Reducing nitrous oxide emission from an irrigated rice field of
North India with nitrification inhibitors
Deepanjan Majumdar, Sushil Kumar
∗, H. Pathak,
M.C. Jain, Upendra Kumar
Division of Environmental Sciences, Nuclear Research Laboratory, Indian Agricultural Research Institute, New Delhi 110012, India
Received 25 August 1999; received in revised form 4 January 2000; accepted 21 March 2000
Abstract
Nitrification inhibitors may be potential management strategy to reduce N2O emissions in irrigated rice (Oryza sativa
L.). A field experiment was conducted to evaluate chemically synthesized as well as locally available neem plant products on N2O emissions, from an irrigated rice at New Delhi, India. Emission of nitrous oxide (N2O) was monitored during 70
days by closed chamber method in rice (var. IR-72) grown on a Typic Ustochrept (cambisol) soil. Treatments were control (no nitrogen), urea alone, urea mixed with different nitrification inhibitors, namely, urea plus dicyandiamide (DCD), neem (powdered Azadirachta indica Juss. seeds) coated urea and nimin (commercial derivative of neem) coated urea. Total N2O–N
emission was highest with urea (59.9 g N2O–N ha−1) and lowest in the control (34.3 g N2O–N ha−1). Total N2O emission
from both nimin coated urea and neem coated urea were not significantly different from urea alone. Urea treated with DCD significantly reduced N2O emissions from urea alone (48.9 g N2O–N ha−1). Nitrogen lost through N2O emission were 0.018,
0.010, 0.016 and 0.013% of total nitrogen applied through urea, urea plus DCD, nimin coated urea and neem coated urea, respectively. Fluxes of N2O were low during flooding but increased markedly during drainage of standing water. After 70
days of transplanting of rice, N2O flux was hardly detectable in any of the treatments. The study indicated that some plant
products, such as neem seeds and nimin which are more readily available with farmers in India, might be useful in mitigating N2O emissions from rice in addition to DCD, which is a widely used nitrification inhibitor. © 2000 Elsevier Science B.V. All
rights reserved.
Keywords: Dicyandiamide; Neem coated urea; Nimin coated urea; Nitrous oxide; Rice
1. Introduction
Nitrous oxide (N2O) is one of the major
green-house gases contributing to global warming (Bouw-man, 1990) and is involved in catalytic destruction of stratospheric ozone (Crutzen, 1970). Fertilizer
appli-∗Corresponding author. Tel.:+91-11-5781490/5786367; fax:+91-11-5766420/5724273.
E-mail address: joy [email protected] (S. Kumar).
cation in rice soils leads to increased N2O emissions
(Eichner, 1990). Agricultural soils fertilized with ni-trogen account for about 81% of the anthropogenic emissions of N2O to the atmosphere (Iserman, 1994).
Houghton et al. (1996) estimated that on the global scale fertilized soils emit 10–17 Tg N2O–N per year.
According to Sharma et al. (1995), N2O–N emissions
from irrigated and upland paddy fields in India is es-timated to be 0.004–0.21 and 0.002–0.01 Tg per year, respectively. Nitrous oxide emission from fertilized
rice has been reported by several other authors (Smith et al., 1982; Buresh and DeDatta, 1990; Lindau et al., 1990; Cai et al., 1997, 1999).
Use of nitrification inhibitors could be one of the mitigation strategies of N2O emissions which can slow
down NH4+ oxidation and thereby limit the loss of
N2O during nitrification and denitrification (Aulakh
et al., 1984). Dicyandiamide (DCD) is one of the most widely used bacteriostatic nitrification inhibitors in agriculture (Zacherl and Amberger, 1990), and decom-poses in soil to nontoxic products (Amberger, 1989). Effects of DCD on N2O emissions have been reported
by de Klein and van Logtestijn (1994) in grassland, Mosier et al. (1996) in wheat (Triticum aestivum L.) and maize (Zea mays L.) and McTaggart et al. (1994, 1997) in ryegrass (Lolium perenne L.), grassland and spring barley (Hordeum vulgare L.).
