*Corresponding author. Tel.:#27-11-489-2911; fax:#27-11-489-2191. E-mail address:[email protected] (H. van der Bank).
Biochemical genetic markers to identify two
morphologically similar South African
Mastomys
species (Rodentia: Muridae)
Andre Smit
!
, Herman van der Bank
!
,
*
, Thomas Falk
"
,
Antonio de Castro
#
!Department of Zoology, Rand Afrikaans University, PO Box 524, Auckland Park, 2006, South Africa
"Zoologisches Institut und Zoologisches Museum, Universita(t Hamburg,
Martin Luther King Pl.3 20146 Hamburg, Germany
#Department of Botany, Rand Afrikaans University, PO Box 524, Auckland Park, 2006, South Africa Received 15 July 1999; received in revised form 25 October 1999; accepted 13 December 1999
Abstract
The two common southern African mice species (Mastomys couchaandM. natalensis) are morphologically almost identical, making"eld identi"cation impossible at present. Specimens from two localities were collected and tissue and blood samples taken. The habitat type of each locality was studied, and a distribution map compiled. A de"nite correlation between biome-type and species range was found to be present. Three isozyme markers were identi"ed: glucose phosphate isomerase in liver, and two general (non-speci"c) protein coding loci in muscle. In addition, we also identi"ed species characteristic haemoglobin components in both species. This is the"rst study to report genetic variation within, and di!erentiation between these species. Our results are of medical importance becauseMastomys couchacarries bubonic plague and M. natalensis carries Lassa Fever. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Rodentia;Mastomys; Markers; Distribution; Haemoglobin; Genetic variation
1. Introduction
The Mastomys species complex of mice is widely distributed in South Africa, especially the so-called Multimammate mice, Mastomys coucha and M. natalensis.
The limits of their distribution are only provisional at this stage (Skinner, 1990), but it is known that they are sympatric in some areas, and allopatric in others.Mastomys coucha acts as a reservoir for the RickettsianYersinia pestis, the organism causing Plague (Dippenaar et al., 1993).
At present, three diagnostic forms of plague are known: bubonic, primary pneu-monic and primary Septicaemic. Bubonic plague, which is the most common type in epidemics, is fatal in about 25}50% of untreated cases. Pneumonic plague, a highly contagious (airborne) form, and Septicaemic plague, a generalised blood infection, are rarer forms, and usually fatal (Roberts et al., 1996). Apart from Bubonic plague, this species complex is also a reservoir for the Banzi and Witwatersrand viruses (Dippenaar et al., 1993) as well as a recently emerged disease in forested West Africa. Lassa fever is an infection caused by an arenavirus, and has a high mortality rate, even in patients with hospital care (15}20% fatal).Mastomys natalensis, being a documented carrier of Lassa Fever also carries a Lassa-like virus called Mopeia. Its e!ect on man is yet to be established or researched (Murray et al., 1995).
Morphologically, both species are almost identical in all visible characteristics, and were originally regarded as one (De Graaf, 1981)M. natalensis. However, Gordon (1984) has shown that, at the very least, both species are distinguishable by ethological and micro-morphological characteristics, by their chromosome number, and charac-teristic haemoglobin variations. More recently, Dippenaar et al. (1993) used multi-variate analysis of cranial characteristics to distinguish between the two species. However, 50% of the specimens collected at a sympatric locality were identi"ed di!erently according to the latter method.
In the present study, we examined new material of two allopatric populations ofM. couchaandM. natalensisaimed at identifying species characteristic genetic markers. Allozyme and haemoglobin variations were analysed comparatively.
2. Materials and methods
Tissue extracts of 24 individuals of M. coucha caught at Montgomery
Park, Johannesburg (26309'22''S, 27358'58''E) and 20 individuals of M. natalensis
caught at La Lucia ridge in Durban North (29344'44''S, 31303'09''E), were analysed electrophoretically using standard horizontal starch gels and homogeneous polyacrylamide gels (Fig. 1). The localities were also studied to determine if there was a relationship between habitat types and the distribution of these two species (Fig. 2). Specimens that were positively identi"ed by Gordon (1984), either chromosomally or via characteristic haemoglobin variation were used as reference.
