Recent studies reveal that relationships among the volvocine algae are more complex than was previously believed. Nevertheless, this group still appears to provide an unrivaled opportunity to analyze an evolutionary pathway leading from unicellularity (Chlamydomonas) to multicellularity with division of labor (Volvox). Significant progress in this regard was made in the past year when two genes playing key roles in Volvox cellular differentiation were cloned, and clues were uncovered regarding their mechanisms of action.
Addresses
Department of Biology, Washington University, Campus Box 1229, St. Louis, MO 63130, USA; e-mail: [email protected] Current Opinion in Plant Biology1999, 2:496–501
1369-5266/99/$ — see front matter © 1999 Elsevier Science Ltd. All rights reserved.
Abbreviations
ECM extracellular matrix
gls gonidialess
lag late gonidia
rbcL rubisco large subunit
regA somatic regenerator
Introduction
Multicellularity has evolved many times on this planet. In most cases, however, the pathway leading from a unicellu-lar ancestor to a multicelluunicellu-lar organism with differentiated cell types has been obscured by the passage of time. Although it is assumed that each of the major groups of conspicuous modern eukaryotes (Figure 1) evolved from a different unicellular ancestor more than a billion years ago [1–3], we can do little more than speculate about what the last unicellular ancestor of each of these groups may have been like, or how the transition to multicellularity may have occurred.
In contrast, within the volvocine algae multicellular organ-isms with differentiated cell types have evolved far more recently — and more than once. Therefore, this group of green flagellates, which includes members of the family Volvocaceae and their close unicellular relative Chlamydomonas reinhardtii, appears to provide an unrivaled opportunity for exploring a pathway leading from unicellu-larity to multicelluunicellu-larity [4•].
The volvocine algae as an evolutionary model
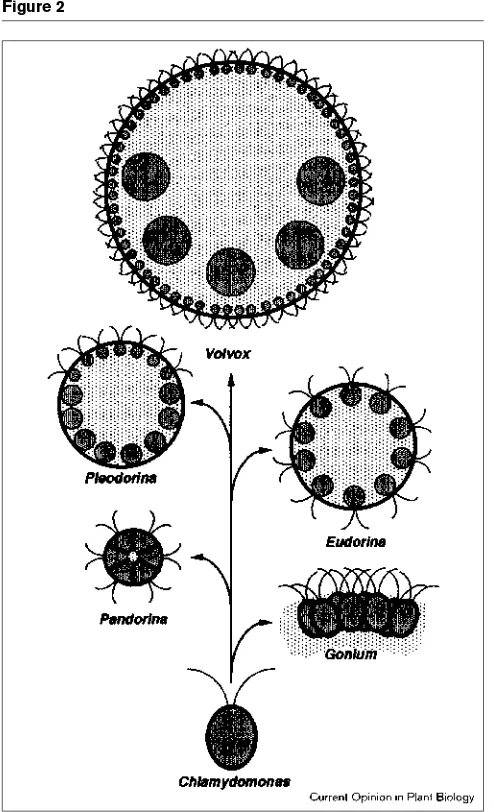
The volvocine algae range in size and complexity from unicellular Chlamydomonas, to Volvox, with its thousands of mortal somatic cells and a few immortal germ cells. It has long been commonplace for textbooks to arrange these algae in a conceptual series such as that in Figure 2, in which there is a regular progression in size, cell number, abundance of extracellular matrix (ECM), and tendency to produce sterile somatic cells. It is usually suggested thatthis might represent the sequence by which Volvox evolved. Although such a proposition contains a germ of truth, it clearly is overly simplistic.
Molecular data indicate that the volvocine algae constitute a surprisingly youthful group of close relatives: Volvox car-teri and C. reinhardtii apparently last shared a common ancestor 50–75 million years ago [5], and the germ-soma division of labor that defines the genus Volvoxhas evolved at least twice and possibly several times in this relatively brief interval [4•,6]. This suggests that the transition from unicellularity to the Volvox type of multicellularity must have a relatively simple genetic basis, and may actually be simple enough to be amenable to detailed analysis.
