68
S
TAMEN DIMORPHISM INR
HODODENDRON FERRUGINEUM(E
RICACEAE):
DEVELOPMENT AND FUNCTION1N
ATHALIEE
SCARAVAGE,
2E
LISABETHF
LUBACKER,
2A
NDRE´P
ORNON,
3B
ERNARDD
OCHE,
2 ANDI
RE` NET
ILL-B
OTTRAUD22Laboratoire de Biologie des Populations d’Altitude CNRS-UMR 5553, Universite´ Joseph Fourier, B.P. 53, F-38041 Grenoble Cedex 09, France; and3Laboratoire d’Ecologie Terrestre CNRS-UMR 5552,
Universite´ Paul Sabatier, Zoologie Ecologie,
Baˆt. 4R3, 118, route de Narbonne, F-31062 Toulouse Cedex 04, France
The function of stamen dimorphism in the breeding system of the alpine shrub Rhododendron ferrugineum was studied in two populations in the French Alps. This species has pentameric flowers with two whorls of stamens: an inner whorl of five long stamens and an outer whorl of short stamens. We studied the development of stamens from buds to mature flowers (measurement of the filament, anther, and style lengths at five successive phenological stages) and compared the size and position of reproductive organs at maturity in control and partially emasculated flowers (removal of level stamens) to determine whether the presence of long-level stamens constitutes a constraint for the development of the short-long-level ones. Stamen dimorphism can be observed early in stamen development, from the bud stage of the year prior to flowering. At this early stage, meiosis had already occurred. Emasculation of the long-level stamens induced the short-level ones to grow longer than in normal conditions. We also performed seven pollination treatments on ten randomly chosen individuals in each population, and the number of seeds following each treatment was recorded. Results from these treatments showed that R. ferrugineum produced spontaneous selfed seeds in the absence of pollinators. However, no seed was produced when short-level stamens were emasculated and pollinators excluded, suggesting that long-level stamens are not responsible for selfing in the absence of pollinators and that reproductive assurance is promoted by short-level stamens.
Key words: Ericaceae; pollination system; reproductive assurance; Rhododendron ferrugineum; stamen development; stamen size.
In alpine and arctic environments, low pollinator activity and diversity should promote characters that result in the as-surance of seed production through self-pollination. Most tem-perate alpine plants need pollinators for pollen deposition on the stigma, and therefore, are predominantly entomophilous (Kevan, 1973; Arroyo, Primack, and Armesto, 1982; Kudo, 1993; Totland, 1994; Jacquemart and Thompson, 1995). How-ever, some animal-pollinated plants growing in severe envi-ronmental conditions have a mixed-mating system and can produce spontaneous selfed seeds. Outcrossing will usually oc-cur when reproductive fitness is not limited by a paucity of animal visits. Where such limitations exist, features that max-imize within-flower selfing (autogamy) may be favored (Rich-ards, 1997). Although autogamy is frequent, it is rarely pre-dominant, probably because most plants growing in harsh con-ditions are long-lived perennials for which constraints on re-productive fitness by seed are low (Richards, 1997). In
Rhododendron parviflorum and R. redowskianum (in Siberia)
spontaneous autogamy occurs in the absence of pollinators (Tikhmenev, 1985), however in some Vaccinium in the Bel-gian Ardennes (V. myrtillus, V. uliginosum, V. vitis-idaea; Jac-quemart and Thompson, 1995), insect-mediated self-pollina-tion accounts for the majority of seeds produced by selfing. In other species, like Rhododendron aureum (in Japanese moun-tains), spontaneous selfing does not occur and insect
pollina-1Manuscript received 3 August 1999; revision accepted 11 April 2000. The authors thank Pascal Thomas for help with the field experiments and John D. Thompson and two anonymous referees for their critical comments and helpful suggestions that greatly improved the first draft of the manuscript, and Paul G. Wolf for improving the English text. This work was financially supported by University Joseph Fourier (Grenoble), the Centre National de la Recherche Scientifique (C.N.R.S.), and the French Ministry of the Environ-ment (E.G.P.N. no. 95.085).
3Author for reprint requests (e-mail: [email protected]).
tion is necessary for seed set (Kudo, 1993). One character that plays a key role in promoting reproductive assurance in self-compatible species is the reduction of stigma-anther separation (Piper, Charlesworth, and Charlesworth, 1986).
The species studied here, Rhododendron ferrugineum L. (Ericaceae), is an evergreen shrub with a mean height of 70 cm that grows at the subalpine level from;1600 to 2200 m. It reproduces both vegetatively (by layering) and sexually. Asexual propagation occurs in closed and mature populations (Pornon et al., 1997) and preferentially down hill (Escaravage et al., 1998). Rhododendron ferrugineum produces an inflo-rescence of 5–22 bright-red nectariferous flowers. The flowers are protandrous (anthers mature before the stigma) and are initiated the year before they mature (Escaravage et al., 1997).