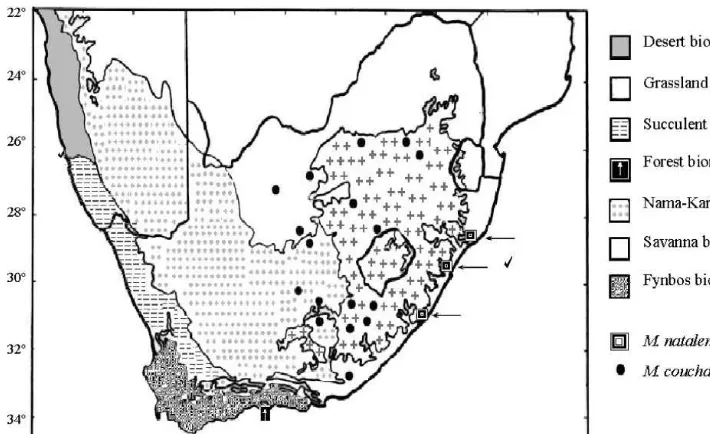
Fig. 1. The distributions ofMastomys couchaandM. natalensisaccording to positively identi"ed specimens. Biome types are denoted by number and the sampling sites of this study are indicated with@.
Allozyme studies were carried out using 12% starch gels, as well as 7% standard polyacrylamide gels (Ferreira et al., 1984). Staining methods are as described by Shaw and Prasad (1970) and Harris and Hopkinson (1976), and the method of interpreta-tion of gel-banding patterns and locus nomenclature as referred to by Van der Bank and Van der Bank (1995). A discontinuous lithium-borate}tris-citric acid bu!er (RW; electrode: pH 8; gel: pH 8.7; Ridgeway et al., 1970) and two continuous bu!ers: a tris-citric acid bu!er system (TC; pH 6.9; Whitt, 1970) and a tris-borate-EDTA bu!er (MF; pH 8.6; Markert and Faulhauber, 1965) were used to study the variation at 34 protein coding loci. Locus abbreviations, enzyme commission numbers, bu!ers, tissues and monomorphic loci are listed in Table 1.
Haemoglobin variations were analysed by isoelectric focusing (IEF) according to Falk et al. (1998). IEF separations were conducted on Servalyte precotes (pH range 3}10, Serva, Heidelberg, Germany). Gels were prefocused at 63C (200}500 V). Subsequently, haemolysate samples (10ll) were applied to an applicator strip positioned 5.0 cm from the anode and the voltage was limited to 1700 V. Separations were "nished when a constant current of maximal 2 mA/gel was reached (after about 2.5 h). Prior to use, haemolysate samples were diluted in distilled water ("nal concentration: 20 mg Hb/ml) and treated with 2-mercaptoethanol (3%) for 1 h at 53C.
IEF separated hemoglobins could be identi"ed by their red colour. In addition, gels were incubated in 4-chloro-1-naphthol/H
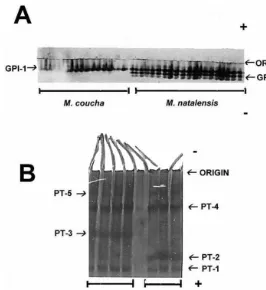
Fig. 2. Isozyme di!erences betweenM. couchaandM. natalensisat (A) GPI (Liver) and (B) PT (muscle) protein coding loci. The GPI-2 and PT-3 loci are absent inM. coucha, but the PT-2 locus is absent inM. natalensis.
Table 1
Locus abbreviations, bu!er systems and enzyme commission numbers (E.C. No) are listed after each protein
Protein: Locus: E.C. No.: Bu!er: Tissue:
Alcohol dehydrogenase ADH-1!-4! 2.6.1.1 RW Muscle, Liver
Creatine kinase CK-1!-3! 2.7.3.2 RW Muscle, Liver
Esterase EST-1, -2!, -3! 3.1.1- MF Muscle, Liver
Glyceraldehyde-3-phosphate dehydrogenase GAP! 1.2.1.12 RW Muscle, Liver
General protein PT-1!, -2, -3, -4!, -5! " Muscle, Blood
Glucose-6-phosphate dehydrogenase GPD-1!-4! 1.1.1.49 RW Muscle, Liver
Glucose-6-phosphate isomerase GPI-1, -2 3.5.1.9 RW Liver
Isocitrate dehydrogenase IDH-1, -2, -3! 1.1.1.42 TC Muscle, Liver Lactate dehydrogenase LDH-1!, -2, -3! 1.1.1.27 RW Muscle, Blood
Malate dehydrogenase MDH! 1.1.1.37 TC Muscle, Liver
Phosphoglucomutase PGML 5.4.2.2 RW Liver
Phosphoglucomutase PGM-1, -2, -3 5.4.2.2 RW Muscle
Superoxide dismutase SOD! 1.15.1.1 RW Muscle, Liver
!Monomorphic loci.