Nitrification inhibition proprieties of neem seed cake have been reported by Reddy and Prasad (1975), Mishra and Chonkar (1978) and Thomas and Prasad (1982) while nitrification inhibition property of nimin (commercial derivative of neem) coated urea has been reported by Vyas et al. (1991). Little work has been done on the effect of neem cake or nimin coated urea on N2O emission.
Nitrification inhibitors may not be highly efficient in lowland rice, as continuous submergence of the field under these conditions make the soil system anaerobic, which suppresses the nitrification process. But in irrigated rice, standing water drains rapidly making the soil fully or partially aerobic, especially in sandy loams. To maintain water levels in rice fields, frequent irrigation has to be given. When fields are irrigated with fresh water, it brings substantial amount of dissolved oxygen and soil may remain partially aerobic for some time even after submergence. In such situations, nitrification inhibitors might be use-ful in inhibiting nitrification and might as well reduce emission of N2O from rice field.
Although DCD has been found as a potent nitrifica-tion inhibitor in irrigated agriculture, little informanitrifica-tion is available on its use in rice to observe its efficacy in reducing N2O emissions. Neem coated urea and
nimin coated urea, are locally and more readily avail-able with the farmers in India, and have nitrification inhibition properties. Hence, the present study was undertaken to evaluate the effects of three nitrifica-tion inhibitors, namely, DCD, neem coated urea and
nimin coated urea on the emissions of nitrous oxide from soil under rice crop.
2. Materials and methods
2.1. Experimental site
The experiment was carried out at the Indian Agri-cultural Research Institute, New Delhi, India. The soil was a Typic Ustocrept (FAO: Cambisol), sandy loam in texture, with pH 7.85, organic carbon 6.8 g kg−1, total N 0.59 g kg−1and EC 0.48 dS m−1. Rice (var. IR 72) was grown during kharif season (monsoon sea-son, June–September, during which rice is cultivated widely in south-east Asia due to high rainfall and high temperature required by rice) of 1998. Twenty five-days old rice seedlings were transplanted in 3 m×3.5 m plots at 15 cm×20 cm spacing (290 rice seedlings per plot) in a randomized block design with the following treatments: urea alone, urea plus DCD (UD), neem coated urea (NU), nimin coated urea (NCU), and no urea (control).
2.2. Treatments
A total of 140 kg N ha−1was applied through
dif-ferent sources, namely, urea alone, urea plus DCD, neem coated urea and nimin coated urea. DCD was added to urea at 15% of N added through urea. Neem coated urea was prepared from neem (Azadirachta indica Juss.) seeds obtained from New Delhi market. The kernels of neem seed were ground to a fine pow-der and coated on urea by mixing 1 kg urea with 200 g neem seed powder using coal tar-kerosene slurry as an adhesive (1:2, w/w). Nimin coated urea was pre-pared by mixing urea with nimin, a product of neem obtained from Godrej India Ltd., Mumbai, India, by the procedure described by Vyas et al. (1991). Basal application of nitrogen at 100 kg ha−1 was given
Irrigation (7±2 cm depth of water) was given at suit-able intervals up to physiological maturity (20 days before harvesting) of the crop.
2.3. Collection of N2O samples
Gas samples were collected using Plexiglas cham-bers (50 cm×20 cm×100 cm) placed on aluminium channels (60 cm×30 cm) inserted permanently in the soils. To make the arrangement airtight, water was poured into the channels. Boxes were kept on the plants for 2 h continuously during the daytime (10:00– 12:00 hours) on the days of sampling. Gas samples were collected through sampling ports fitted at the top of the chamber using gas tight syringes. The samples were transferred to 15 ml vacutainer tubes, brought to the laboratory and analyzed for N2O by a
gas chromatograph (Hewlett-Packard, Series 5590), fitted with Porapack-N column and electron capture detector (ECD). Nitrogen was used as carrier gas with a flow rate of 20 ml min−1. Oven, injector and detector temperatures were set at 50, 120, 350◦C, respectively. A sample of 500 ppb N2O was used as
standard. Total emissions of N2O–N during the
en-tire period were estimated by multiplying the average emissions of two consecutive sampling dates by the number of days in between. Soil redox potential of soil was measured in situ by portable pH–millivolt meter (Phillips, PW9424).