Rhododendron ferrugineum is a self-compatible species,
main-ly pollinated by bees, bumble bees, and diptera. Spontaneous selfing occurs in the absence of pollinators (Escaravage et al., 1997). A study of the floral morphology revealed herkogamy (spatial separation between anther and stigma) with different stamen lengths: short-level stamens below or at the same height as the style and long-level ones above the style (Es-caravage et al., 1997). The term stamen dimorphism will be used subsequently. Stamens are mediafixed (filament links to the back of the anther) and are located on two whorls (five stamens per whorl) with long- and short-level stamens on the inner and outer whorls, respectively (E. Flubacker, personal observation). This has not been described in other
Rhododen-dron species and the question that arises is: what are the
var-iability caused by timing and position of stamen origin prob-ably accounts for variation in stamen length within flowers of heterostylous species that have two whorls of stamens (Rich-ards and Barrett, 1992). In some arctic Primula species, hom-ostyly has replaced heterhom-ostyly (Kelso, 1992). It is suggested that both restrictions of pollination efficiency and develop-mental constraints (imposed by the need for reciprocity in or-gan position and to limit stigma-anther separation) are in-volved in this evolutionary process (Richards and Barrett, 1992).
In this study, we focused on stamen dimorphism and its possible consequences on the breeding system of
Rhododen-dron ferrugineum. The aims of this paper are (1) to describe
the development of the stamens in Rhododendron ferrugineum (from the bud stage to full bloom) to determine at what phe-nological stage stamen dimorphism can first be detected, and (2) to determine the role of long- and short-level stamens in the pollination of the species.
MATERIALS AND METHODS
The study site—This study was carried out during the summer of 1997 at two mountain sites in the northwestern French Alps (Belledonne range). The first was ‘‘Collet d’Allevard’’ (458259N, 68109E) located at 1680 m on a north-facing slope (50%), and the second was ‘‘Chamrousse’’ (458259 N, 508559E) located at 1770 m on a west-facing slope (62%). In both sites, the populations are composed of Rhododendron ferrugineum with a cover of 40%, mixed with other Ericaceous shrubs, such as Vaccinium myrtillus, V. uligi-nosum, and V. vitis-idaea.
Experimental design—Stamen development—To study patterns of stamen development, we randomly selected ten individuals in each population. On each individual we conducted the following experiments: we collected three inflorescences per individual at five successive phenological stages [inflores-cence buds (t0), young inflores[inflores-cences (t012 d), medium-aged inflorescences (t014 d), advance-aged inflorescences (t016 d), and inflorescences with flowers in full bloom (t018 d)]. One flower per inflorescence was dissected and measured under a dissecting microscope, together with the filaments, an-thers, and style.
Effects of population, individual, flower, whorl, and position of stamens on anther and filament length in mature flowers were statistically tested by per-forming five-way mixed analysis of variance using PROC GLM (SAS, 1990). Population, whorl, and position of stamens were treated as fixed effects; in-dividual and flower were random effects. Population, inin-dividual, and flower, and position and whorl were nested (see Table 2).
Associations between anther height of the short- and long-level stamens and length of short- and long-level filament were tested by a correlation anal-ysis (SAS, 1990). Data were ln transformed for normality. All these tests were performed for each developmental stage. The mean daily growth rate, com-puted as the length of each stamen at the end of the last phenological stage (full bloom) divided by the number of days elapsed between bud breaking and full bloom (8 d), was compared by performing a Tukey HSD multiple-range test using SPSS for Windows, release 9.0.0.
Effects of partial emasculation—To determine whether the presence of long-level stamens (inner whorl) limits the development of short-level stamens (outer whorl), we selected three other inflorescences on the same ten individ-uals in which we partially emasculated three flowers at the bud stage. Anthers of long-level stamens (inner whorl) were removed (but their filaments re-mained) and short-level stamens (outer whorl) were left intact. It would have been interesting to remove the stamens of the outer whorl to compare the results, but unfortunately this experiment was difficult to perform without damaging the flower. The other flowers of the inflorescence were unmanipu-lated. When flowers reached their mature size, we collected all the treated inflorescences. We dissected the partially emasculated flowers under a
dis-secting scope and measured the style, anther, and filament lengths of the intact stamens of outer whorl, and the filament length of emasculated stamens (inner whorl). We dissected and performed the same measurements on one unemas-culated flower of each inflorescence as a control. In both experiments, stamens were numbered according to their position in the flower. Stigma-anther sep-aration was calculated as filament length minus style length.