haemoglobin components by their pseudoperoxidase activity. The staining solution consisted of 60 ml methanol and 340 ml PBS (pH 7.4) containing 120 mg 4-chloro-1-naphthol and 1 ml 30% H
2O2(Miribel and Arnoud, 1988). Gels were also
counter-stained with Coomassie Brilliant Blue G-250 (Serva). The following pI marker proteins (Serva) were used: horse myoglobin: pI 6.90 and 7.40 and lectins of Lens culinaris: pI 7.80, 8.00, and 8.30.
3. Results and discussion
3.1. Ecology and distribution
It was not previously possible to accurately describe the geographic distribution and habitat requirements of either species, as a result of the extreme morphological similarity ofM. natalensisandM. coucha,and the fact that the genetic composition of only a relatively small number of individuals has been studied. It does however seem likely that di!erences in the habitat requirements of the two species do exist. Fig. 2 shows the localities of the M. coucha and M. natalensis populations positively identi"ed by means of morphometric analyses conducted by Dippenaar et al.(1993), the chromosomal and haemoglobin analyses by Gordon (1984), as well as one population of each species identi"ed during the genetic study presented here, in relation to the boundaries of the various biomes recognised within the southern African Subregion by Rutherford and Westfall (1993). These localities represent only a fraction of the known localities forMastomyswithin South Africa and do not include other southern African material.
The boundaries of the biomes as depicted in Fig. 2, were determined by Rutherford and Westfall (1993) on the basis of dominant and co-dominant plant life forms in climax systems, at a scale of 1 : 10, 000, 000. Biomes determined on the basis of vegetation have been shown to correspond with zoogeographical patterns, though the correlation seems to depend strongly on the animal group (Rutherford and Westfall, 1993). Some correspondence has been found for mammals. (Rautenbach, 1978), and this is to be expected as vegetation not only determines the structural nature of the habitat for animals (Odum, 1971), but also re#ects the prevailing climatic conditions, such as rainfall and temperature, which also a!ect mammals both directly and indirectly.
The localities included in Fig. 2 indicate thatM. natalensisis clearly a species of the Savanna biome, and more particularly of the moist warm regions of this biome.M. couchaseems to be a species which occurs predominantly in the Grassland biome, but also extends into cool, dry areas of the Savanna biome, and the moister parts of the Nama-Karoo biome. The localities within the Nama-karoo biome probably represent areas which were historically part of the Grassland biome, but have since been invaded by vegetation of the Nama-Karoo biome, and can thus be termed &false' Karoo (Rutherford and Westfall, 1993).
Table 2
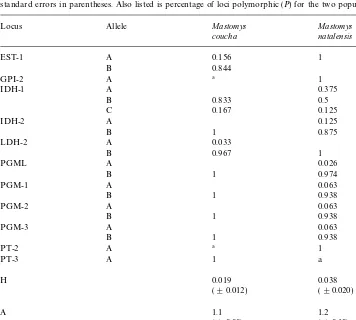
Allele frequencies, average heterozygosity per locus (H) and mean number of alleles per locus (A) with standard errors in parentheses. Also listed is percentage of loci polymorphic (P) for the two populations
Locus Allele Mastomys Mastomys
Hectorspruit in this region, which is situated in close proximity to the boundary between the Grassland and the Savanna biomes. An analysis of distribution patterns, based on a far larger number of localities would be required to establish whether there are indeed major habitat di!erences between these two species.
