*Corresponding author. Tel.:#81-43-265-3111; fax:#81-43-266-2481. E-mail address:[email protected] (Y. Mikanagi)
Anthocyanins in
#
owers of genus
Rosa, sections
Cinnamomeae
(
"
Rosa),
Chinenses,
Gallicanae
and
some modern garden roses
Yuki Mikanagi
!
,
*, Norio Saito
"
, Masato Yokoi
#
,
Fumi Tatsuzawa
#
!Natural History Museum and Institute, Chiba, 955-2 Aoba-cho, Chuo-ku, Chiba, 260-8682 Japan
"Meiji-gakuin University, Totsuka-ku, Yokohama, Kanagawa, 244-8539 Japan
#Faculty of Horticulture, Chiba University, 648 Matsudo, Chiba, 271-8510 Japan Received 17 March 1999; accepted 15 November 1999
Abstract
Forty-four taxa of three sections (Cinnamomeae("Rosa) 26,Chinenses8 andGallicanae10) and eight modern garden roses in the genusRosawere surveyed for their#oral anthocyanins. Eleven anthocyanins: 3-glucosides and 3,5-diglucosides of cyanidin (Cy), pelargonidin (Pg) and peonidin (Pn), 3-rutinosides and 3-o-coumaroylglucoside-5-glucosides of Cy and Pn, and Cy 3-sophoroside, were isolated from #owers of these taxa and identi"ed by chemical and spectroscopic techniques. Four anthocyanins: Cy 3-rutinoside, Pn 3-rutinoside, Pn 3-o -coumaroylglucoside-5-glucoside and Cy 3-sophoroside were found for the"rst time in Rosa
#owers.
Investigated sections of wild roses showed characteristic distribution of anthocyanins. Cy 3,5-diglucoside was the dominant anthocyanin detected in all three sections, but accumulation of Pn 3,5-diglucoside distinguished sectionsCinnamomeaefrom other sections, and the occur-rence of Cy 3-glucoside separates sectionChinensesfrom others.
Cy 3-sophoroside was detected in large amount in some taxa of sectionCinnamomeae: e.g.,R. moyesiiand its related cultivars, andR. rugosacv. Salmon Pink. The acylated Cy glycoside was found in all sections and also in some modern garden roses, while the acylated Pn glycoside was detected in the sectionCinnamomeae, but not in sectionsChinensesandGallicanae. According to anthocyanin distribution patterns, eight groups were classi"ed chemotaxonomically in genus
Rosa. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Rosa; Rosaceae; Floral anthocyanins; Cyanidin 3-sophoroside; Cyanidin 3-rutinoside; Peonidin 3-rutinoside; Acylated anthocyanins; Chemotaxonomy
1. Introduction
Flower colour investigation of roses so far have shown that four anthocyanins, 3-glucosides and 3,5-diglucosides of cyanidin (Cy) and peonidin (Pn), can be detected in #owers of wild Rosa species, and also pelargonidin (Pg) 3-glucoside and Pg 3,5-diglucoside are detected in Rosa cultivars (WillstaKtter and Nolan, 1915; Harborne, 1961, 1967; Arisumi, 1963, 1967; Yokoi, 1974, 1975; Yokoi et al., 1979; Saito et al., 1982; Mikanagi et al., 1990,1994,1995; Biolley et al., 1992, 1994a,b; Raimond et al., 1995). In addition, one acylated anthocyanin,o-coumaroylcyanidin 3,5-diglucoside, was reported to be present inR. cv. Frensham (Arisumi, 1967). To our knowledge, only one paper has reported that Pg derivatives were found in some wild Rosa taxa (Eugster and MaKrki-Fischer, 1991).
In our previous investigations we could not "nd Pg derivatives in wild taxa (Mikanagi et al., 1995). We have already surveyed over 200 taxa ofRosaincluding some old garden roses, and about 120 taxa contained anthocyanins. Wild taxa in section Cinnamomeae seem to contain more unknown anthocyanins than other sections, and we consider that a study of anthocyanins of sectionCinnamomeae is necessary. Therefore, in the present study, we have investigated the anthocyanins in Rosa, the main purpose being the structural elucidation of these unknown com-pounds. In particular, we have examined the wild roses of sectionCinnamomeaein Japan, and compared them with those in other countries, as well as old garden roses and some modern garden roses.
In the study, Cy 3-rutinoside, Pn 3-rutinoside, Pn 3-o-coumaroylglucoside-5-glucoside and Cy 3-sophoroside were found for the"rst time inRosa#owers, and in total, eleven anthocyanins were detected. We wish to report the structure and distribution of these pigments.
2. Materials and methods
2.1. Plant materials
Fresh or dried petals of 26 taxa in section Cinnamomeae: eight taxa in section Chinenses, 10 taxa in sectionGallicanaeand eight modern garden roses in genusRosa were collected from various sources as listed in Table 1, arranged by sections and groups. Names of species, varieties, hybrids and cultivars are those by Rehder (1949), Hara (1957), Satake et al. (1989) and Cairns (1993), and they were con"rmed by YM. Voucher specimens of wild roses were deposited at the Natural History Museum and Institute, Chiba (CBM).