Such optimism is dampened only slightly by the accumu-lating molecular evidence that the volvocacean family tree is far more complicated than was suspected a generation ago. Volvox is not the only volvocine taxon that is poly-phyletic. For example, the phylogenetic reconstruction shown in Figure 3, which is based on rubisco large subunit (rbcL) sequences, supports the long-standing assumption that Eudorina,Pleodorinaand Volvoxare all closely related but at the same time it reveals the striking degree to which relationships within this section of the family Volvocaceae may deviate from the simple ones suggested by Figure 2. The data summarized in Figure 3 indicate clearly, for example, that the genus Eudorina is polyphyletic, and that its type species, E. elegans, is paraphyletic. Such deviations from monophyly had already been suspected when only a few Eudorina isolates had been analyzed [6], but the extent of these deviations from monophyletic expectations
Evolution of multicellularity in the volvocine algae
David L Kirk
Figure 1
has become increasingly apparent as more and more puta-tively ‘congeneric’ and ‘conspecific’ isolates have been analyzed [7]. Therefore, we may anticipate a plethora of additional complications when Pleodorina and Volvox species are sampled with similar intensity, and when the 12–14 species of Volvox that are currently missing from Figure 3 are added.
Relationships at the opposite end of the volvocine com-plexity spectrum are no simpler. Chlamydomonasturns out to be a highly paraphyletic genus comprising many dis-tinct clades [8], and C. reinhardtii is found to be much more closely related to the volvocaceans than it is to most Chlamydomonas species [8–10], including those species once thought to be its closest relatives [10,11]. Although
analysis of rbcL data provide little support for the idea that all species previously known as Goniumshould be moved from the Volvocaceae to two new families [12], they do indicate that these species define two entirely separate clades [6]; moreover, phylogenetic analysis at a higher level of resolution demonstrated that Gonium pectorale con-sists of six reproductively isolated subclades [13••]. But this is modest compared to the extraordinary reproduc-tive, chromosomal and molecular diversity that has been found within the morphologically uniform species Pandorina morum [14–16].
In short, it appears that many volvocacean taxonomic cate-gories identify developmental and/or organizational grades, rather than phylogenetic clades. However, reconstructions such as the one in Figure 3 (and others not shown) indicate that transitions among these grades — in both directions — must be easily accomplished and thus must have a relatively simple genetic basis. This notion is supported by mutation-al studies: a single mutation is adequate to convert Volvox powersiiinto a mimic of Pleodorina californica[17], and two mutations abolish germ-soma differentiation in V. carteriand convert it into a mimic of Eudorina[18].
Thus, an image of a highly branched, spreading bush has replaced the earlier image of an upright, relatively Figure 2
A conventional representation of ‘the volvocine lineage’, with organisms drawn at progressively decreasing magnification from bottom to top. The diameters of the biflagellate cells would be in the range of 5–10µm in each organism. Dark gray shading represents the cell bodies, light gray shading represents the ECM and thick black lines represent the tripartite layer that is discussed below.
Figure 3
unbranched tree as a metaphor for volvocacean family his-tory. But although this clearly means that reconstructing the complete family history of the Volvocaceae will be a much more challenging task than was once imagined, it has done little to diminish optimism that it will be easier to elucidate the molecular genetic origins of multicellularity and cellular differentiation in the volvocine algae than in any other lineage yet identified.
From unicells to colonies: sharing extracellular
materials
The prime feature distinguishing the volvocaceans from Chlamydomonas is that the glycoprotein based ‘cell walls’ of volvocaceans are linked together in some species-spe-cific manner to form an extracellular matrix (ECM); however, many components of Chlamydomonas walls and volvocacean ECM are clearly homologous. The most con-served extracellular feature of the group is a layer that has a characteristic crystalline appearance when viewed en face [19], but is more commonly seen as a dark–light–dark pro-file in electronmicrograph thin sections, and is therefore usually called the ‘tripartite’ layer. This layer has an extremely similar organization [19–21], and contains very similar proteins [22–24] in all volvocine algae that have been carefully examined; however, the disposition of this
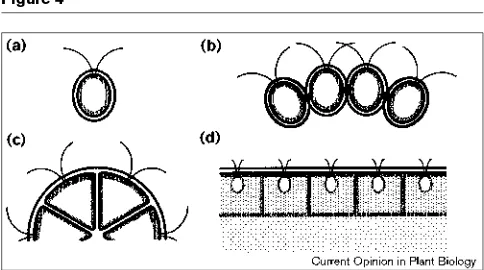
layer with respect to the cell bodies varies in a taxon-spe-cific way (Figure 4).
