in oligoasthenospermic rats treated with acetyl-l-carnitine were significantly higher (P <0.05) than untreated rats, further support our observation. The positive correlation observed between lactate and the percentage of progressively motile spermatozoa after 24, 48 and 72 h of raw semen storage, in conjunction with the positive correlation between AC and total number of motile morphologically normal spermatozoa, could indicate that these metabolites influence sperm cell viability. In this respect, it is interesting to note that, in boar spermatozoa, lactate is the major mitochondrial substrate for ATP production (Jones, 1997; Jones and Bubb, 2000); furthermore, lactate and pyruvate contribute to hold the acetylation state of carnitine through acetyl-CoA formation (Casillas, 1973). The correlation between AC/LC ratio and spermatozoa motility in the second ejaculate, observed at 48 and 72 h of storage, further supports this observation.
Both AC and lactate are precursors of the intramitochondrial acetyl-CoA pool, whereas only AC represents a true reservoir of activated acetyl groups (Smith et al., 1985). Thus, endogenous AC could guarantee gamete’s viability in ejaculated spermatozoa (Jeulin and Lewin, 1996).
5. Conclusion
The seminal characteristics of the Italian Maremmano stallion did not differ from those of other saddle breed horses. The percentage of progressively motile and morphologically normal live spermatozoa was on the borderline of the minimum recommended by Colen-brander et al. (1992). Indeed, our results suggest that semen quality evaluation have to be included among the criteria recommended for the selection of young stallions, especially when they could be assigned to artificial insemination plans.
refrigeration, suggests that carnitine may contribute towards improving the maintenance of spermatozoa viability during in vitro storage.
Acknowledgements
Research supported by funds from the “Ministry of the University, Scientific Research and Technologies”, Rome, Italy; “Sport Horse Research Center”, University of Perugia, Italy; Sigma tau s.p.a, Pomezia, Rome, Italy. We also acknowledge Professor Alberto Gaiti for advice and criticisms and Dr. Orlando Ghirardi for statistical analysis input.
References
Abdel-aleem, S., Saved-Ahmed, M., Nada, M.A., Hendrickson, S.C., St Louis, J., Lowe, J.E., 1995. Stimulation of non-oxidative glucose utilisation byl-carnitine in isolated myocytes. J. Mol. Cell Cardiol. 27, 2465–2472. Amann, R.P.M., 1981. A critical review of methods for evaluation of spermatogenesis from seminal characteristics.
J. Androl. 2, 37–58.
Bartellini, M., Canale, D., Izzo, P.L., Giorgio, P.M., Menchini, P., Menchini-Fabbris, G.F., 1987. L-carnitine and acetylcarnitine in human sperm with normal and reduced motility. Acta Eur. Fertil. 18, 29–31.
Bedford, S.J., Graham, J.K., Amann, R.P., Squires, E.L., Pickett, B.W., 1995. Use of two freezing extenders to cool stallion spermatozoa to 5◦C with and without seminal plasma. Theriogenology 43, 939–953.
Bieber, L.L., Emaus, R., Valkner, K., Farrell, S., 1982. Possible functions of short-chain and medium-chain carnitine acyltransferases. Fed. Proc. 41, 2858–2862.
Bieber, L.L., 1988. Carnitine. Ann. Rev. Biochem. 57, 261–283.
Bielanski, W., Dudek, E., Bittmar, A., Kosiniak, K., 1982. Some characteristics of common abnormal forms of spermatozoa in highly fertile stallions. J. Reprod. Fertil. Suppl. 32, 21–26.
Blom, E., 1950. Eine schnellfarb methode mit eosin und nigrosin zur unterscheidung von lebenden und toten spermien. Wien. Tierärztl. Wschr. 37, 441–442.
Borman, M.S., du Toit, D., Otto, B., Muller, H., Hurter, P., du Plessis, D.J., 1989. Seminal carnitine, epididymal function and spermatozoal motility. S. Afr. Med. J. 75, 20–21.
Bremer, J., 1983. Carnitine: metabolism and function. Physiol. Rev. 63, 1420–1480.
Brooks, D.E., 1979. Carnitine, acetylcarnitine and activity of carnitine acetyltransferase in seminal plasma and spermatozoa of men, rams and rats. J. Reprod. Fertil. 56, 667–673.
Brooks, D.E., 1980. Carnitine in the male reproductive tract and its relation to the metabolism of the epididymis and spermatozoa. In: McGarry, J.D., Frenkel, R.A. (Eds.), Carnitine Biosynthesis Metabolism and Function. Academic Press, New York, pp. 219–235.
Bruns, K.A., Casillas, E.R., 1990. Partial purification and characterisation of an acetylcarnitine hydrolase from bovine epididymal spermatozoa. Arch. Biochem. Biophys. 277, 1–7.