2.4. Soil analysis
Soil samples were collected from a depth of 0–15 cm soil and immediately extracted with 2 M KCl and analyzed for NH4+–N by the indophenol blue
method (Keeney and Nelson, 1982). NO3−–N content
of soils was estimated by the phenol disulphonic acid method (Ghosh et al., 1983) after extracting the soil with distilled water.
2.5. Statistical analysis
Analysis of variance and Duncan’s multiple range test (DMRT) were conducted to determine the degree of variability and critical difference between the means of total N2O emissions. Statistical analyses were done
using the statistical package MSTAT-C (Version 1.41),
developed by Crop and Soil Science Division, Michi-gan State University, USA.
3. Results and discussion
3.1. Emissions of nitrous oxide
Emissions of N2O started on first day after
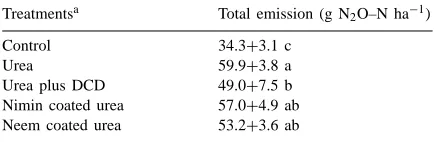
trans-planting (DAT) in all the treatments (Fig. 1). N2O
fluxes (i.e. rate of emission per unit area per day) from control were low throughout the study period, never exceeding 216mg N2O–N m−2per day. Mean
N2O flux during the study period was higher with urea
as compared to urea with inhibitors, indicating the inhibitory role of the nitrification inhibitors in N2O
emission. Top dressing with N on 50 DAT resulted in appearances of small peaks of N2O in all the
treat-ments (Fig. 1). The N2O fluxes were significant up to
70 DAT and were not detectable thereafter. Temporal variation of N2O fluxes was high in all the treatments
(CV values ranged between 109–151%).
Total N2O–N emission during 70 days with urea
was significantly higher than that of control, urea plus DCD, whereas it was at par with neem coated urea, nimin coated urea (Table 1). Total emissions with urea, nimin coated urea, neem coated urea and urea plus DCD were 75, 66, 56 and 43%, respectively, higher than control. Total N2O–N emissions were in
the range of 0.010 and 0.018% of the total nitrogen applied through different treatments and were lower than the values reported by other workers. The N2O–N
emissions were reported to vary between 0.01–0.55% of the total nitrogen applied in rice (Smith et al.,
Table 1
Total nitrous oxide emissions (mean±S.E.) with fertilizer urea and different nitrification inhibitors from a rice field in India during 70 days
Treatmentsa Total emission (g N2O–N ha−1)
Control 34.3+3.1 c
Urea 59.9+3.8 a
Urea plus DCD 49.0+7.5 b Nimin coated urea 57.0+4.9 ab Neem coated urea 53.2+3.6 ab
1982; Minami, 1987; Cai et al., 1997). Studies have shown that total N2O emissions accounted for 0.49%
(Mahmood et al., 1998), 0.77% (Skiba et al., 1996), 0.42–0.55% (Henault et al., 1998) of applied N to wheat crop. Higher loss of N through N2O emission
in wheat is argued to be due to higher production of N2O, via vigorous rate of nitrification in aerobic soil
conditions under wheat.
3.2. Effect of water content and Ehon N2O emissions
Emissions of N2O were influenced by water
con-tent and soil Ehof the plots (Fig. 1). Decreasing
wa-ter levels were accompanied by high soil Eh and
in-creased N2O emissions. Under submergence, the soil
Eh values were highly negative and N2O emissions
were low. Flooded paddy soils have an aerobic surface layer and an anaerobic subsurface layer (Reddy and Patrick, 1976) where N2O can be produced, via
nitri-fication of ammonium to nitrate and denitrinitri-fication of accumulated nitrate, respectively. Natural drainage in this sandy loam soil was high and the field dried up intermittently during the crop growth. Natural drain-ing of standdrain-ing water, accompanied by high soil Eh,
increased N2O emissions. Shao (1993) also reported
significant increase in N2O emission after drainage of
standing water. This could be the result of nitrifica-tion, which took place in aerobic soil after drainage of standing water, indicated by increased soil Eh.