In this experiment, the effects of population, individual, flower, treatment (flower partially emasculated or not), and position of stamens on filament length of each stamen (emasculated or not) were tested by five-way mixed analysis of variance (SAS, 1990) with population, emasculation, and whorl of stamens as fixed effects, individual and flower as random effects, and population, individual, and flower nested. The treatment effect tested for an effect of partial emasculation on the whole, whereas the effect of emasculation on the longer filaments was tested by the treatment3whorl interaction. For each site and treatment (partial emasculation or not) the whorl effect on fil-ament length and stigma-anther separation was tested by one-way analysis of variance (SAS, 1990).
At the end of the flowering period (mid-July), floral primordia for the next flowering season are initiated. To determine when meiosis of pollen grains occurred, we collected two flowers (from different inflorescences) per indi-vidual, on ten individuals in each population every week from mid-July to mid-August. We dissected the flowers and crushed the anthers on a slide in a drop of Alexander’s stain (Alexander, 1969), and observed the slide under a microscope. As pollen is released in tetrads in the Ericaceae, observation of single cells indicates that meiosis had not yet occurred.
Pollination trials—To study the respective roles of short- and long-level stamens in sexual reproduction, we (1) quantified the number of pollen grains per anther in the two whorls and (2) performed a series of pollination treat-ments. To quantify pollen production we collected one inflorescence bud on each of ten individuals and preserved it in 70% ethanol in May 1997. One flower from each bud was dissected under a dissecting scope. Each stamen was measured according to its position (Fig. 1) and pollen grains were quan-tified after acid extraction from the anther using a hemacytometer according to Escaravage et al. (1997). Pollen production was analyzed by performing five-way mixed analysis of variance as described in the stamen development analysis (see above). The association between the number of pollen grains and stamen length was tested by a correlation analysis (SAS, 1990). We per-formed seven pollination treatments (summarized in Table 1) on ten other randomly selected individuals in each population. Each individual received all seven treatments, and each treatment was performed on two inflorescences, on at least five flowers per inflorescence. We thus studied a total of 140 inflorescences and 700 flowers at each site.
In treatments T5 and T7, we emasculated the five long-level stamens (inner whorl), while in treatments T4 and T6 we emasculated the five short-level stamens (outer whorl). In treatments T6 and T7, nylon mesh bags were used to exclude pollinators, whereas treatments T4 and T5 were open-pollinated. We performed three control treatments: in treatment T3 all stamens were emasculated (cross-pollination control), in T2 flowers were unmanipulated and inflorescences were bagged (self-pollination control), and T1 consisted of un-manipulated (intact) inflorescences. To avoid self-pollen contamination, emas-culation was performed; 2 d after the inflorescence bud opened. At this stage, inflorescences were very small and the flowers were still closed. Five flowers per inflorescence (except for T1 and T2) received one of the seven treatments. All other flowers (between one to five flowers) were removed. This manipulation did not disturb the development of the remaining flowers (Escaravage et al., 1997). Fruits were collected shortly before dehiscence (ear-ly September) and the seeds counted.
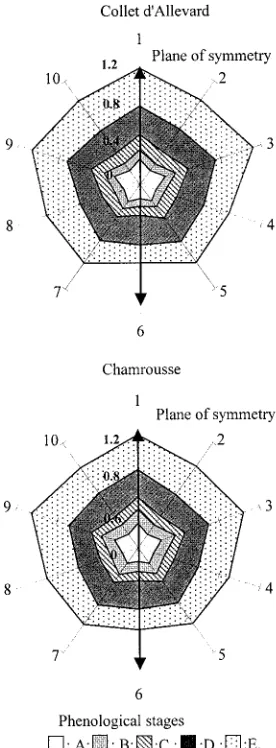
Fig. 1. Mean sizes (in cm) of the different stamens according to their positions for different phenological stages of the flower. Odd-numbered sta-mens (long-level) are on the inner whorl, even-numbered stasta-mens (short-level) on the outer whorl. A: inflorescence buds, B: young inflorescences, C: me-dium-aged inflorescences, D: advance-aged inflorescences, E: inflorescences with flowers in full bloom.
TABLE1. Pollination tests used in the study of the pollination system of R. ferrugineum.
Code
Level of stamens
remaining Bagging No. of flowers/inflo.
T1
TABLE2. Results of analysis of variance to detect the effect of population, individual, flower, whorl, and stamen position on anther and filament lengths in mature flowers.
Stamen development—All floral organs are preformed in
July of the year preceding flowering (N. Escaravage, personal observation). Anthers are relatively voluminous and seem
more advanced in their development than filament, style, and petals. At this stage anthers are positioned in staggered rows (one anther high, one low, etc.), in such a way that stamen dimorphism can already be observed early in development. Moreover, pollen tetrads are observed in anthers from the sec-ond week of August on. Therefore, meiosis occurred in buds preformed in the summer the year prior to flowering.