3.2. Geneticvariation
Table 3
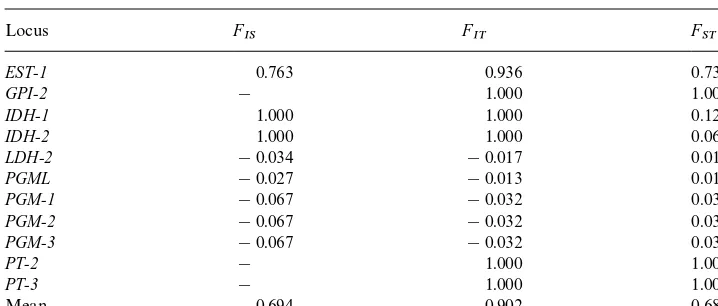
Summary ofF-statistics at all polymorphic loci for both species
Locus F
Fixed allele mobility di!erences between the two populations studied were obtained at three loci:GPI-2in liver,PT-2andPT-3in muscle tissue. The products ofGPI-2and
PT-3 were absent in M. coucha, whereas the products of PT-2 were absent in M. natalensis(Fig. 2a,b). While it is possible thatPT-2and-3are di!erent alleles of the same locus, the large separation distance between the bands suggests that they represent individual loci. A study of more individuals from di!erent populations may reveal polymorphism, and is required to verify this conclusion. Products of these three isozyme loci are useful to identify individuals from either of the populations studied. Moreover, signi"cant allelic frequency di!erences (P(0.05) were identi"ed atEST-1.
Allelic frequencies forM. couchadeviated from Hardy}Weinberg equilibrium atEST-1
andIDH-1, while those forM. natalensisdeviated atIDH-1and-2.These are probably due to small sample sizes.
Fvalues (Wright, 1978) can be used to estimate the amount of genetic di! erenti-ation between species. A meanF
ST value of 0.68 suggests a large amount of genetic di!erentiation between the two species studied, con"rming their present taxonomic status and the results of Gordon (1984). Moreover, our estimate for meanF
IS and
F
ITvalues also suggests a high degree of positive assortative mating and inbreeding, and further demonstrates very little or no gene#ow between both species in the wild. Gordon (1984) also found no evidence of hybrids in nature, but he was able to mate them in captivity. However, these hybrids were infertile when back-crossed. Nei's unbiased genetic distance (1978) was calculated to be 0.123. This measurement estimates the number of allelic substitutions per locus that have occurred since both species have diverged. This estimate falls outside Ayala's (1982) estimate for genetic distances between local populations of the same species of mammals (D"0.058), but well inside of his estimate for subspecies (D: 0.232). It is also low when compared with aDvalue of 0.46 betweenPeromyscus species (Rodentia: Cricetidae), but compares
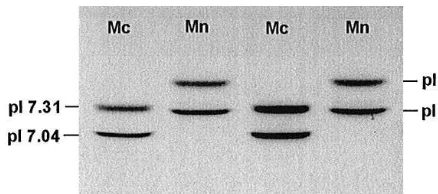
Fig. 3. IEF separation of haemoglobin phenotypes ofM. coucha(Mc) andM. natalensis(Mn) (pI"isoelec-tric point).
1982). Thus our calculation may appear low, but it does fall into the range of rodent variation, and could most probably be attributed to an on-going process of speciation. Unbiased heterozygosity values (Nei, 1978) were calculated to be 0.019 and 0.038 for
M. couchaandM. natalensis,respectively (Table 2). However, both of these values are less than that calculated by Selander et al. (1971) for Peromyscus polionotus
(H"0.053), and Ayala (1982) for mammals (average"0.051).
In addition, we also determined the degree of haemoglobin variation in both populations ofM. coucha andM. natalensis. Characteristically, all samples investi-gated revealed heterogeneous haemoglobin patterns (Fig. 3). Two haemoglobin com-ponents of di!ering pI, ranging between pH 7.52 and 7.04, were detected by thin-layer isoelectric focusing of individual samples. Characteristic variations in haemoglobin phenotypes were found to occur in samples of the two species studied. Mastomys couchaspecimens were characterised each by a unique combination of two di!erent haemoglobin components with pIs of 7.04 and 7.31, whereasM. natalensisspecimens displayed two haemoglobin components with pIs of 7.25 and 7.52. Haemoglobin phenotypes appeared to be consistent within both species. Essentially, these"ndings are in accordance with previous studies on the haemoglobin types of both species of the genus Mastomys studied here (Gordon, 1984). However, based on starch gel separations, only one distinct haemoglobin component could be identi"ed for each of the species and de"nite species characteristic di!erences in haemoglobin pro"les remained questionable. It should also be noted that isoelectric focusing of haemoglo-bins enables an identi"cation of both mice species without the use of reference samples, only pI marker proteins are required.