N2O fluxes had significant correlation with
corre-sponding daily soil Eh in all the treatments (values
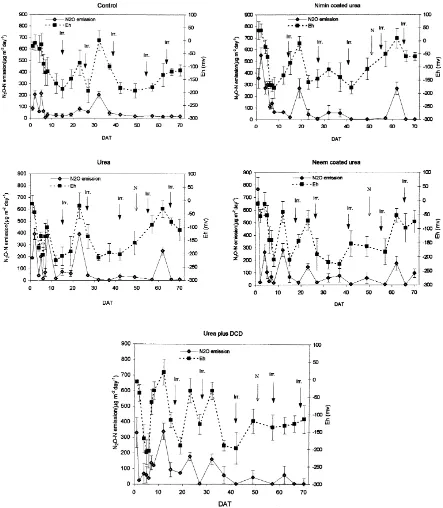
of ‘r’ being 0.79, 0.66, 0.76, 0.70, 0.60 for control, urea, urea plus DCD, nimin coated urea and neem coated urea, respectively, ∗∗ p<0.01). Nitrification might have been vigorous at times when sufficient NH4+–N in soil was present and N2O may have been
produced (Fig. 2). Accumulated nitrate disappeared quickly from surface soil layers due to leaching and crop removal, apart from its conversion to N2O and
N2 and thereby depleting the precursor of N2O
pro-duction through denitrification (Fig. 3). Cai et al., (1997) reported that N2O emissions were low under
continuous flooding while under mid season aeration emissions increased markedly, giving a pulse-like N2O emissions pattern. Flessa and Beese (1995)
ob-served very little N2O emitted during water-logging
while N2O emissions peaked when waterlogged soil
columns were drained. Smith and Patrick (1983)
Fig. 2. Soil NH4+content in a rice field with and without fertilizer nitrogen and different nitrification inhibitors, e.g., NCU, NU and UD over 70 DAT (bars indicate mean N2O–N flux±S.D. for five treatments on each DAT).
showed that alternate anaerobic and aerobic cycling considerably increased N2O emissions relative to
constant anaerobic or aerobic conditions.
3.3. Effect of nitrification inhibitors on N2O
emissions
The nitrification inhibitors played an important role in reducing N2O emissions from the rice field. The
percent inhibition of total N2O emission was
calcu-lated using the following equation:
N2O inhibition(%)=
The highest inhibition of total N2O emission (43%)
was recorded from plots treated with urea plus DCD followed by neem coated urea (26%) and nimin coated urea (11%) in the current study. Urea plus DCD was able to reduce N2O emission significantly while neem
coated urea and nimin coated urea did not reduce it significantly from urea alone (Table 1). DCD has been reported to have reduced N2O emission by 40% in
a dry sandy loam soil, while in wet conditions no inhibition was noticed (Skiba et al., 1993).
DCD was most efficient in inhibiting nitrification of ammonium followed by neem coated urea and nimin coated urea. This inhibitory effect was visible only up to a period of 6 weeks (Figs. 2 and 3) during which DCD was able to maintain highest soil NH4+content.
Due to slower nitrification indicated by higher levels of NH4+, soil NO3−was also lower up to this period with
DCD application. NH4+–N increased immediately
af-ter urea application due to hydrolysis of urea and it decreased subsequently. Up to a period of 2 weeks, NO3−concentration in urea treated plots were higher
than plots treated with inhibitors. Joseph and Prasad (1993) have reported higher inhibition of nitrification of NH4+by DCD than neem cake. Nimin coated urea
was less efficient than urea plus DCD and neem coated urea in preserving soil NH4+.There was considerable
inter-replicate variation in NH4+ and NO3− content
of the soil, as indicated by S.D. values (Figs. 2 and 3). The study showed that N2O emissions may not be a
serious concern from the economic point of view con-sidering the low percentage of applied N lost through N2O emissions. But its high global warming
poten-tial and total annual emission load to the atmosphere by widespread rice cultivation in south Asia, may add significantly to enhanced greenhouse effect. So, efforts are needed to mitigate N2O emission, as
agri-culture needs increasingly higher fertilizer N to meet production demands. Use of nitrification inhibitors is an important mitigation option for N2O emissions.