Table 1
Plant materials (wild taxa, old garden roses and modern garden roses)
Taxa Sources
Wild taxa and old garden roses SectionCinnamomeae("sectionRosa)
(No. in Table 5)
Rosa acicularisLindl. (R01) Monbetsu, Hokkaido, Japan (wild) R. arkansanaPorter (R02) Keisei Rose Nursery, Japan (cultivated) R. bellaRehder and Wilson (R03) Keisei Rose Nursery, Japan (cultivated)
R. cinnamomeaL. (R04) Keisei Rose Nursery, Japan (cultivated)
R. forrestianaBoulenger (R05) Keisei Rose Nursery, Japan (cultivated)
R. marretiiLeHv. (R06) Monbetsu, Hokkaido, Japan (wild)
Sugadaira, Japan (wild) Teshikaga, Japan (wild)
Keisei Rose Nursery, Japan (cultivated) R. moyesiiHemsl. & Wilson (R07) The Garden of Roses, UK (cultivated)
Keisei Rose Nursery, Japan (cultivated) R. moyesiicv. Arthur Hillier (R08) The Garden of Roses, UK (cultivated)
Keisei Rose Nursery, Japan (cultivated) R. moyesiicv. Eddie's Crimson (R09) The Garden of Roses, UK (cultivated)
Keisei Rose Nursery, Japan (cultivated) R. moyesiicv. Fargesii (R10) The Garden of Roses, UK (cultivated) R. moyesiicv. Geranium (R11) The Garden of Roses, UK (cultivated) Keisei Rose Nursery, Japan (cultivated) R. moyesiicv. Highdownensis (R12) The Garden of Roses, UK (cultivated) R. moyesiicv. Hillieri (R13) The Garden of Roses, UK (cultivated) R. nipponensisCreHp. (R14) Mt. Fuji, Japan (wild)
Mt. Haku-san, Japan (wild) Mt. Tsurugi-san, Japan (wild) Mt. Shibutsu-san, Japan (wild)
R. nutkanaPresl. (R15) Keisei Rose Nursery, Japan (cultivated)
R. pendulinavar.oxyodon(Boissier) Rehder (R16)
Keisei Rose Nursery, Japan (cultivated) R. rugosaThunb.exMurray (R17) Sado Isl., Japan (wild)
Abashiri, Hokkaido, Japan (wild) Chiba University, Japan (cultivated) Keisei Rose Nursery, Japan (cultivated) R. rugosavar.plenaRegel (R18) Keisei Rose Nursery, Japan (cultivated) R. rugosacv. Maikwai (R19) Keisei Rose Nursery, Japan (cultivated) R. rugosacv. Rosaraie de l'Hay (R20) The Garden of Roses, UK (cultivated) R. rugosacv. Salmon Pink (R21) Keisei Rose Nursery, Japan (cultivated) R. rugosacv. Scabrosa (R22) Keisei Rose Nursery, Japan (cultivated) R. rugosacv. Scarlet (R23) Keisei Rose Nursery, Japan (cultivated) R. sweginzowiiKoehne (R24) The Garden of Roses, UK (cultivated) R. willmottiaeHemsl. (R25) Keisei Rose Nursery, Japan (cultivated)
R. xiwaraSieb. (R26)! Sado Isl., Japan (wild)
Keisei Rose Nursery, Japan (cultivated) SectionChinenses
R. chinensisJacq. Keisei Rose Nursery, Japan (cultivated)
R. chinensisvar.minima(Sims) Voss Keisei Rose Nursery, Japan (cultivated)
2.2. Isolation of acylated anthocyanins
Fresh#owers (ca. 1.0 kg) ofRosacv. Red Meillandina were extracted with MAW (10l; MeOH}HOAc}H2O, 9 : 1 : 10). The extract was concentrated to 500 ml. The concentrated extract was puri"ed by Diaion HP-20 CC, PC, TLC and HPLC as previously reported (Saito et al., 1995). Solvents used were 15% HOAc, BAW (n-BuOH}HOAc}H2O, 4 : 1 : 5), 5% HOAc}MeOH and MAW for CC, PC and TLC. Prep. HPLC was run on a Waters C18 (19/]150 mm) column at 403C with a#ow rate of 4 ml min~1monitoring at 530 nm. Solvent system used a linear gradient elution for 40 min from 40 to 85% solvent B (1.5% H3PO4, 20% HOAc, 25% MeCN in H2O) in solvent A (1.5% H3PO4in H2O). The evaporated residues were dissolved Table 1 (continued)
Taxa Sources
R. chinensisvar.spontaneaRehder & Wilson Keisei Rose Nursery, Japan (cultivated) R. chinensiscv. Fabvier Keisei Rose Nursery, Japan (cultivated) R. chinensiscv. Miss Lowe Keisei Rose Nursery, Japan (cultivated) R. chinensiscv. Mutabilis Keisei Rose Nursery, Japan (cultivated) R. chinensiscv. Pompon de Paris Keisei Rose Nursery, Japan (cultivated) R. chinensiscv. Slater's Crimson China Keisei Rose Nursery, Japan (cultivated) SectionGallicanae
