B R I E F R E P O R T
Lack of secondary microthrombosis after thrombin-induced
stroke in mice and non-human primates
M . G A U B E R T I , S . M A R T I N E Z D E L I Z A R R O N D O , C . O R S E T and D . V I V I E N
Inserm UMR-S U919, Serine Proteases and Pathophysiology of the Neurovascular Unit, Inserm, Universite Caen Basse-Normandie, GIP Cyceron, Caen, France
To cite this article: Gauberti M, Martinez de Lizarrondo S, Orset C, Vivien D. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates.J Thromb Haemost2014;12: 409–14.
Summary.Background:Secondary microthrombosis is a major pathophysiologic mechanism leading to brain dam-age following transient mechanical vascular occlusion (TMVO), the most widely used experimental stroke model. Whether secondary microthrombosis also occurs in non-TMVO stroke models represents an important issue for clinical translation of antimicrothrombosis thera-peutic strategies.Objectives:To assess the occurrence and the pathogenic role of secondary microthrombosis in two thrombin-induced stroke models in mice and non-human primates (Macaca mulatta).Methods:Stroke was induced in mice and non-human primates by intra-arterial admin-istration of recombinant thrombin. This method induces the formation of a fibrin-rich thrombus, which is sponta-neously dissolved in the following hours by the endoge-nous fibrinolytic system. Perfusion-weighted imaging and fluorescent-lectin microangiography were performed after recanalization to detect secondary microthrombosis. Moreover, to investigate its pathogenic role, thrombin-induced stroke was thrombin-induced in bradykinin receptor B1 (B1R) knockout mice, which are protected from the thromboinflammation responsible for secondary micro-thrombosis in TMVO models. Results:Reperfusion was stable and complete in all mice and non-human primates tested, revealing no secondary decrease in cerebral blood flow. No evidence of secondary microthrombosis was found in the two models. Accordingly, deficiency in B1R did not protect the mice from brain damage after throm-bin-induced stroke. Conclusions:Our data demonstrate that secondary microthrombosis does not occur after thrombin-induced stroke. In view of this, the pathophysi-ologic roles of hematpathophysi-ologic players promoting or
protect-ing against secondary microthrombosis (such as factor XII, von Willebrand factor, and T cells) deserve to be re-evaluated in non-TMVO stroke models.
Keywords: bradykinin; coagulation; inflammation; ischemia; stroke; thrombosis.
Introduction
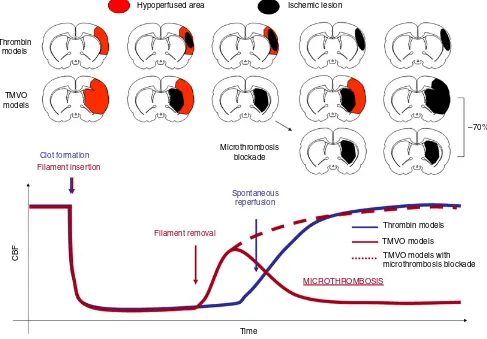
Numerous recent studies have demonstrated the involve-ment of secondary microthrombosis in the developinvolve-ment of cerebral ischemic lesions following transient mechanical vascular occlusion (TMVO) in mice. Notably, inhibition of microthrombosis with different strategies (including factor XII inhibition [1], glycoprotein [GP]Iba blockade [2], regulatory T-cell depletion [3], and kininogen/bradyki-nin blockade [4,5]) reduces ischemic lesion size by ~70% in TMVO models, suggesting that secondary microthrom-bosis may constitute a promising therapeutic target for acute stroke treatment.
Secondary microthrombosis occurs within minutes after reperfusion in TMVO models, once the occluding fila-ment has been removed. It induces delayed cerebral blood flow (CBF) reduction, secondary ischemia, and lesion growth, which is responsible for ~70% of the final ische-mic lesion size [1]. These findings led to the concept of stroke being a thromboinflammatory disorder [6]. How-ever, clinical studies have demonstrated that secondary ischemia and lesion growth are modest or non-existent after reperfusion [7–9]. Moreover, serious concerns have been raised about the clinical relevance of TMVO models [10]. Therefore, whether secondary microthrombosis also occurs in non-TMVO models represents a critical issue for clinical translation of antimicrothrombosis strategies.