Locally and readily available plant products like neem
cake, nimin, etc., could be the cheaper resource for poor farmers for reducing N2O emission as against the
expensive nitrification inhibitors, especially in devel-oping countries. Further, these nitrification inhibitors may reduce nitrate leaching to the groundwater under rice cultivation and reduce health risks.
4. Conclusions
1. The study indicated that application of fertilizer N through urea in irrigated rice could lead to a significant increase in N2O emissions as compared
to no fertilizer application.
2. DCD reduced N2O emission significantly from the
rice field when used along with urea.
3. Neem or nimin coated urea showed no significant reduction in N2O emission.
4. Although nitrogen lost through N2O emission from
the rice field was as low as 0.01% by the lone ap-plication of urea, but total N2O emission from rice
cultivation in South Asia may be worth reckoning, considering large area under rice. This emphasizes the need for further research on N2O emission from
rice cultivation.
References
Amberger, A., 1989. Research on DCD as a nitrification inhibitor and future outlook. Commun. Soil Sci. Plant Anal. 20, 1933– 1955.
Aulakh, M.S., Rennie, D.A., Paul, E.A., 1984. Acetylene and N-serve effects upon N2O emissions from NH4+ and NO3− treated soils under aerobic and anaerobic conditions. Soil Biol. Biochem. 16, 351–356.
Bouwman, A.F., 1990. Exchange of greenhouse gases between terrestrial ecosystems and atmosphere. In: Bouwman, A.F. (Ed.), Soils and Greenhouse Effect. Wiley, Chichester, pp. 61–127. Buresh, R.J., DeDatta, S.K., 1990. Denitrification losses from
puddled rice soils in the tropics. Biol. Fertil. Soils 9, 1–13. Cai, Z., Xing, G., Yan, X., Xu, H., Tsuruta, H., Yagi, K., Minami,
K., 1997. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilizers and water management. Plant Soil 196, 7–14.
Cai, Z.C., Xing, G.X., Shen, G.Y., Xu, H., Yan, X.Y., Tsuruta, H., Yagi, K., Minami, K., 1999. Measurements of CH4 and N2O emissions from rice paddies in Fengqiu, China. Soil Sci. Plant Nutr. 45, 1–13.
de Klein, C.A.M., van Logtestijn, R.S.P., 1994. Denitrification and N2O emission from urine-affected grassland soil. Plant Soil 163, 235–242.
Eichner, M.J., 1990. Nitrous oxide emissions from fertilized soils: summary of available data. J. Environ. Qual. 19, 272–280. Flessa, H., Beese, F., 1995. Effects of sugarbeet residues on soil
redox potential and nitrous oxide emissions. Soil Sci. Soc. Am. J. 59, 1044–1051.
Ghosh, A.B., Bajaj, J.C., Hasan, R., Singh, D., 1983. Soil and Water Testing Methods. Division of Soil Science and Agricul-tural Chemistry, Indian AgriculAgricul-tural Research Institute, New Delhi.
Henault, C., Devis, X., Lucas, J.L., Germon, J.C., 1998. Influence of different agricultural practices (type of crop, form of N-fertilizer) on soil nitrous oxide emissions. Biol. Fertil. Soils 27, 299–306.
Houghton, J.T., Meira Filho, L.G., Callander, B.A., Harris, N., Kattenberg, A., Maskell, K., 1996. Climate Change 1995 — The Science of Climate Change. Cambridge University Press, Cambridge.
Iserman, K., 1994. Agriculture’s share in the emissions of trace gases affecting the climate and some cause oriented proposals for reducing this share. Environ. Pollut. 83, 95–111. Joseph, P.A., Prasad, R., 1993. The effect of dicyandiamide and
neem cake on the nitrification of urea derived ammonium under field condition. Biol. Fertil. Soils 15, 149–152.