In Gonium, as in Chlamydomonas, the tripartite layer sur-rounds each cell and is separated from the plasmalemma by only a very thin, nondescript ‘inner wall’; however, in Goniumthe walls of neighboring cells are linked together (Figure 4a,b), with the nature of the linkages differing in a species-specific manner [25,26]. A transition occurs in Pandorina, where the tripartite layer is partially split, such that its outer leaflet is continuous over the surface of the colony, although its inner leaflet is still wrapped around each cell body (Figure 4c) [27]. This transition is taken a step further in the rest of the volvocaceans, in which the entire tripartite layer is always continuous over the surface (Figure 4d). In the larger volvocaceans (Eudorina toVolvox) the ECM accounts for 90–99% of the volume of the organ-ism, and it exhibits a wide variety of interesting taxon-specific internal specializations [28]. The vast major-ity of this ECM appears to be an extensive evolutionary amplification and elaboration of what in Chlamydomonas is the remarkably unimpressive ‘inner wall’ (Figure 4a,d).
It would be surprising, therefore, if the architecturally complex ECM of the larger volvocaceans was not signifi-cantly more complex biochemically than the cell wall of C. reinhardtii, which contains >30 kinds of glycoproteins, some of which are glycine-rich but most of which are hydroxyproline-rich [23,24]. Thus, the ten ECM proteins of V. carteri that have been characterized to date by cDNA cloning and sequencing [29••] constitute an impressive and important start — but only a start — toward a full mol-ecular characterization of the VolvoxECM.
The most striking feature shared by the extracellular gly-coproteins of C. reinhardtiiand V. carteriis their modularity. One of the most recurrent themes is a hydroxyproline-rich, rod-like module of variable length attached at one or both ends to various globular modules, which often results in molecules capable of extensive self-assembly [24,29••]. It has been postulated that the propensity of the genes encoding ECM modules for rapid diversification has resulted in their exploitation for entirely different, but no less important evolutionary ends: to encode species-specif-ic sex proteins — sexual agglutinins in the case of Chlamydomonas [30] and species-specific sex-inducing pheromones in the case of Volvox[29••].
The conversion of a Chlamydomonas cell wall to a volvocine ECM undoubtedly was facilitated by the unique pattern of cell division that the green flagellates exhibit: instead of undergoing binary fission as most cells do, green flagellates undergo ‘multiple fission’. That is, after growing 2n–fold in volume, they divide rapidly n times within the mother-cell wall, to produce 2ndaughter cells (where the maximum value of nis a species charac-teristic) [4•,31]. In unicellular green flagellates, cytokinesis is complete, so sister cells are able to move Figure 4
A schematic representation of the way in which certain extracellular materials (predominantly glycoproteins and sulfated polysaccharides) are arranged in selected volvocine taxa. Not drawn to scale. (a)Chlamydomonas reinhardtii. The concentric black–white–black ellipses at the periphery represent the ‘trilaminar’ layer of the cell wall, while the stippled ellipse represents the more amorphous-looking inner wall, and the central clear space represents the cell body. (b)Gonium pectorale.A wall resembling that of Chlamydomonas surrounds each cell body, but specializations (black rectangles) attach the outer layers of neighboring walls to one another at specific points. Such
relative to one another while they are still within the mother-cell wall and are forming their own cell walls [31]; as a result they develop as untethered, free individuals.
In contrast, in all volvocaceans that have been carefully examined, cytokinesis is incomplete, and cytoplasmic bridges that are formed in each division furrow hold all sis-ter cells in fixed relationships until afsis-ter the deposition of ‘wall’ components has begun [4•]. With the cells held together in this way while extracellular materials are being deposited, ‘walls’ invariably become fused in species-spe-cific ways to form the ECM that ties volvocacean cells into a coherent unit.
Thus, the critical first step in evolution of volvocine mul-ticellularity was probably the invention of a mechanism for delaying separation of sister cells until wall materials had been deposited around and between them. A recent-ly discovered flagellate, Gonium dispersum, appears to be trying to make up its mind right now whether or not it will permanently adopt such a mechanism. G. dispersum divides two ways: one way results in sister cells being held together between divisions, and results in formation of colonies similar to those of other Gonium species; the other way permits cells to move about between divi-sions, and generates Chlamydomonas-like unicellular progeny [32]. Further study of this interesting schizoid flagellate is warranted.