R. gallicaL. Keisei Rose Nursery, Japan (cultivated)
R. gallicacv. Cardinal de Rich :"lieu Keisei Rose Nursery, Japan (cultivated) R. gallicacv. Rosa Mundi Keisei Rose Nursery, Japan (cultivated)
R. gallicacv. Shigyoku Keisei Rose Nursery, Japan (cultivated)
R. gallicacv. Violacea Keisei Rose Nursery, Japan (cultivated)
R. xcentifoliacv. Bullata Keisei Rose Nursery, Japan (cultivated) R. xcentifoliacv. Muscosa Keisei Rose Nursery, Japan (cultivated)
R. xdamascenaMiller Keisei Rose Nursery, Japan (cultivated)
R. xdamascenacv. Bifera Keisei Rose Nursery, Japan (cultivated) R. xdamascenacv. Gloire de Guilan Keisei Rose Nursery, Japan (cultivated) Modern garden roses
Hybrid Tea
R. cv. La France Keisei Rose Nursery, Japan (cultivated)
R. cv. OleH Keisei Rose Nursery, Japan (cultivated)
R. cv. Papa Meilland Keisei Rose Nursery, Japan (cultivated)
Tokyo Metropolitan Jindai Botanical Park (cultivated)
R. cv. Seika Tokyo Metropolitan Jindai Botanical Park (cultivated)
Floribunda
R. cv. Frensham Keisei Rose Nursery, Japan (cultivated)
R. cv. Orange Bunny Tokyo Metropolitan Jindai Botanical Park (cultivated)
Miniature and Polyantha
R. cv. Red Meillandina Keisei Rose Nursery, Japan (cultivated)
R. cv. The Fairy Keisei Rose Nursery, Japan (cultivated)
!R. xiwarais considered to be a natural hybrid betweenR. rugosaandR. multiyoraThunb. ex Murray (sectionSynstylae)
in a small volume of 5% HOAc}EtOH followed by the addition of excess of Et2O, and then dryed to give pigment powder: Cy 3-o-coumaroylglucoside-5-glucoside ca. 10 mg, Pn 3-o-coumaroylglucoside-5-glucoside ca. 5 mg.
2.3. Preparation of other anthocyanins
Three anthocyanins were also puri"ed from R. cv. Red Meillandina extracts as follows; Cy 3,5-diglucoside ca. 30 mg, Cy 3-glucoside ca. 5 mg and Pn 3,5-diglucoside ca. 6 mg. The other six pigments were extracted from dry or fresh petals ofR. moyesii and its cultivars andR. cv. Orange Bunny (ca. 30}80 g) with MAW at room temper-ature and "ltered. After concentration the extact, the pigments were puri"ed by preparative PC (BAW and 15% HOAc) and TLC (BAW and 15% HOAc). Then, six pigments (ca. 0.1}10 mg) were obtained as follows; Cy sophoroside and Pn 3-glucoside were obtained from the mixed red#owers ofR. moyesiiand its cultivars. Cy 3-rutinoside and Pn 3-rutinoside were obtained fromR. moyesiicv. Arthur Hillier. Pg 3,5-diglucoside and Pg 3-glucoside were obtained from orange-red#owers ofR. cv. Orange Bunny.
2.4. Analyses of anthocyanins
Fresh or driedRosapetals (fresh weight of each taxon ca. 1}3 g) were extracted with HOAc}MeOH-H2O (1 : 10 : 9) and the extracts were analyzed for the anthocyanin distribution. The anthocyanin distribution in each taxon was examined by HPLC and 2D-TLC with comparison to authentic anthocyanins (Mikanagi et al., 1995).
HPLC was run on a Waters C18 (4.6/]250 mm) column at 403C with a#ow rate of 1 ml min~1monitoring at 530 nm for anthocyanins. Solvent system used were as follows: a linear gradient elution for 85 min from 20 to 80% solvent B (1.5% H3PO4, 20% HOAc, 25% MeCN in H2O) in solvent A (1.5% H3PO4in H2O). Retention times of anthocyanins detected in this study are shown in Table 2.
2D-TLC was carried out on Avicel cellulose plates (Funakoshi) in BuHCl (n-BuOH}2M-HCl 1 : 1, top layer) for the"rst direction and AHW (HOAc}HCl}H2O 15 : 3 : 82) for the second. Identi"cation of the structure of these anthocyanins was based on the standard methods of TLC, HPLC, UV spectroscopy and NMR spectro-scopy (Harborne, 1967, 1984; Saito and Harborne, 1992; Saito et al., 1995; Tatsuzawa et al., 1996.).