Carter, A.L., Stratman, F.W., Hutson, S.M., Lardy, H.A., 1980. The role of carnitine and its esters in sperm metabolism. In: McGarry, J.D., Frenkel, R.A. (Eds.), Carnitine Biosynthesis Metabolism and Function. Academic Press, New York, pp. 251–269.
Casano, R., Orlando, C., Caldini, A.L., Barni, T., Natali, A., Serio, M., 1987. Simultaneous measurement of seminall-carnitine,a,1-4-glucosidase, and glycerylphophorylcholine in azoospermic and oligozoospermic
patients. Fertil. Steril. 47, 324–328.
Casillas, E.R., 1973. Accumulation of carnitine by bovine spermatozoa during maturation in the epididymis. J. Biol. Chem. 248, 8227–8232.
Chiodi, P., Ciani, S., Kentroti, S., Maccari, F., Vernadakis, A., Angelucci, L., Ramacci, M.T., 1994. Carnitine and derivatives in the central nervous system of chick embryo. Int. J. Biochem. 26, 711–720.
Colenbrander, R., Puyk, H., Zandee, A.R., Parlevliet, J., 1992. Evaluation of stallion for breeding. Acta Vet. Scand. Suppl. 88, 29–37.
Cooper, T.G., Gudermann, T.W., Yeung, C.H., 1986a. Characteristics of the transport of carnitine into the cauda epididymis of the rat as ascertained by luminal perfusion in vitro. Int. J. Androl. 9, 348–358.
Cooper, T.G., Yeung, C.H., Weinbauer, G.F., 1986b. Transport of carnitine by the epididymis of the cynomolgus macaque (Macaca fascicularis). J. Reprod. Fertil. 77, 297–301.
Dowsett, K.F., Pattie, W.A., 1982. Characteristics and fertility of stallion semen. J. Reprod. Fertil. Suppl. 32, 1–8. Dowsett, K.F., Pattie, W.A., 1987. Variation in characteristics of stallion semen caused by breed, age, season of
year and service frequency. J. Reprod. Fertil. Suppl. 35, 645–647.
Dowsett, K.F., Knott, L.M., 1996. The influence of age and breed on stallion semen. Theriogenology 46, 397–412. Foster, C.V.L., Harris, R.C., Pouret, E.J.M., 1989. Survey of plasma free carnitine levels in 74 thoroughbred horses
at stud and in training. Equine Vet. J. 21, 139–141.
Golan, R., Weissenberg, R., Lewin, L.M., 1984. Carnitine and acetylcarnitine in motile and immotile human spermatozoa. Int. J. Androl. 7, 484–494.
Jasko, D.J., Little, T.V., Lein, D.H., Foote, R.H., 1991. Determination of stallion semen quality and its relationship with fertility. J. Reprod. Fertil. Suppl. 44, 649–650.
Jasko, D.J., 1992. Evaluation of stallion semen. Vet. Clin. N. Am. — Equine Practice 8, 129–148.
Jeulin, C., Lewin, L.M., 1996. Role of freel-carnitine and acetylcarnitine in postgonadal maturation of mammalian spermatozoa. Human Reprod. Update 2, 87–102.
Jones, R., 1978. Comparative biochemistry of mammalian epididymal plasma. Comp. Biochem. Physiol. B 61, 365–370.
Jones, A.R., 1997. Metabolism of lactate by mature boar spermatozoa. Reprod. Fertil. Dev. 9, 227–232. Jones, A.R., Bubb, W.A., 2000. Substrates for endogenous metabolism by mature boar spermatozoa. J. Reprod.
Fertil. 119, 129–135.
Jones, R.C., Murdoch, R.N., 1996. Regulation of the motility and metabolism of spermatozoa for storage in the epididymis of eutherian and marsupial mammals. Reprod. Fertil. Dev. 8, 553–568.
Lamprecht, W., Heinz, F., 1984. Pyruvate. In: Bergmeyer (Ed.), Methods of Enzymatic Analysis, 3rd Edition. Vol. VI. Verlag Chemie, Weinheim, pp. 570–577.
Leone, M., Costa, M., Palmero, S., Capitanio, G.L., 1989. Biological basis for the treatment of oligoasthenospermia withl-acetylcarnitine (LAC). In: Proceedings of the 2nd European Winter Conference on Gynaecology and Obstetrics, Madonna di Campiglio, Italy, Off. J. Int. Soc. Gynaecol. Endocrinol. 3 (Suppl. 1), 65 (abstract). Lewin, L.M., Pace Shalev, D., Weissenberg, R., Soffer, Y., 1981. Carnitine and acylcarnitines in semen from
azoospermic patients. Fertil. Steril. 36, 214–218.
Magistrini, M., Seguin, F., Beau, P., Akoka, S., Le Pape, A., Palmer, E., 1995a.1H nuclear magnetic resonance
analysis of stallion genital tract fluids and seminal plasma: contribution of the accessory sex glands to the ejaculate. Biol. Reprod., Monograph Series No. 1, 599–607.