From colonies to multicellular organisms:
dividing life’s labors
V. carteri has become the premier model for studies of volvocine ontogeny and phylogeny because of its genetic accessibility and the clarity with which it can be used to pose two of the central unanswered questions of biology: how is a program for the development of cells of entirely different phenotypes programmed in a genome and how does such a program evolve?
In distinction to colonial volvocaceans such as Pandorina or Eudorina, V. carteriis a bona fide multicellular organism with a complete division of labor between two interde-pendent cell types [4•,33]. The ~2,000 biflagellate somatic cells of V. carteriare specialized for motility but are terminally differentiated and programmed to die after serving their purpose. The ~16 gonidia (asexual reproduc-tive cells), in marked contrast, are completely non-motile, programmed for division and reproduction, and potential-ly immortal. When mature, each gonidium divides repeatedly to produce an embryo containing all of the cells of both types that will be present in an adult of the next generation. A key step in germ-soma differentiation occurs in mid-embryogenesis, when visibly asymmetric divisions set apart large ‘gonidial initials’ from small ‘somatic initials’. Many types of evidence indicate that it is this difference in size, and not any difference in cyto-plasmic quality, that determines which pathway of differentiation each cell will follow [34].
Mutational and Mendelian analyses have led to the pro-posal that three major types of gene program V. carteri germ-soma differentiation [18]. First the gonidialess (gls) genes act in the cleaving embryo to cause asymmetric divi-sion. Then a pair of negative regulators are activated differentially: the somatic regenerator (regA) gene product acts in small cells to repress reproductive development, while the late gonidia (lag) gene products act in the large cells to repress somatic development.
The simple logic underlying such a negative-regulatory program becomes apparent when viewed in an evolution-ary context. In volvocine algae from Chlamydomonas to Eudorina that have a single cell type, every cell first develops as a biflagellate (somatic-like) cell, and then later loses its flagella, rounds up, and transforms into a reproductive (gonidia-like) cell that initiates a series of rapid cleavage divisions [4•,31]. Thus, the basic program of volvocine development is a sequential one: first bifla-gellate, then reproductive. In V. carteri, however, this sequential program has been converted into a dichoto-mous one by repressing the biflagellate phase in gonidia (with the lag genes), and the reproductive phase in somatic cells (with the regAgene). When one of the lag genes is inactivated, presumptive gonidia revert to the ancestral ‘first biflagellate, then reproductive’ program, and when the regAgene is inactivated the somatic cells do the same [35]. Thus, it seems clear that these genes once played as important a role in the evolution of V. carterias they now play in its development.
Detailed analysis of such genes awaited development of molecular-genetic tools of the sort now considered com-monplace in other systems. The concerted efforts of the three laboratories now actively engaged in Volvoxresearch have resulted in development of such a tool kit: this now includes a pair of selectable markers [36,37•], a nuclear transformation system [38], a transposon suitable for gene tagging [39], and reporter and inducible-promoter con-structs [40]. Transformation has been used to modify carbohydrate metabolism with a foreign gene [41], and to achieve gene replacement by homologous recombination [42]. More recently, transposon tagging has been used to clone two key genes controlling germ-soma differentiation: glsA, a gene required for asymmetric division [43••] and regA, the gene whose product represses reproductive development in somatic cells [44••].
in shifting the division apparatus to an off-center site in an asymmetrically dividing cell is under investigation (SM Miller, personal communication).
RegA, the protein encoded by regA, is a nuclear protein with many features of a transcriptional repressor [44••]. Earlier studies had identified a set of 18 genes defined by ‘maturation-abundant gonidial cDNAs’ [45], which have expression patterns suggesting that they are under either direct or indirect negative regulation by regA: transcripts of these 18 genes accumulate to high levels in both gonidia and regA– somatic cells but are undetectable in wild-type (regA+) somatic cells after regAexpression begins [18,45,46]. It was initially a surprise to learn that the first three of these genes to be sequenced represented nuclear genes encoding key chloroplast proteins [47] but this observation has now been extended substantially. All 16 of these 18 candidate target genes that have recognizable polypeptides encode known or putative chloroplast proteins [48•]. These include components of both light-harvesting complexes, the oxy-gen-evolving complex, the electron transport chain, the chloroplast ATP-synthase, and the Calvin cycle, as well as a regulator of chloroplast translation.