2.5. Reference anthocyanins
Table 2
Chromatographic and spectral properties of anthocyanins in#owers of genusRosa
Anthocyanins! Rf values (]100) Spectral data in 0.1% HCl}MeOH HPLC
Rt (min)
FAB-MS [M]`
BAW BuHCl 1% HCl AHW jmax (nm) E440/E
.!9(%) AlCl3
2 24 15 4 14 269, 528 24 # 37.4 449
5 10 4 7 22 270, 526 16 # 33.1 611
7 23 15 5 33 282, 531 24 # 39.6 *
9 19 14 20 40 283, 526 29 # 35.0 611
10" 31 22 6 23 280, 310, 525 19 # 49.2, 56.0 757
3 27 20 5 18 280, 527 28 0 43.5 463
6 20 5 10 30 278, 524 13 0 19.4 625
8 24 9 11 33 279, 528 29 0 37.8 *
11# 33 25 8 28 270, 310, 525 15 0 54.0, 60.9 771
1 30 30 7 22 269, 509 43 0 41.8 433
4 23 7 17 36 267, 507 21 0 35.4 595
!For key to abbreviation, see Materials and Methods: (1) Pg 3-glucoside; (2) Cy 3-glucoside; (3) Pn 3-glucoside; (4) Pg 3,5-diglucoside; (5) Cy 3,5-diglucoside; (6) Pn 3,5-diglucoside; (7) Cy 3-rutinoside; (8) Pn 3-rutinoside; (9) Cy 3-sophoroside; (10) Cy 3-o-coumaroylglucoside-5-glucoside; (11) Pn 3-o -coumaroylgluco-side-5-glucoside.
"HPLC Rt 49.2 min"cis-form, 56.0"trans-form. #HPLC Rt 54.0 min"cis-form, 60.9"trans-form.
892
Y.
Mikanagi
et
al.
/
Biochemical
Systematics
and
Ecology
28
(2000)
887
}
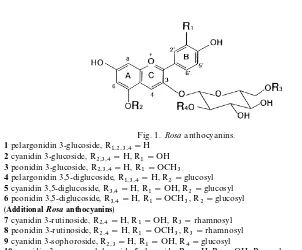
Fig. 1. Rosaanthocyanins.
1pelargonidin 3-glucoside, R1,2,3,4"H
2cyanidin 3-glucoside, R2,3,4"H, R1"OH
3peonidin 3-glucoside, R2,3,4"H, R1"OCH3
4pelargonidin 3,5-diglucoside, R1,3,4"H, R2"glucosyl
5cyanidin 3,5-diglucoside, R3,4"H, R1"OH, R2"glucosyl
6peonidin 3,5-diglucoside, R3,4"H, R1"OCH3, R2"glucosyl (AdditionalRosaanthocyanins)
7cyanidin 3-rutinoside, R2,4"H, R1"OH, R3"rhamnosyl
8peonidin 3-rutinoside, R2,4"H, R1"OCH3, R3"rhamnosyl
9cyanidin 3-sophoroside, R2,3"H, R1"OH, R4"glucosyl
10cyanidin 3-o-coumarylglucoside-5-glucoside, R4"H, R1"OH, R2"glucosyl, R3"o-coumaryl
11 peonidin 3-o-coumarylglucoside-5-glucoside, R4"H, R1"OCH3, R2"glucosyl, R3"o -coumaryl
2.6. Mass and NMR spectroscopy
Positive and negative ion FAB mass spectra of pigments were measured by the JEOL JMS SX-102 mass spectrometer, positive FAB-MS in HCl}MeOH#glycerol
and negative FAB-MS in glycerol. The detailed structures of Cy 3-sophoroside and 3-o-coumaroylglucoside-5-glucoside of Cy and Pn were determined by the analysis of 1H-NMR and1H-1H COSY spectra.1H-NMR spectra of pigments were measured by JEOL FX-400 spectrometer in 10% TFA-90% DMSO-d6.
3. Results and discussion
In the survey of anthocyanins inRosa #owers of 52 taxa by HPLC analysis, 11 major anthocyanin peaks were observed as shown in Fig. 1, and Tables 2 and 4 (pigments1}11). Among them, the 3-glucosides and 3,5-diglucosides of Cy, Pn and Pg (1, 2, 3, 4, 5and 6) were con"rmed with authentic samples and con"rmed the former studies (Arisumi, 1963, 1967; Yokoi, 1974, 1975; Yokoi et al., 1979; Saito et al., 1982 and Mikanagi et al., 1994). In most taxa, Cy 3,5-diglucoside (5) was detected as the major constituent. However, some taxa in sectionCinnamomeaecontained larger amounts of Pn 3,5-diglucoside (6) than 5, and some taxa in section Chinenses contained larger amounts of Cy 3-glucoside (2) than5.