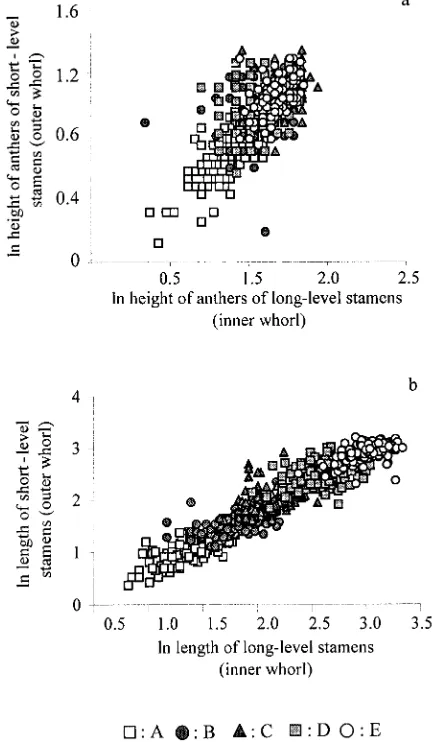
de-Fig. 2. (a) Height of anthers of the long-level stamens as a function of height of anthers of the short-level stamens for each phenological stage (A: r250.6319, N5200, y50.9082x10.0883, P,0.001; B: r250.1012, N5187, y50.3773x10.5638, P,0.05; C: r250.1332, N5199, y5 0.4371x10.5353, P,0.001; D: r250.0171, N5196, y50.1649x1 0.7666, non significant; E: r250.0214, N5194, y50.1756x10.214, non significant) and (b) length of long-level filament as a function of length of short-level filament for each phenological stage (A: r250.6122, N5200, y
50.7982x10.0711, P,0.001; B: r250.2779, N5189, y50.5079x1 0.650, P,0.001; C: r250.1854, N5199, y50.536x 10.8092, P, 0.001; D: r250.2080, N5194, y50.4802x11.2314, P,0.001; E: r2
50.3867, N5191, y50.644x10.9567, P,0.001).
Fig. 3. Mean daily stamen growth rates of each stamen. Odd-numbered stamens are on the long-level inner whorl and even-numbered stamens are on the short-level outer whorl. Vertical bars indicate standard deviation. Values that share the same letter are not significantly different at 5% threshold. Means were compared by a Tukey HSD multiple-range test.
TABLE3. Results of analysis of variance to detect effects of popula-tion, individual, flower, treatment (partial emasculation of long-lev-el stamens), and whorl on filament length in mature flowers.
Source of variation df MS F
Population
Individual (population) Flower [individual (population)] Treatment
Treatment3whorl
Treatment3whorl3population Error
1 18 36 1 2 3 883
157.81 25.68 8.73 799.67 87.26 3.26 1.22
6.13* 2.99* 7.16*** 259.02*** 26.09***
2.67*
Note: ***P,0.001, *P,0.05.
velopment of the flower (Fig. 2b). For each stamen, the daily mean growth rate is shown in Fig. 3. Stamen 6 (the shortest stamen) has the slowest growth rate, whereas stamens 1, 3 and 9 (the longest stamens) have the fastest.
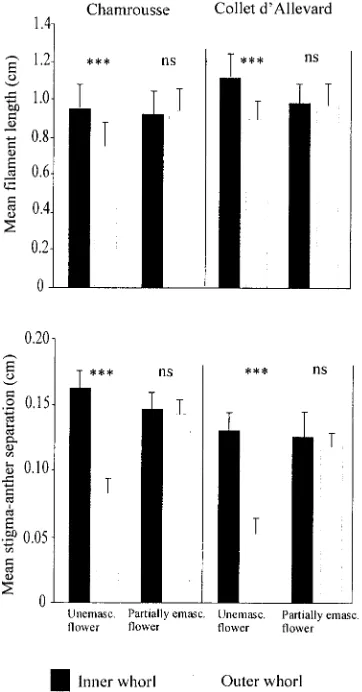
In partially emasculated flowers, treatment (effect on the whole flower) and treatment 3 whorl interaction (specific to the emasculated whorl) have a significant effect on filament length (Table 3; P , 0.001). At the end of the growth of partially emasculated flowers, mean length of the filaments of stamens on the outer whorl (unemasculated) had increased compared to unemasculated flowers. In both populations, the filament length difference between whorls disappeared. For partially emasculated flowers, stamen filaments of the outer whorl were not different from those of the inner whorl but
were significantly longer than those of unemasculated flowers (P,0.001; Fig. 4). In unemasculated flowers, stamens of the inner, long-level whorl showed a greater stigma-anther sepa-ration than stamens of the outer, short-level whorl (P,0.001). In partially emasculated flowers the increased growth of the stamens of the outer whorl significantly increased the stigma-anther separation (Fig. 4); as a result this distance was similar to that measured between anthers of the longest stamens (inner whorl) and stigma in unemasculated flowers.