Magistrini, M., McDonnel, S.M., Seguin, F., Beau, P., Palmer, E., 1995b. Analysis of sex gland markers in equine seminal plasma of in copula and ex copula-induced ejaculates: quantification by magnetic resonance spectroscopy. In: Proceedings of the 2nd International Workshop on Erection and Ejaculation in Horses and Man, Mount Joy, PA, pp. 35–37.
Magistrini, M., Vidament, M., Clement, F., Palmer, E., 1996. Fertility prediction in stallions. Anim. Reprod. Sci. 42, 181–188.
Magistrini, M., Lindeberg, H., Koskinen, E., Beau, P., Seguin, F., 1998. Biophysical and 1H Magnetic
resonance spectroscopy (1H MRS) characteristics of fractionated stallion ejaculates. In: Proceedings of the 7th International Symposium on Equine Reproduction, University of Pretoria, South Africa, pp. 27–28. Menchini-Fabris, G.F., Canale, D., Izzo, P.L., Olivieri, L., Bartelloni, M., 1984. Freel-carnitine in human semen:
its variability in different andrologic pathologies. Fertil. Steril. 42, 263–267.
Milkowsky, A.L., Babcock, D.F., Lardy, H.A., 1976. Activation of bovine epididymal sperm respiration by caffeine: its transient nature and relationship to utilisation of acetylcarnitine. Arch. Biochem. Biophys. 176, 250–255. Noll, F., 1984.l-(+)-Lactate. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis, 3rd Edition. Vol. 6.
Verlag Chemie, Weinheim, pp. 582–592.
Parlevliet, J.M., Colenbrander, B., 1999. Prediction of first season stallion fertility of 3-year old Dutch Warmblood with prebreeding assessment of percentage of morphologically normal live sperm. Equine Vet. J. 31, 248–251. Pearson, D.J., Tubbs, P.K., Chase, J.F.A., 1974. Carnitine and acylcarnitines. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis, 2nd Edition. Vol. 4. Verlag Chemie, Weinheim, Academic Press, New York, pp. 1758–1771.
Pickett, B.W., Sullivan, J.J., Seidel, J., 1975. Reproductive physiology of the stallion. V. Effect of frequency of ejaculation on seminal characteristics and spermatozoal output. J. Anim. Sci. 40, 917–923.
Pickett, B.W., Faulkner, L.C., Seidel Jr., G.E., Berndtson, W.E., Voss, J.L., 1976. Reproductive physiology of the stallion. Seminal and behavioural characteristics. VI. J. Anim. Sci. 43, 617–625.
Pickett, B.W., Amann, R.P., McKinnon, A.O., Squires, E.L., Voss, J.L., 1989. Management of the stallion for maximum reproductive efficiency, II. Bulletin 5, Colorado State University, Fort Collins, CO, USA. Pickett, B.W., 1993. Factors affecting sperm production and sperm output. In: McKinnon, A.O., Voss, J.L. (Eds.),
Equine Reproduction. Lea and Febiger, Philadelphia, London, pp. 689–704.
Setchell, B.P., Maddocks, S., Brooks, D.E., 1994. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil, E., Neill, J.D. (Eds.), The Physiology of Reproduction, Vol. 1. Raven Press, New York, pp. 1063–1175.
Sighieri, C., Ducci, M., Della Longa, A., Frateschi, T.L., Mariani, A.P., Martelli, F., 1991. Carnitine and acetylcarnitine in stallion spermatozoa. Atti Società Italiana di Scienze Veterinarie XLV, 489–492.
Smith, M.B., Babcock, D.F., Lardy, H.A., 1985. A31PNMR study of the epididymis and epididymal sperm of the
bull and hamster. Biol. Reprod. 33, 1029–1040.
Statistic Package for Social Science (SPSS), 1997. SPSS Advanced Statistics 7.5. SPSS Inc., Chicago, IL. Stradaioli, G., Chiacchiarini, P., Monaci, M., Verini Supplizi, A., Martino, G., Pieramati, C., 1995. Reproductive
characteristics and seminal plasma carnitine concentration in maiden Maremmano stallions. In: Proceedings of the 46th Annual Meeting of the EAAP, Prague, Czech Republic, Vol. 1, p. 357 (abstract).
Uziel, G., Garavaglia, B., Di Donato, S., 1988. Carnitine stimulation of pyruvate dehydrogenase complex (PDHC) in isolated human skeletal muscle mitochondria. Muscle Nerve 11, 720–724.
Varner, D.D., Blanchard, T.L., Love, C.L., Garcia, M.C., Kenney, R.M., 1988. Effects of cooling rate and storage temperature on equine spermatozoal motility parameters. Theriogenology 29, 1043–1054.
Wang, H.Y., Baxter Jr., C.F., Schulz, H., 1991. Regulation of fatty acid beta-oxidation in rat heart mitochondria. Arch. Biochem. Biophys. 289, 274–280.