These unanticipated observations regarding the putative targets of RegA regulation have led to the following working hypothesis: RegA represses reproductive devel-opment in somatic cells by repressing genes whose products are required for chloroplast biogenesis, thereby preventing these cells from growing enough to repro-duce. V. carteri is an obligate photoautotroph whose growth is photosynthesis limited. Each somatic cell inherits ~0.04% of the maternal chloroplast during goni-dial cleavage; but if unable to make additional chloroplast, it cannot grow significantly. And if it cannot grow, it cannot reproduce.
Of the many additional observations that are judged to be consistent with this hypothesis [48•], the most compelling is that a significant number of somatic cells that are mor-phologically indistinguishable from their neighbors lack any detectable chloroplast DNA [49]. This seems to demonstrate quite clearly that chloroplast biogenesis is not required for normal somatic cell development.
Conclusions
The family Volvocaceae is phylogenetically far more com-plex than was once believed. Nevertheless, it still holds great promise as a model for studying the evolution of multicellularity and cellular differentiation. V. carteri, which has been studied more intensely than any other member of the family, has begun to reveal the details of its genetic program for germ-soma differentiation. This rais-es two fascinating qurais-estions to be addrrais-essed in the future: what are the evolutionary roots of the gls, regA and lag genes that now control cellular differentiation in V. carteri and do other Volvox species that arose by independent phylogenetic pathways exhibit a genetic program for
germ-soma differentiation similar to that of V. carteri? Or have they discovered some entirely different way to solve the same evolutionary challenge?
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Devereux R, Loeblich AR III, Fox GE: Higher plant origins and the phylogeny of green algae.J Mol Evol1990, 31:18-24.
2. Wainwright PO, Hinkle G, Sogin ML, Stickel SK: Monophyletic origins of the Metazoa: an evolutionary link with fungi.Science
1993, 260:340-342.
3. Sogin ML: Early evolution and the origin of eukaryotes.Curr Biol
1991, 1:457-463.
4. Kirk DL: Volvox: Molecular-Genetic Origins of Multicellularity and
• Cellular Differentiation, vol 381. Cambridge: Cambridge University Press; 1998.
A comprehensive treatise on the ontogeny and phylogeny of Volvox, partic-ularly Volvox carteri.Most of the topics that are touched on in the present review are discussed in considerably more detail in this monograph. 5. Rausch H, Larsen N, Schmitt R: Phylogenetic relationships of the
green alga Volvox carterideduced from small-subunit ribosomal RNA comparisons.J Mol Evol1989, 29:255-265.
6. Nozaki H, Itoh M, Sano R, Uchida H, Watanabe MM, Kuroiwa T:
Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data.J Phycol
1995, 31:970-979.
7. Nozaki H, Ito M, Uchida H, Watanabe MM, Kuroiwa T:
Phylogenetic analysis of Eudorina species (Volvocaceae, Chlorophyta) based on rbcL gene sequences. J Phycol 1997,
33:859-863.
8. Buchheim MA, Chapman RL: Phylogeny of the colonial green flagellates: a study of 18S and 26S rRNA sequence data.
BioSystems 1991, 25:85-100.
9. Larson A, Kirk MM, Kirk DL: Molecular phylogeny of the volvocine flagellates.Mol Biol Evol1992, 9:85-105.
10. Coleman AW, Mai JC: Ribosomal DNA ITS-1 and ITS-2 sequence comparisons as a tool for predicting genetic relatedness.
J Mol Evol 1997, 45:168-177.
11. Ettl H: Die Gattung ChlamydomonasEhrenberg.
Beih Nova Hedwigia 1976, 49:1-1122. [Title translation: The genus
ChlamydomonasEhrenberg.]
12. Nozaki H, Itoh M: Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from cladistic analysis based on morphological data. J Phycol 1994,30:353-365.
13. Fabry S, Köhler A, Coleman AW: Intraspecies analysis: Comparison
•• of ITS sequence data and gene intron sequence data with breeding data for a worldwide collection of Gonium pectorale.
J Mol Evol1999, 48:94-101.