To address this question, we first investigated whether microthrombosis occurs in other recently reported models of acute ischemic stroke, induced by intra-arterial injec-tion of recombinant thrombin in mice and non-human primates [11,12]. Then, we studied the impact of thromboinflammation blockade, by genetic deletion or Correspondence: Denis Vivien, Inserm UMR-S U919, GIP Cyceron,
Bd H. Becquerel, 14074 Caen, France.
Tel.: +33 2 31 47 01 60; fax: +33 2 31 47 02 22. E-mail: vivien@cyceron.fr
Received 28 October 2013 Manuscript handled by: M. Levi
pharmacologic blockade of the bradykinin receptor B1 (B1R), on stroke outcome in the mouse model.
Material and methods
Mice
Male Swiss mice (28 animals weighing 30–35 g) were pur-chased from the CURB (Caen, France). Five male B1R-deficient mice (B1R / , aged 8 weeks) from a C57BL6/J background [13] were provided by INSERM U1048, housed at the CURB, and compared with five male C57BL6/J mice (aged 8 weeks; CURB). Mice were housed with a 12-h light–dark cycle with free access to food and water. We performed in situthromboembolic occlusion in
anesthetized mice (2% isoflurane in 33 : 67 O2/N2O [33% : 67%] with the rectal temperature maintained at 37°C) by intra-arterial injection of thrombin, as previously described [11]. The operator performing surgery was una-ware of the genetic background and treatment group of the mice. Pharmacologic inhibition of B1R was performed by intravenous injection of R-715 (1 mg kg 1; Tocris, Bristol, UK) 20 min after stroke onset [5].
Non-human primates
Experiments were performed in five male rhesus macaques (Macaca mulatta) aged 5–6 years and with body weights
ranging from 7 kg to 11 kg. An experimental protocol was submitted to the Regional Ethics Committee for Animal Experimentation (Normandy), and approval was granted to conduct the study (referral No. N/02-03-08/03/02-11). Experiments were performed in accordance with French ethical laws (act No. 87-848; Ministere de l’Agriculture et de la For^et) and European Communities Council Direc-tives (86/609/EEC and 2010/63/EU) guidelines for the care and use of laboratory animals. We performed in situ
thromboembolic occlusion in anesthetized non-human pri-mates as previously described [12].
Magnetic resonance imaging (MRI) analysis
Experiments in mice were carried out on a Pharmascan 7 T/12-cm system with surface coils (Bruker, Wissembourg,€
France). T2-weighted images were acquired by use of a multislice multiecho sequence: echo time (TE)/repetition time (TR), 51/2500 ms; and spatial resolution, 709 709500 lm3 [14]. Lesion sizes were quantified on T2-weighted images withIMAGEJsoftware (v1.45r; National Institute of Health, USA). For perfusion-weighted imag-ing, a gradient-echo fast imaging with steady-state preces-sion sequence was used, with the following parameters: TR/TE, 10/5 ms (Flip Angle =8°); temporal resolution, 911 ms; and spatial resolution, 1009100 975lm3 (half-scan). Mice were injected with 30 lL of a 0.5 Msolution of Dotarem (Guerbet, Aulnay-sous-Bois, France) during
repetitive image acquisitions. Then,DR2*images were gen-erated with an in-house-created macro inIMAGEJ. CBF was estimated by measurement of the ratio of ipsilateral and contralateral DR2*, as described previously [15]. Experi-ments in non-human primates were performed as previ-ously described [12]. All quantifications were performed blind to the experimental data.