Table 3
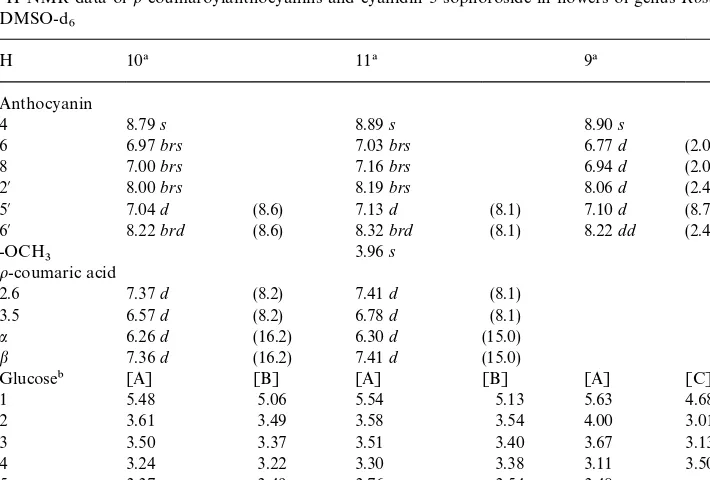
1H-NMR data ofo-coumaroylanthocyanins and cyanidin 3-sophoroside in#owers of genusRosausing DMSO-d6
H 10! 11! 9!
Anthocyanin
4 8.79s 8.89s 8.90s
6 6.97brs 7.03brs 6.77d (2.0)
8 7.00brs 7.16brs 6.94d (2.0)
2@ 8.00brs 8.19brs 8.06d (2.4)
5@ 7.04d (8.6) 7.13d (8.1) 7.10d (8.7)
6@ 8.22brd (8.6) 8.32brd (8.1) 8.22dd (2.4, 8.7)
-OCH3 3.96s
o-coumaric acid
2.6 7.37d (8.2) 7.41d (8.1)
3.5 6.57d (8.2) 6.78d (8.1)
a 6.26d (16.2) 6.30d (15.0)
b 7.36d (16.2) 7.41d (15.0)
Glucose" [A] [B] [A] [B] [A] [C]
1 5.48 5.06 5.54 5.13 5.63 4.68
2 3.61 3.49 3.58 3.54 4.00 3.01
3 3.50 3.37 3.51 3.40 3.67 3.13
4 3.24 3.22 3.30 3.38 3.11 3.50
5 3.37 3.49 3.76 3.54 3.48
6a 3.93 3.68 4.26 3.73 3.89}3.72 3.79}3.63
6b 4.33 3.76 4.48 3.80
!(10) cyanidin 3-o-coumaroylglucoside-5-glucoside; (11) peonidin 3-o-coumaroylglucoside-5-glucoside; (9) cyanidin 3-sophoroside.
"All the observed vicinal coupling constants were ca. 7.0}10.0 Hz.
this study. Furthermore, the occurrence of four anthocyanins, Cy 3-rutinoside (7), Pn 3-rutinoside (8), Cy 3-sophoroside (9) and Pn 3-o-coumaroylglucoside-5-glucoside (11), are recorded for the"rst time in genusRosa(Fig. 1). Contents of7,8,10and11 were small, but9accumulated in large amounts in some taxa in sectionCinnamomeae. The distribution of anthocyanins in each section is described in detail.
3.1. Structural elucidation of anthocyanins
3,5-diglucoside (4), Cy 3,5-diglucoside (5) and Pn 3,5-diglucoside (6). Pigments7and 8were identi"ed by HPLC only as Cy 3-rutinoside (7) and Pn 3-rutinoside (8). Among these pigments, the 3-rutinosides of Cy (7) and Pn (8) were found for the"rst time in the#owers of Rosa. Pigments9,10,11were identi"ed to be Cy 3-sophoroside (9), Cy 3-o-coumaroylglucoside-5-glucoside (10) and Pn 3-o-coumaroylglucoside-5-glucoside (11) by similar process of pigments1}8. Pigments9and11have not been reported in Rosapreviously.
Furthermore, the detailed structures of these three pigments (9, 10and 11) were con"rmed by the analysis of 1H-NMR and 1H-1H COSY spectra (Table 3). The FAB-mass spectrum of 9 gave its molecular ion [M]` at 611 m/z C25H27O16 (calculated, 611.529) indicating one molecule of cyanidin and two molecules of glucose. In the1H-NMR spectrum, the signals of six protons in the cyanidin moiety were easily assigned by the analysis of the1H-1H COSY spectrum of9(Table 3), and those of the sugar moiety appeared in the region 3.13}5.63 ppm. Two typical doublets were found to be two anomeric protons atd5.63 (d,J"7.5 Hz, Glc A) andd4.68 (d,
J"7.5 Hz, Glc C). Also, a proton atd4.00 (t,J"8.3 Hz) being shifted to a lower"eld
was directly correlated to the H-1A of glucose A by analysis of its 1H-1H COSY spectrum. Thus, this proton was assigned to the H-2Aof Glc A. Therefore, Glc C is attached to the OH-2 of Glc A through a glucosidic bond. Consequently, 9 was determined to be Cy 3-[2-O-(b-D-glucopyranosyl)-b-D-glucopyranoside], which has not been reported inRosa.