The authors use sequences of the rDNA internal transcribed spacers (ITS) [10], and three spliceosomal introns to perform two parallel high-resolution phylogenetic analyses of G. pectorale isolates from five continents. The trees generated by these two approaches have extremely similar topologies and sort these 25 isolates into the same two major clades and six subclades. Pairwise tests then demonstrate that only members of the same subclade are able to mate and produce fertile progeny.
14. Coleman AW: Sexual isolation in Pandorina morum.J Protozool
1959, 6:249-264.
15. Coleman AW, Zollner J: Cytogenetic polymorphism within the species Pandorina morum Bory de St. Vincent (Volvocaceae).
Arch Protistenkd1977, 119:224-232.
16. Coleman AW, Suarez A, Goff LJ: Molecular delineation of species and syngens in volvocacean green algae (Chlorophyta).J Phycol
1994, 30:80-90.
17. Vande Berg WJ, Starr RC: Structure, reproduction and differentiation in Volvox gigasand Volvox powersii.
18. Tam L-W, Kirk DL: The program for cellular differentiation in Volvox carterias revealed by molecular analysis of development in a gonidialess/somatic regenerator mutant.Development 1991,
112:571-580.
19. Roberts K: Crystalline glycoprotein cell walls of algae; their structure, composition and assembly.Phil Trans R Soc Lond, Biol Sci1974, 268:129-146.
20. Goodenough UW, Heuser JE: Molecular organization of cell-wall crystals from Chlamydomonas reinhardtiiand Volvox carteri.
J Cell Sci 1988, 90:717-733.
21. Adair WS, Steinmetz SA, Mattson DM, Goodenough UW, Heuser JE:
Nucleated assembly of Chlamydomonasand Volvoxcell walls.
J Cell Biol1987, 105:2373-2382.
22. Adair WS, Appel H: Identification of a highly conserved hydroxyproline-rich glycoprotein in the cell walls of Chlamydomonas reinhardtii and two other Volvocales.Planta
1989, 179:381-386.
23. Adair WS, Snell WJ: The Chlamydomonas reinhardtiicell wall: structure, biochemistry, and molecular biology.In Organization and Assembly of Plant and Animal Extracellular Matrix.Edited by Adair WS, Mecham RP. San Diego: Academic Press; 1990:15-84. 24. Woessner JP, Goodenough UW: Volvocine cell walls and their
constituent glycoproteins: an evolutionary perspective.
Protoplasma 1994, 181:245-258.
25. Nozaki H: Ultrastructure of the extracellular matrix of Gonium (Volvocales, Chlorophyta).Phycologia1990, 29:1-8.
26. Nozaki H, Itoh M, Watanabe MM, Kuroiwa T: Ultrastructure of the vegetative colonies and systematic position of Basichlamys (Volvocales, Chlorophyta).Eur J Phycol 1996, 31:67-72. 27. Fulton AB: Colony development in Pandorina morum. II. Colony
morphogenesis and formation of the extracellular matrix.Dev Biol
1978, 64:236-251.
28. Kirk DL, Birchem R, King N: The extracellular matrix of Volvox: a comparative study and proposed system of nomenclature.
J Cell Sci 1986, 80:207-231.
29. Sumper M, Hallmann A: Biochemistry of the extracellular matrix of
•• Volvox.Int Rev Cytol 1998, 180:51-85.
In a comprehensive and lucid review, the authors summarize all that is known to date (principally as a result of contributions from their own laboratory) regarding the organization, composition, assembly, and roles played in the life of the organism by the V. carteriextracellular matrix (ECM). Of particular interest are their observations regarding sequence relationships between a family of ECM glycoproteins and the extraordinarily powerful pheromone that triggers sexual reproduction in V. carteri.
30. Goodenough UW: Chlamydomonas mating interactions.In
Microbial Cell–Cell Interactions. Edited by Dworkin M. Washington DC: American Society for Microbiology; 1994:71-112.
31. Harris EH: The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989.
32. Batko A, Jakubiec H: Gonium dispersum sp. nov., a new species of Gonium from Poland.Arch Hyrobiol Suppl1989, 82:39-47. 33. Starr RC: Control of differentiation in Volvox.Dev Biol Suppl1970,
4:59-100.