Number of pollen grains per stamen—Long-level stamens
contain significantly more pollen grains than short-level sta-mens (Table 4), and all effects are significant. A positive cor-relation exists between stamen length and the number of pollen grains in both populations (P , 0.001 with r2 5 0.211 and
0.273 for Collet d’Allevard and Chamrousse, respectively). At Collet d’Allevard the mean number of pollen grains was 16 000 (64498 SD) and 12 257 (63761 SD) for long- and short-level stamens, respectively. At Chamrousse, mean pollen grain production was 21 152 (64563 SD) and 15 974 (63861 SD) for long- and short-level stamens, respectively.
Effect of partial emasculation on seed
Fig. 4. Mean filament length and anther-stigma separation for the inner and outer stamen whorls. One-way analysis of variance indicated significant differences among whorls in unemasculated flowers but not in flowers where the stamens of the inner whorl were removed. Vertical bars indicate standard deviation.
TABLE4. Results of analysis of variance to detect the effect of whorl, position, population, individual, and flower on the number of pollen grains.
Source of variation df MS F
Whorl
Position (whorl) Population
Individual (population)
Flower (individual3population)
1 8 1 18 18
31147.6 254.8 35143.6 2657.1 304.2
316.1*** 2.6** 13.3** 8.7*** 3.1*** Whorl3population
Whorl3individual (population)
Whorl3flower (individual3population) Error
1 18 18 654
1301.7 236.7 297.3 98.5
13.2*** 2.4*** 3.0***
Note: ***P,0.001, **P,0.01.
TABLE5. Results of analysis of variance for each population, to detect individual, inflorescence, and treatment effects on the seed number per fruit.
Source of variation df
Population
Chamrousse
MS F
Collet d’Allevard
MS F
Seeds/fruit Individual
Inflorescence (individual) Treatment
Treatment3inflorescence (individual) Error
9 10 6 60 49
536.8 676.6 14053.7 280.5 261.6
0.8 ns 2.5* 53.7***
1.1 ns
1297.2 491.9 20110.3 376.9 927.6
2.6 ns 0.5 ns 21.6***
0.4 ns
Note: ***P,0.001, *P,0.05, ns5non-significant.
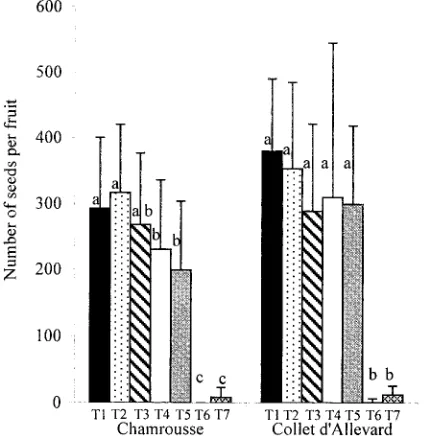
0.001, Table 5). The greatest number of seeds was produced following open pollination and when pollinators were exclud-ed and flowers left intact (T1 and T2; Fig. 5). At Chamrousse, the number of seeds decreased when at least one stamen whorl was emasculated (T4 and T5; Fig. 5), whereas at Collet d’Allevard the mean number of seeds produced was not af-fected significantly. In contrast when short stamens were emas-culated and inflorescences bagged, very few seeds were pro-duced (T7; Fig. 5). The same result was obtained when long
stamens were removed (T6; Fig. 5). This effect was likely due to the extra growth of short-level stamens following removal of long-level ones (Fig. 4).
DISCUSSION
In R. ferrugineum two stamen levels are observed: the long-level stamens of the inner whorl and the short-long-level stamens of the outer whorls. Long-level stamens produce more pollen grains than short-level stamens. This stamen dimorphism can be detected as early as June of the year preceding flower mat-uration, and pollen grains are formed in August of the same year. When long-level stamens are removed early the flower-ing year, the short-level stamens grow longer. Stamen dimor-phism has not been described in other Rhododendron species but is widespread among heterostylous taxa. As in tristylous species of Pontederia (Richards and Barrett, 1987) and
Ei-chhornia (Richards and Barrett, 1984) and in distylous species
of Primula (Stirling, 1932), stamen dimorphism in R.
ferru-gineum appears in premeiotic stamens and is maintained
throughout phenological stages.