The FAB-mass spectra of10and 11gave their molecular ions [M]`at 757 and 771m/z which correspond to C36H37O18 (calculated, 757.674) and C37H39O18 (calculated, 771.701), respectively, indicating10to be composed of Cy, two molecules of glucose and one molecule ofo-coumaric acid, and also11to be composed of Pn, two glucose and one o-coumaric acid. In the 1H-NMR spectra, both signals of pigments 10 and 11 were superimposable except signals of the 3@-OCH3 of 11 as shown in Table 3. Signals from the sugar moieties of10and11were observed in the region ofd3.22}5.54, and all vicinal coupling constants of four glucose moieties were at 7.0}10.0 Hz. The chemical shifts and the large coupling constants of four anomeric protons appeared atd5.48 (J"7.4 Hz, Glc A of10),d5.06 (J"7.4 Hz, Glc B of10),
d5.54 (J"7.4 Hz, Glc A of11) and d5.13 (J"7.3 Hz, Glc B of 11), showing four
glucose units to beb-D-glucopyranosides. The down"eld shift of the methylenes of Glc A of10(d3.93 and 4.33) and11(d4.26 and 4.48) indicated theo-coumaroyl moieties to be attached to the OH-6 of glucose A in both pigments. In the chemical shifts of
o-coumaroyl moieties of10and11, the two pairs of ole"nic protons (10,d6.26 and
7.36;11d6.30 and 7.41) had large coupling constants (J"16.2,10andJ"15.0,11).
3.2. Distribution of Cy 3-sophoroside,Cy 3-rutinoside and Pn 3-rutinoside
In this survey 26 taxa of section Cinnamomeae, 24 taxa (92%) contained Cy 3-sophoroside (9), 14 taxa (54%) contained Cy 3-rutinoside (7), and 19 taxa (73%) contained Pn 3-rutinoside (8) (Table 4). R. moyesiiand its cultivars contained large amounts of9. In particular, the blood-red coloured#owers ofR. moyesiicv. Geranium contained9as 56% of total anthocyanins. This pigment is probably associated with the special colour of that cultivar. One more taxa in sectionCinnamomeae,R. rugosa cv. Salmon Pink, contained9(8%). This cultivar is a wild mutant ofR. rugosawhich was found in east Hokkaido, Japan, and identi"ed by Suzuki. We suspect that the glycosylation enzyme for the 5-position of this mutant is lacking, and consequently it accumulates9and Cy 3-glucoside (2) instead of Cy 3,5-diglucoside (5). The occurrence of 9 was mainly restricted to section Cinnamomeae, but some old garden roses in sectionGallicanaealso contained this pigment in small amounts.
Pigment 7 was detected in sectionsCinnamomeaeandChinensesin small amounts. Pigment8was found in almost every taxa we analyzed, but the content was small and except for some taxa in sectionCinnamomeae, could be detected by HPLC only. The occurrences of these pigments7,8and9is new forRosa(Eugster and MaKrki-Fischer, 1991; Biolley et al., 1994a,b; Mikanagi et al., 1995). It is supposed that the reason these pigments were not distinguished in earlier studies is due to the very close Rf values of 7and8to5on TLC (Table 2) and the limited distribution of9in sectionCinnamomeae (Table 4).
In our previous studies (Mikanagi et al., 1990, 1995), it was shown that the major #ower#avonols of sectionCinnamomeaewere the 3-sophorosides of kaempferol (K) and quercetin (Q), whereas those of sections Chinenses and Gallicanae were 3-glucosides of K and Q. In this study, Cy 3-sophoroside (9) was detected in section Cinnamomeae, but hardly at all in sectionsChinensesand Gallicanae. This suggests that the same glycosylation process occurs for#avonols and anthocyanins inRosa. The restriction of distribution of9to section Cinnamomeaesuggests the possibility that it can be used as a chemical marker for sectionCinnamomeaeidenti"cation.
In addition, there was a pigment which seems to be Pn 3-sophoroside because the Rt. 41.2 min on HPLC agrees with that of the authentic anthocyanin (Table 4, Pigment No.]). The amount of this anthocyanin is very small, but its distribution is mainly in sectionCinnamomeae.
3.3. Distribution of acylated anthocyanins
being the major pigment in the#owers ofR. moyesiiand its cultivars,R. pendulinavar. oxyodon,R. sweginzowii,R. willmottiae(sectionCinnamomeae),R. chinensiscv. Fabvier, cv. Slater's Crimson China (sectionChinenses),R. gallicacv. Violacea,R. x centifolia cv. Muscosa (sectionGallicanae),R. cv. Frensham,R. cv. Red Meillandina andR. cv. The Fairy (modern garden roses). On the other hand,11has been found only in the #owers of sectionCinnamomeae, mainly inR. acicularis,R. arkansana,R. cinnamomea, R. moyesii and its cultivars, R. rugosa cv. Maikwai and cv. Roseraie de l'Hay, R. sweginzowii,R. willmottiae (sectionCinnamomeae) and a modern garden rose,R. cv. Red Meillandina.
In the#owers ofR. acicularis,R. bella,R. moyesiicv. Arthur Hillier andR. nutkana two unknown anthocyanins (UK1andUK2in Table 4) were detected. From the Rts of both pigments, they are suspected to be polyacylated anthocyanins (Strack and Wray, 1994). We are attempting to resolve the chemical structure of these anthocyanins.