Fluorescein isothiocyanate (FITC)–lectin microangiography
Twenty-four hours after thrombin-induced middle cere-bral artery (MCA) occlusion, mice were injected intrave-nously with FITC-conjugated lectin [16] (100lg per mouse). Two minutes after injection, mice were killed by transcardiac perfusion with cold heparinized saline fol-lowed by paraformaldehyde and picric acid. Brains were postfixed and cryoprotected before being frozen in Tis-sue-Tek. Cryostat sections (10lm) were collected on polylysine-coated slides before being analyzed with fluo-rescence microscopy. Sections were coincubated overnight with goat anti-collagen type IV (1 : 1500; Southern Biotech, Birmingham, AL, USA) or chicken anti-microtu-bule-associated protein 2 (1 : 8000; Abcam, Paris, France). Primary antibodies were revealed with Fab′2 fragments of donkey anti-goat or anti-chicken IgG linked to tetramethylrhodamine isothiocyanate (1 : 500; Jackson ImmunoResearch, West Grove, PA, USA). Washed sec-tions were coverslipped with antifade medium containing 4′,6′-diamidino-2-phenylindole, and images were digitally captured with a Leica DM6000 microscope-coupled cool-snap camera and visualized with METAVUE5.0 software (Molecular Devices, Sunnyvale, CA, USA). All quantifi-cations were performed blind to the experimental data.
Results and Discussion
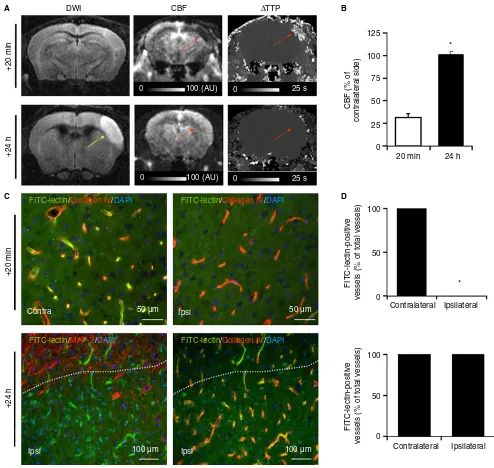
We induced ischemic stroke in mice and in non-human primates (M. mulatta) byin situintra-arterial
whereas 78% of the microvessels are occluded in TMVO models at this time point [2]. These results demonstrated that secondary microthrombosis does not occur after thrombin-induced stroke.
We then wanted to investigate whether differences in microthrombosis have therapeutic implications. As genetic
deletion of B1R prevents the thromboinflammatory reaction responsible for microthrombosis in TMVO models [4–6], we investigated whether B1R / mice [13] are protected from stroke in a thromboembolic model. We chose to block B1R because it is not involved in the initial thrombin-induced clot formation, in contrast to, DWI
+20 min
125
100
100
50
0
100
50
0 75
50
25
0
20 min
Contralateral Ipsilateral
Contralateral Ipsilateral
CBF (% of
contralateral side)
FITC-lectin-positive
vessels (% of total vessels)
FITC-lectin-positive
vessels (% of total vessels)
24 h *
*
+20 min
+24 h
+24 h
CBF
0 100 (AU)
0
FITC-lectin/Collagen IV/DAPI
FITC-lectin/MAP-2/DAPI
FITC-lectin/Collagen IV/DAPI
FITC-lectin/Collagen IV/DAPI 100 (AU)
0 25 s
0 25 s
Contra Ipsi
Ipsi
50 µm
100 µm Ipsi 100 µm
50 µm ∆TTP
A B
C D
+2 h
0 0
0
100 (AU) 15 s
0 15 s
100 (AU)
+2 h
+24 h
DWI CBF
CBF (% of contralateral side)
∆TTP +24 h
125
100
75
50
25
0
2 h 24 h
*
A B
Fig. 2.Lack of secondary cerebral blood flow (CBF) reduction after thrombin-induced stroke and reperfusion in non-human primates. (A) Representative magnetic resonance angiograms, demonstrating spontaneous recanalization 24 h after stroke onset in non-human primates sub-jected to thrombin-induced middle cerebral artery occlusion (top, yellow arrow). Representative magnetic resonance image (3 T) of non-human primates 2 h and 24 h after intra-arterial thrombin injection, showing a cortical ischemic lesion (yellow arrows) and demonstrating full recovery of CBF after spontaneous arterial recanalization (red arrows,n=5). (B) Corresponding quantification of CBF.P<0.05 was considered to indicate statistical significance. Data are presented as meanstandard error of the mean. AU, arbitrary units; DWI, diffusion weighted imaging; TTP, time to peak.