Fig. 5. Mean number of seeds produced per fruit. Vertical bars indicate standard deviation. For each site the treatment effect was tested by a Scheffe´ multiple-range test. For each site, values that share the same letter are not significantly different at 1% threshold.
personal observation). In alpine environments, early anther formation and spermatogenesis could be a strategy to allow the flower to become functional rapidly and could be an ad-aptation to the short optimal reproductive period, allowing the plant to flower as soon as the temperature increases. The for-mation of pollen grains in the season preceding flowering is risky since a severe winter could occur and freeze the buds. However, this risk is reduced because flowers are protected in bud scales and plants of R. ferrugineum usually spend the winter under thick snow cover that buffers temperature effects (Larcher and Siegwolf, 1985).
Two main categories of causes can explain stamen dimor-phism: (1) ‘‘proximate’’ and (2) ‘‘ultimate’’ causes that refer to developmental and selective aspects, respectively. Under the first category filament expansion can be regulated, through an-thers, by growth hormones such as cytokinin (Hess and Morre´, 1978), gibberellins (Greyson and Tepfer, 1967), or auxin (Kon-ing, 1983). Greyson and Tepfer (1967) argued that inhibition of filament growth following emasculation in Nigella
hispan-ica could be accompanied by inhibition of both cell elongation
and cell division in the epidermis. In R. ferrugineum, emas-culation of the inner whorl results in extra growth of the outer whorl, yet emasculation does not affect the growth of the inner whorl. This suggests that, if hormonal control is involved it is only in the one direction. Testing for hormonal control can be challenging because it is often difficult to distinguish between hormonal control and homeostatic responses.
Monopolization of space by anthers of long stamens could represent a sufficient barrier to the development of the short stamens to create a mechanical resistance to filament cell di-vision. Thus, the different sizes should disappear when the spatial constraint imposed by the long stamen is removed. However the size difference between long- and short-level sta-mens lies primarily in filament length and filament growth occurs when flowers open and space is not as limiting. Daily stamen growth rate is similar across all stamens except for the
shortest, stamen 6, and the longest ones, stamens 1, 3, and 9 (Fig. 3). We detected no difference in stamen growth rate be-tween inner and outer whorls (data not shown). This is differ-ent from several heterostylous species (Richards and Barrett, 1984, 1987) in which differences in stamen length among morphs arise through differences in the relative growth rate and the duration of growth. However, some species of
Pon-tederiaceae, Lythraceae, and Oxalidaceae initiate two
tem-porally separated stamen sizes. The difference in time of origin establishes a size differential between stamen series that is maintained throughout development. This mechanism could account for the two stamen levels in R. ferrugineum.
Short stamens might benefit from resources normally allo-cated to the development of long stamens (inner whorl). How-ever, this suggestion seems unlikely since differences in nu-trient availability between populations, individuals, inflores-cences, and flowers would induce variation in stamen size within the two whorls. On the contrary, stamen hierarchy in relation to their position in whorls appears to be similar in both populations.
In the second category, ultimate causes, the two stamen lev-els may have different functions in pollination in R.
ferrugi-neum. Our results indicate that long-level stamens do not
par-ticipate autonomously in self-pollination in the absence of pol-linators since no seeds were produced when short stamens are removed (T6; Fig. 5). Because unemasculated flowers could self in the absence of pollinators, spontaneous self-pollination is likely due to short-level stamens. However, our experiments provided no formal proof. The treatment in which long-level stamens were removed also produced no seeds in the absence of pollinators (T7; Fig. 5), but, when short-level stamens were in the corolla alone, they grew to the height of long-level stamens. Thus they function similarly to long-level stamens and are inefficient in autonomous selfing. Spatial separation between the stigma and the anther of the longest stamen is on average 1.50 mm. This distance seems to be sufficient to avoid self-pollination (Webb and Lloyd, 1986), especially in R.
fer-rugineum where pollen is sticky and dehiscence poricidal.
The distance between stigmas and anthers of short-level sta-mens is smaller at Collet d’Allevard than at Chamrousse. This may explain why plants at Collet d’Allevard produced more seeds in treatment T5. The pollination mechanism in open-pollinated treatment T1 is less obvious, and we do not know the proportion of seeds sired by self- and cross-pollination, or which stamen level is involved. However in this species the autofertility index is high (0.92; Escaravage et al., 1997), sug-gesting that autogamy could account for the majority of seeds produced.
In a previous study on the genotypic structure of a R.