As the occurrence of acylated anthocyanins is considered to be important for the stability and bluing e!ect of#ower colour (Lu et al., 1992; Dangles et al., 1993; Saito et al., 1995; Figueiredo et al., 1996), the accumulation of acylated anthocyanins in #owers of Rosa is expected to be important for new#ower colour creation. Further studies of the newly discovered Rosa anthocyanins (7,8,9,],10,11,UK1andUK2) are necessary for rose breeding in the future.
In summary, the taxa investigated in this study can be divided into eight groups on the basis of their hydroxylation, methylation, glycosylation and acylation patterns as shown in Table 5. Modern garden roses reveal heterogeneity in anthocyanin patterns which re#ect their complicated origins, but we place them in group VIII for the sake of convenience. Taxa in sectionsChinensesandGallicanaewere placed in groups VI and VII, respectively by patterns of anthocyanins as shown in Table 5. Group VI is characterized with much accumulation of Cy 3-glucoside (2) and VII is characterized with remarkable concentration of Cy 3,5-diglucoside (5) in the petals.
Most taxa of sectionCinnamomeaeare grouped as a homogeneous unit by mor-phological characters, but anthocyanin constituents of taxa in this section show a range of patterns of methylation, glycosylation and acylation, and are divided into "ve groups. Grossi et al. (1998) wrote that through the analyses of#ower #avonols and leaf enzymes, three evolutionary trends could be recognized, corresponding to the sectionsSynstylae, Pimpinellifolliaeand Cinnamomeae pro parte. The half of section Cinnamomeaeare the most advanced and constitute the nucleus of the evolution. Unfortunately, we have no data about sectionsSynstylaeand Pimpinelifoliaewhich contain only a few cyanic species. However, our results showed that section Cinna-momeae has a very diverse anthocyanin distribution, and may support Grossi's hypothesis for the evolution of roses.
Table 4
Anthocyanin distribution in#owers of genusRosa
Rosa]iwara(R26) 52 ] 0 ] ] ] 46 0 0 ] ] 1 0 0 0 0 Rts of&]'pigments are identical to each pigments, but chemical structures are not yet con"rmed.
Table 5
Hydroxylation, methylation, glycosylation and acylation patterns of anthocyanins in #owers of genus Rosa (value(0.5%"`!a, 0.5%( value(1%"`#a, 1%(value(10%"`La, 10%(value"`Ua)
Hydroxylation, methylation, glycosylation and acylation patterns SectionCinnamomeae (sectionRosa)
Hydroxylation B-ring 3@- & 4@-OH (Cyanidin & peonidin)
U U U U U U U U
4@-OH (Pelargonidin)- ! ! ! ! ! ! ! U,!
Methylation Aglycone 3@-O-Me (Peonidin) U U U ! ! # ! U,!
Glycosylation Aglycone 3-O-monogly (3-glu) ! L U ! U U # L
3-O-digly (3-rut & 3-sop) L L L ! U # # !
Acylation Sugar 6-O-Ac (3-o -coumaroyl-glu-5-glu)
L L ! # L L # L,!
!Patterns of anthocyanins were divided into eight groups as follows, R-numbers indicate Rosa taxa of sectionCinnamomeaeshown in Table 1. IAcicularisgroup - R01, R02, R03, R04, R09, R15, R17, R18, R19, R20, R22, R23, R24, R25 and R26;
IIMoyesiiArthur Hillier group - R08, R10 and R12; IIIRugosaSalmon Pink group - R21;
IVNipponensisgroup - R05, R06, R14 and R16; VMoyesiigroup - R07, R11 and R13;
VIChinensesgroup; VIIGallicanaegroup; VIII Modern garden roses group.
(R26) belong to the Acicularis group (group I in Table 5). This group has the largest number of taxa, and they contain mainly 3,5-diglucosides of Cy and Pn and lack the 3-glucoside and 3-sophoroside of Cy and Pn. AlthoughR. nipponensisis sometimes treated as a variety ofR. acicularis(R. acicularisLindl. var.nipponensisHook."l.), the di!erence of anthocyanin distribution between these species is clearly shown in this result.
Acknowledgements
We are grateful to the secretary general of the Royal National Rose Society, UK, Mr. S. Suzuki and Mr. H. Hirabayashi in Keisei Rose Nursery, Japan, Mr. Ichikawa in Tokyo Metropolitan Jindai Botanical Park, Japan for provision of plant materials. We also thank Miss M. Yoshikawa and Miss F. Hayano for their help in collecting plant materials and chemical analysis. The authors greatfully acknowledge Dr. N.B. Clark and Dr. Y. Yazaki at CSIRO Forestry and Forest Products for his assistance with the English expression in this manuscript. Financial support by &Fujiwara Natural History' is greatly acknowledged.
References
Arisumi, K., 1963. Studies on the#ower colours inRosa, with special references to the biochemical and genetic analyses and to the application of those results to the practical breeding. I. Sci. Bull. Fac. Agric. Kyushu 20 (2), 131}149.
Arisumi, K., 1967. Studies on the#ower colours inRosa, with special references to the biochemical and genetic analyses and to the application of those results to the practical breeding. III. Bull. Fac. Agr. Yamaguchi University No. 18, 1077}1089.