In conclusion, this study provides evidence that the populations studied of these two species of the genus Mastomys are more genetically distinct than previous studies have shown. Gordon (1984) demonstrated the mobility di!erences between
required to con"rm the presence of these markers between both species. These results may be valuable for the routine identi"cation of these two medically important species.
Acknowledgements
We thank Sasol far funding this project.
References
Avise, J.C., Aquadro, C.F., 1982. A Comparative Summary of Genetic Distances in the Vertebrates: Patterns and Correlations. Evol. Biol. 15, 151}185.
Ayala, F.J., 1982. Population and Evolutionary Genetics. Benjamin/Cummings Publishing Company, Menlo Park, CA.
De Graa!, G., 1981. The Rodents of Southern Africa. Butterworths, Durban and Pretoria.
Dippenaar, N.J., Swanepoel, P., Gordon, D.H., 1993. Diagnostic morphometrics of two medically impor-tant southern African rodents,Mastomys natalensisandM. coucha(Rodentia: Muridae). South African J. Sci. 89, 300}303.
Falk, T.M., Abban, E.K., Oberst, S., Villwock, W., Pullin, R.S.V., Renwrantz, L., 1996. A biochemical laboratory manual for species characterization of some tilapiine"shes. ICLARM Education Series, Vol. 17, Manila, Philippines.
Falk, T.M., Villwock, W., Renwrantz, L., 1998. Heterogeneity and subunit composition of the haemoglo-bins of 5 tilapiine species (Teleostei, Cichlidae) of the genera Oreochromis and Sarotherodon. J. Comparative Physiol. B 168, 9}16.
Ferreira, J.T., Grant, W.S., Avtalion, R.R., 1984. Workshop on Fish Genetics. CSIR Special Publications, Pretoria, p. 72.
Gordon, D.H., 1984. Evolutionary genetics of the praomys (Mastomys) natalensis species complex (Ro-dentia: Muridae). Ph.D. Thesis, Witswatersrand University.
Harris, H., Hopkinson, D.A., 1976. Handbook of Enzyme Electrophoresis in Human Genetics. North-Holland, Amsterdam.
Nei, M., 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583}590.
Markert, C.L., Faulhaber, I., 1965. Lactate dehydrogenase isozyme patterns of"sh. J. Expl. Zool. 159, 319}332.
Miribel, L., Arnoud, P., 1988. Electrotransfer of proteins following polyacrylamide gel electrophoresis
*nitrocellulose versus nylon membranes. J. Immunol. Methods 107, 253}259.
Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., 1995. Manual of Clinical Microbiol-ogy, 6th Edition. ASM Press, Washington, DC.
Odum, E.P., 1971. Fundamentals of Ecology. Saunders, Philidelphia
Rautenbach, I.L., 1978. Ecological distribution of the mammals of the Transvaal. Ann. Trans. Mus. 31 (10), 131}156.
Ridgeway, G.J., Sherbourne, S.W., Lewis, R.D., 1970. Polymorphism in the esterses of Atlantic herring. Trans. Am. Soc. 99, 147}151.
Roberts, L.S., Janovy, J., 1996. Foundations of Parasitology, 5th Edition. WCB Publishers, London. Rutherford, M.C., Westfall, R.H., 1993. Biomes of Southern Africa. Memoirs of the Botanical Survey of
South Africa, No. 63.
Shaw, C.R., Prasad, R., 1970. Starch gel electrophoresis of enzymes}a compilation of recipes. Biochem. Genet. 4, 297}320.
Skinner, J.D., Smithers, R.H.N., 1990. The Mammals of the Southern African Subregion, 2nd Edition. University of Pretoria, Pretoria.
Van der Bank, F.H., Van der Bank, M., 1995. An estimation of the amount of genetic variation in a population of the BulldogMarcusenius macrolepidotus(Mormyridae). Water S. A. 21, 265}268. Whitt, G.S., 1970. Developmental genetics of the lactate dehydrogenase enzyme of"sh. J. Expl. Zool. 175,
1}35.