Keeney, D.R., Nelson, D.W., 1982. Nitrogen-inorganic forms. In: Page, A.L., Miller, R.H., Keeney, D.R. (Eds.), Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America, Inc., Madison, WI, USA, pp. 674–676.
Lindau, C.W., Patrick Jr., W.H., Delaune, R.D., Reddy, K.R., 1990. Rate of accumulation and emission of N2, N2O and CH4 from flooded rice soil. Plant Soil 129, 269–276.
Mahmood, T., Ali, R., Malik, K.A., Shamsi, S.R.A., 1998. Nitrous oxide emissions from an irrigated sandy-clay loam cropped to maize and wheat. Biol. Fertil. Soils 27, 189–196.
McTaggart, I.P., Clayton, H., Smith, K.A., 1994. Nitrous oxide flux from fertilized grassland: strategies for reducing emissions. In: van Ham, J., Janssen, L.J., Swart, R.J. (Eds.), Non-CO2Greenhouse Gases. Kluwer Academic Publishers, The Netherlands, pp. 421–426.
McTaggart, I.P., Clayton, H., Parker, J., Swan, L., Smith, K.A., 1997. Nitrous oxide emissions from grassland and spring barley following N fertilizer application with and without nitrification inhibitors. Biol. Fertil. Soils 25, 261–268.
Minami, K., 1987. Emission of nitrous oxide (N2O) from agro-ecosystem. Jpn. Agric. Res. Q. 21, 21–27.
Mishra, K.C., Chonkar, P.K., 1978. Possible utilization of neem cake for inhibiting nitrification in soil. J. Indian Soc. Soil Sci. 26, 90–92.
Mosier, A.R., Duxbury, J.M., Freney, J.R., Heinemeyer, O., Minami, K., 1996. Nitrous oxide emissions from agricultural fields: assessment, measurement and mitigation. Plant Soil 181, 95–108.
Reddy, K.R., Patrick Jr., W.H., 1976. Yield and nitrogen utilization by rice as affected by method and time of application of labeled nitrogen. Agron. J. 68, 965–969.
Reddy, R.N.S., Prasad, R., 1975. Studies on mineralization of urea, coated urea and nitrification inhibitor treated urea in soil. J. Soil Sci. 26, 305–312.
Shao, K.S., 1993. Preliminary study on the relationship of agricultural management measures and methane emission flux from rice paddy near Beijing area. Rural Eco-Environ. (Suppl.), 19–22.
Sharma, C., Gupta, P.K., Parashar, D.C., 1995. Nitrous oxide estimates from paddy fields and forests in India. Indian J. Radio Space Phys. 24, 311–313.
Skiba, U., Smith, K.A., Fowler, D., 1993. Nitrification and denitrification as sources of nitric oxide and nitrous oxide in a sandy loam soil. Soil Biol. Biochem. 11, 1527–1536. Skiba, U., Hargreaves, K.J., Beverland, I.J., Oneill, D.H., Fowler,
D., Moncrieff, J.B., 1996. Measurement of field scale N2O emission fluxes from a wheat crop using micrometeorological techniques. Plant Soil 181, 139–144.
Smith, C.J., Patrick Jr., W.H., 1983. Nitrous oxide emissions as affected by alternate anaerobic and aerobic conditions from soil suspensions enriched with (NH4)2SO4. Soil Biol. Biochem. 15, 693–696.
Smith, C.J., Brandon, M., Patrick Jr., W.H., 1982. Nitrous oxide emissions following urea-N fertilization of wetland rice. Soil Sci. Plant Nutr. 28, 161–171.
Thomas, J., Prasad, R., 1982. Studies on mineralization of neem and sulfur coated and N-serve treated urea. Fertil. News 27, 39–53.
Vyas, B.N., Godrej, N.B., Mistry, K.B., 1991. Development and evaluation of neem extract as a coating for urea fertilizer. Fertil. News 2, 19–25.