Biolley, J.-P., Jay, M., Barbe, J.-P., 1992. Chemometric Approach (Flavonoids) in an automatic recognition of modern rose cultivars. Biochem. Syst. Ecol. 20, 697}705.
Biolley, J.-P., Jay, M., Forkmann, G., 1994a. Pigmentation patterns of modern rose mutants throw light on the#avonoid pathway inRosa]hybrida. Phytochemistry 36, 1189}1196.
Biolley, J.-P., Jay, M., Viricel, M.-R., 1994b. Flavonoid diversity and metabolism in 100Rosa]hybrida cultivars. Phytochemistry. 35, 413}419.
Cairns, T. (Ed.), 1993.Modern Roses 10. The American Rose Society, Louisiana.
Dangles, O., Saito, N., Brouillard, R., 1993. Anthocyanin intramolecular copigment e!ect. Phytochemistry 34, 119}124.
Eugster, C.H., MaKrki-Fischer, E., 1991. The Chemistry of rose pigments. Angew Chem. Int. Ed. Engl. 30, 654}672.
Figueiredo, P., Elhabiri, M., Toki, K., Saito, N., Dangles, O., Brouillard, R., 1996. New aspects of anthocyanin complexation. Intramolecular copigmentation as a means for colour loss? Phytochemistry 41, 301}308.
Grossi, C., Raymond, O., Jay, M., 1998. Flavonoid and enzyme polymorphisms and taxonomic organisa-tion ofRosasections:Carolinae,Cinnamomeae,PimpinellifoliaeandSynstylae. Biochem. Syst. Ecol. 26, 857}871.
Hara, H., 1957. On some double-#owered cultivated plants of old Chinese origin. J. Jpn. Bot. 32 (10), 313}315.
Harborne, J.B., 1984. Phytochemical Methods, 2nd Edition. Chapman & Hall, London, 288p.
Lu, T.S., Saito, N., Yokoi, M., Shigihara, A., Honda, T., 1992. Acylated pelargonidin glycosides in the red-purple#owers ofPharbitis nil. Phytochemistry 31, 289}295.
Mikanagi, Y., Saito, N., Yokoi, M., 1990. Flavonoid distribution in the#owers of genus Rosa, sections Synstylae,CinnamomeaeandPimpinellifoliae. Flavonoids in biology and medicine III. Current issues in Flavonoid Research. National University of Singapore, pp. 89}96.
Mikanagi, Y., Yokoi, M., Saito, N., Ueda, Y., Hirabarashi, H., Suzuki, S., 1994. Flower #avonoid distribution inRosa rugosaThunb. ex Murray and interspeci"c Rosa hybrids. J. Jpn. Soc. Hort. Sci. 62 (4), 857}866.
Mikanagi, Y., Yokoi, M., Ueda, Y., Saito, N., 1995. Flower#avonol and anthocyanin distribution in SubgenusRosa. Biochem. Syst. Ecol. 23, 183}200.
Raimond, O., Biolley, J.-P., Jay, M., 1995. Fingerprinting the selection process of ancient roses by means of #oral phenolic metabolism. Biochem. Syst. Ecol. 23, 555}565.
Rehder, A., 1949. Bibliography of Cultivated Trees and Shrubs Hardy in the Cooler Temperate Regions of the Northern Hemisphere, Jamaica Plain, pp. 296}317.
Saito, N., Harborne, J.B., 1992. Correlations between anthocyanin type, pollinator and#ower colour in the Laviatae. Phytochemistry 31, 3009}3015.
Saito, N., Yokoi, M., Suzuki, S., Hirabayashi, H., Kawabata, Y., 1982. Variation in the#ower colour and pigments among F1 hybrids of rose cultivars. Bull. Inst. General Educ. Meiji Gakuin University 14, 19}45.
Saito, N., Tatsuzawa, F., Yoda, K., Yokoi, M., Kasahara, K., Iida, S., Shigihara, A., Honda, T., 1995. Acylated cyanidin glycosides in the violet-blue#owers of Ipomoea purpurea. Phytochemistry 40, 1283}1289.
Satake et al., 1989. Wild Flowers of Japan, Woody Plants. (in Japanese) Heibonsha Ltd. Tokyo. Strack, D., Wray, V., 1994. 1 The anthocyanins. The Flavonoids. Advances in research since 1986, pp. 1}22. Tatsuzawa, F., Saito, N., Yokoi, M., 1996. Anthocyanins in the#owers ofCymbidium. Lindleyana 11 (4),
214}219.
WillstaKtter, R., Nolan, T.J., 1915. Untersuchungen uKber die Anthocyane. II. UGber den Farbstott der Rose. Liebigs Ann. Chem. 408, 1}14.
Yokoi, M., 1974. Color and pigment distribution in ornamental plants V. Anthocyanin distribution in rose cultivars. Tech. Bull. Fac. Hort., Chiba University 22, 12}24.
Yokoi, M., 1975. Color and pigment distribution in cultivars of selected ornamental values of plants. Trans. Fac. Hort., Chiba University 14, 1}65.