fer-rugineum population, Escaravage et al. (1998) showed that
1995). Therefore, in R. ferrugineum, pollination by short-level stamens could act as reproductive assurance in the absence of pollinators that would prevent outcrossing. Reproductive as-surance is promoted in numerous plants growing in severe environments. In arctic Primula species, it is common to see a shift in breeding system from distyly to homostyly and at least facultative autogamy (Kelso, 1992; Mazer and Hultgard, 1993; Miller et al., 1994). Armeria maritima has also lost dis-tyly in the tundra portion of its range (Vekemans et al., 1990). Anther position can affect pollen removal through its influ-ence on the timing of anther dehiscinflu-ence (Harder and Barrett, 1993). However, all anthers of R. ferrugineum flowers dehisce before the flower opens (as in R. ponticum; Percival, 1955), whereas stigmas become receptive when the flower opens (Es-caravage et al., 1997). No difference in the timing of anther dehiscence among whorls has been observed (N. Escaravage, personal observation). Anther position can also determine the placement of pollen on a pollinator’s body, which influences the likelihood of pollen loss through grooming (Faegri and van der Pijl, 1979). It seems that the advantages of a particular anther position depend on the morphological and behavioral characteristics of a plant’s dominant pollinators (Harder and Barrett, 1993). For bee-pollinated species with tubular flowers, anther placement in the opening of the perianth mouth seems to be the best position for pollen removal. King and Buchmann (1995) suggested that Rhododendron spp. are seldom buzz-pollinated because the flowers are too large. However, R.
fer-rugineum has relatively small flowers and stamens are short
and located in the perianth mouth, where bees and bumble bees can be in contact with all of the anthers. So far, this pollination aspect has not been addressed in R. ferrugineum and should be studied in future investigations.
Rhododendron ferrugineum seems to fit well with the usual
theories concerning plants that grow in drastic environments: (1) this species spreads vegetatively and (2) has a high sexual reproduction potential (0.4–2.5 million seeds/m2of heathland;
Pornon et al., 1997). Thus, even if seedling establishment oc-curs rarely, episodic recruitment is sufficient to maintain a high level of genotypic diversity in the populations (Escaravage et al., 1998); Also, (3) all floral organs are formed in the year previous to flowering allowing the species to flower as soon as conditions become favorable and (4) R. ferrugineum repro-duces both through self- and cross-pollination (Escaravage et al., 1997) with one level of stamens (the short ones) devoted to self-pollination when pollinators are scarce.
The stamen dimorphism in flowers of R. ferrugineum has been studied under both developmental and selective aspects. According to Gould and Lewontin (1979), evolutionary biol-ogists focus exclusively on immediate adaptation to local con-ditions and tend to ignore developmental constraints. In this study we showed that stamen dimorphism in R. ferrugineum flowers may originate from developmental constraints. How-ever, it also becomes adaptive by favoring reproductive assur-ance.
LITERATURE CITED
ALEXANDER, M. P. 1969. Differential staining of aborted and nonaborted pollen. Stain Technology 44: 117–122.
ARROYO, M. T. K. 1974. Electrophoretic studies of genetic variation in nat-ural population of allogamous Limnanthes alba and autogamous Lim-nanthes flocossa (Limnanthaceae). Heredity 35: 153–164.
———, R. PRIMACK,ANDJ. J. ARMESTO. 1982. Community studies in pol-lination ecology in the high temperate Andes of central Chile. I.
Polli-nation mechanisms and altitudinal variation. American Journal of Botany 69: 82–97.
AYDELOTTE, A. R.,ANDP. K. DIGGLE. 1997. Analysis of developmental preformation in the alpine herb Caltha leptosepala (Ranunculaceae). American Journal of Botany 84: 1646–1657.
BELL, K. L.,ANDL. C. BLISS. 1980. Plant reproduction in high arctic en-vironment. Arctic and Alpine Research 12: 1–10.
BLISS, L. C. 1971. Arctic and alpine plant life cycles. Annual Review of Ecology and Systematics 2: 405–438.
CHEUNG, M.,ANDR. SATTLER. 1967. Early floral development of Lythrum salicaria. Canadian Journal of Botany 45: 1609–1618.
DIGGLE, P. K. 1997. Extreme preformation in alpine Polygonum viviparum: an architectural and developmental analysis. American Journal of Botany 84: 154–169.
ESCARAVAGE, N. 1997. Syste`me de reproduction et strate´gie de colonisation de Rhododendron ferrugineum L. (Ericaceae); (e´tage subalpin; Alpes du Nord), thesis, University of Grenoble, France.
———, A. PORNON, B. DOCHE,ANDI. TILL-BOTTRAUD. 1997. Breeding system in an alpine species: Rhododendron ferrugineum L. (Ericaceae) in the French Northern Alps. Canadian Journal of Botany 75: 736–743. ———, S. QUESTIAU, A. PORNON, B. DOCHE, ANDP. TABERLET. 1998. Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) popula-tion inferred from AFLP markers. Molecular Ecology 7: 975–982. FAEGRI, K.,ANDL. VANDERPIJL. 1979. The principles of pollination
ecol-ogy, 3rded. Pergamon, Oxford, UK.
GANDERS, F. R. 1979. The biology of heterostyly. New Zealand Journal of Botany 17: 607–635.