34. Kirk MM, Ransick A, McRae SE, Kirk DL: The relationship between cell size and cell fate in Volvox carteri.J Cell Biol1993,
123:191-208.
35. Kirk DL: Genetic control of reproductive cell differentiation in Volvox.In Experimental Phycology 1: Cell Walls and Surfaces, Reproduction and Photosynthesis.Edited by Wiessner W, Robinson DG, Starr RC. Berlin: Springer-Verlag; 1990:81-94.
36. Gruber H, Goetinck SD, Kirk DL, Schmitt R: The nitrate reductase-encoding gene of Volvox carteri: map location, sequence and induction kinetics.Gene 1992, 120:75-83.
37. Hallmann A, Rappel A: Genetic engineering of the multicellular
• green alga Volvox: a modified and multiplied bacterial antibiotic resistance gene as a dominant selectable marker.Plant J1999,
17:99-109.
This paper is important both as a demonstration of features that can be engi-neered into foreign genes to enhance their expression in V. carteri, and as a description of a much-needed second selectable marker for use in transfor-mation studies of Volvox.
38. Schiedlmeier B, Schmitt R, Müller W, Kirk MM, Gruber H, Mages W, Kirk DL: Nuclear transformation of Volvox carteri.
Proc Natl Acad Sci USA1994, 91:5080-5084.
39. Miller SM, Schmitt R, Kirk DL: Jordan,an active Volvox transposable element similar to higher plant transposons. Plant Cell1993,
5:1125-1138.
40. Hallmann A, Sumper M: Reporter genes and highly regulated promoters as tools for transformation experiments in Volvox carteri.Proc Natl Acad Sci USA1994, 91:11562-11566. 41. Hallmann A, Sumper M: The Chlorellahexose/H+symporter is a
useful selectable marker and biochemical reagent when expressed in Volvox. Proc Natl Acad Sci USA1996, 93:669-673. 42. Hallmann A, Rappel A, Sumper M: Gene replacement by
homologous recombination in the multicellular green alga Volvox carteri.Proc Natl Acad Sci USA1997, 94:7469-7474.
43. Miller SM, Kirk DL: glsA, a Volvoxgene required for asymmetric
•• division and germ cell specification, encodes a chaperone-like protein.Development1999, 126:649-658.
This paper and its companion [44••] report the first uses of an inducible
transposon (see [39]) to tag and recover a developmentally important Volvox
gene. The glsAgene, which plays an essential role in the asymmetric divi-sions that set apart germ and somatic cell lineages, is shown to encode a protein that contains two protein-binding sites, and that associates with the mitotic spindle in dividing cells.
44. Kirk MM, Stark K, Miller SM, Müller W, Taillon BE, Gruber H,
•• Schmitt R, Kirk DL: regA, a Volvoxgene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein.
Development1999, 126:639-647.
This paper and its companion [43••] report the first uses of an inducible
transposon (see [39]) to tag and recover a developmentally important Volvox
gene. The regA gene, which prevents reproductive development in somatic cells, is shown to be a nuclear protein with features of an active transcrip-tional repressor that is present in somatic cells, but not gonidia, for most of the life cycle.
45. Tam L-W, Kirk DL: Identification of cell-type-specific genes of Volvox carteriand characterization of their expression during the asexual life cycle.Dev Biol 1991, 145:51-66.
46. Tam L-W, Stamer KA, Kirk DL: Early and late gene expression programs in developing somatic cells of Volvox carteri.
Dev Biol1991, 145:67-76.
47. Choi G, Przybylska M, Straus D: Three abundant germ line-specific transcripts in Volvox carteriencode photosynthetic proteins.
Curr Genet1996, 30:347-355.
48. Meissner MK, Stark K, Cresnar B, Kirk DL, Schmitt R: Volvox
• germline-specific genes that are putative targets of RegA repression encode chloroplast proteins. Curr Gent 1999, in press. This paper extends an analysis begun in [47], and shows that all of the puta-tive target genes of regA[44••] that have recognizable open reading frames
are nuclear genes encoding chloroplast proteins. This leads to the hypothe-sis that regA exerts its effect on germ-soma differentiation by repressing chloroplast biogenesis in somatic cells.
49. Coleman AW, Maguire MJ: A microspectrophotometric analysis of nuclear and chloroplast DNA in Volvox.Dev Biol 1982,