GOULD, S. J.,ANDR. C. LEWONTIN. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proceedings of the Royal Society of London, B 205: 581–598. GREYSON, R. I.,ANDS. S. TEPFER. 1967. Emasculation effects on the stamen
filament of Nigella hispanica and their partial reversal by gibberellic acid. American Journal of Botany 54: 971–976.
HARDER, L. D.,ANDS. C. H. BARRETT. 1993. Pollen removal from tristylous Pontederia cordata: effects of anther positions and pollinators speciali-zation. Ecology 74: 1059–1072.
———,AND———. 1995. Pollen dispersal and mating patterns in animal-pollinated plants. In D. G. Lloyd and S. C. H. Barrett [eds.], Floral biology: studies on floral evolution in animal-pollinated plants, 140–190. Chapman and Hall, New York, New York, USA.
HESS, E.,ANDD. J. MORRE´. 1978. Fine structural analysis of the elongation zone of Easter lily (Lilium longiflorum) staminal filaments. Botanical Gazette 139: 312–321.
HOLSINGER, K. E. 1992. Ecological models of plant mating systems and the evolutionary stability of mixed mating systems. In R. Wyatt [ed.], Ecol-ogy and evolution of plant reproduction. New approaches, 169–191. Chapman and Hall, New York, New York, USA.
JACQUEMART, A. L., ANDJ. D. THOMPSON. 1995. Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the Upper Ardennes, Belgium. Canadian Journal of Botany 74: 210–221. KELSO, S. 1992. The genus Primula as a model for evolution in Alaska flora.
Arctic and Alpine Research 24: 82–87.
KEVAN, P. 1973. Flower insects, and pollination ecology in the Canadian high arctic. Polar Record 16: 667–674.
KING, M.,ANDS. L. BUCHMANN. 1995. Bumble bee-initiated vibration re-lease mechanism in Rhododendron pollen. American Journal of Botany 82: 1407–1411.
KONING, R. E. 1983. The role of auxin, ethylene and acid growth in filament elongation in Gaillardia grandiflora (Asteraceae). American Journal of Botany 70: 602–610.
KUDO, G. 1993. Relationship between flowering time and fruit set of the entomophilous Rhododendron aureum (Ericaceae), inhabiting snow patches. American Journal of Botany 80: 1300–1304.
LARCHER, W.,ANDR. SIEGWOLF. 1985. Development of acute frost drought in Rhododendron ferrugineum at the alpine timberline. Oecologia 67: 298–300.
MAZER, S. J.,ANDU. M. HULTGARD. 1993. Variation and covariation among floral traits within and among four species of Northern European Primula (Primulaceae). American Journal of Botany 80: 474–485.
PERCIVAL, M. S. 1955. The presentation of pollen in certain angiosperms and its collection by Apis mellifera. New Phytologist 54: 353–368. PIPER, J. G., B. CHARLESWORTH,ANDD. CHARLESWORTH. 1986. Breeding
system evolution in Primula vulgaris and the role of reproductive assur-ance. Heredity 56: 207–217.
PORNON, A., N. ESCARAVAGE, I. TILL-BOTTRAUD, ANDB. DOCHE. 1997. Variation of reproductive traits in Rhododendron ferrugineum L. (Eri-caceae) populations along a successional gradient. Plant Ecology 130: 1–11.
RICHARDS, A. J. 1997. Plant breeding systems, 2nded. Chapman and Hall, London, UK.
RICHARDS, J. H.,ANDS. C. H. BARRETT. 1984. The developmental basis of tristyly in Eichhornia paniculata (Pontederiaceae). American Journal of Botany 71: 1347–1363.
———,AND———. 1987. Development of tristyly in Pontederia cordata (Pontederiaceae). I. Mature floral structure and patterns of relative growth rate of reproductive organs. American Journal of Botany 74: 1831–1841. ———,AND———. 1992. The development of heterostyly. In S. C. H. Barrett [ed.], Evolution and function of heterostyly, 85–127. Springer-verlag, Berlin, Germany.
SAS. 1990. SAS user’s guide: SAS Institute, Cary, North Carolina, USA. STIRLING, J. 1932. Studies of flowering in heterostyled and allied species.
Part I. The Primulaceae. Publication of the Hartley Botanical Laboratory 8: 3–42.
TIKHMENEV, E. A. 1985. Pollination and self-pollination potential of ento-mophilic plants in arctic and mountain tundras of the Northeastern USSR. Soviet Journal of Botany 15: 166–172.
TOTLAND, O. 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic and Alpine Research 26: 66–71.
VEKEMANS, X., C. LEFEBVRE, L. BELALIA,ANDP. MEERTS. 1990. The evo-lution and breakdown of the heteromorphic incompatibility system of Armeria maritima revisited. Evolutionary Trends in Plants 4: 15–23. WEBB, J. C.,ANDD. G. LLOYD. 1986. The avoidance of interference between
the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178.