Saline
R-715
25 30
25
20
15
10
5
0
NS NS
WT B1R–/– Saline R-715
20
15
10
5
0
WT
B1R–/–
Lesion size (mm
3)
Lesion size (mm
3) A
B
C
D
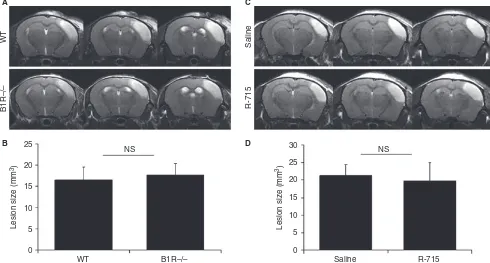
for example, FXII or platelet GPIba. We demonstrated
that wild-type and B1R / mice show similar ischemic lesion sizes 24 h after stroke (Fig. 3A,B). Similarly, B1R antagonist (R-715, 1 mg kg 1, 20 min after stroke induction) failed to influence ischemic lesion size (Fig. 3C,D).
Altogether, these data provide evidence that micro-thrombosis does not occur after thrombin-induced stroke in either mice or non-human primates (Fig. 4). Accord-ingly, blockade of the thromboinflammatory reaction by either genetic deletion or pharmacologic inhibition of B1R failed to protect against stroke in the mouse model. As blockade of the kininogen–bradykinin axis is also not beneficial in a permanent ischemic stroke model [4], the available data suggest that thromboinflammation block-ade through B1R inhibition is ineffective in permanent and thrombin-induced transient ischemic strokes, thus questioning the potential impact of such therapeutic strat-egy in stroke patients.
In view of this, the pathophysiologic roles of hemato-logic players promoting or protecting against micro-thrombosis (such as FXII, von Willebrand factor, platelet GPIba, and T-cells) deserve to be re-evaluated in
non-TMVO stroke models. Nevertheless, secondary micro-thrombosis may represent a potential therapeutic target in patients treated with endovascular procedures, as late secondary injury occurs in 18% of them [19]. Interest-ingly, these patients show transient reversal of the cyto-toxic edema after reperfusion, a specific feature of TMVO models [10]. As stroke is a polyetiologic disease, our results cannot be extrapolated to all stroke subtypes. In particular, the role of microthrombosis in lacunar stroke pathogenesis was not assessed in the present study, and remains to be investigated.
Addendum
M. Gauberti designed and performed research, and wrote the manuscript. S. Martinez de Lizarrondo and C. Orset provided scientific suggestions and contributed to manuscript review. D. Vivien supervised the study.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Hypoperfused area
Microthrombosis blockade
–70%
Thrombin models
TMVO models
TMVO models with microthrombosis blockade
Spontaneous reperfusion
Filament removal
MICROTHROMBOSIS
Time Thrombin
models
TMVO models
Clot formation Filament insertion
CBF
Ischemic lesion
References
1 Pham M, Kleinschnitz C, Helluy X, Bartsch AJ, Austinat M, Behr VC, Renne T, Nieswandt B, Stoll G, Bendszus M. Enhanced cortical reperfusion protects coagulation factor XII-deficient mice from ischemic stroke as revealed by high-field MRI.Neuroimage2010;49: 2907–14.
2 Momi S, Tantucci M, van Roy M, Ulrichts H, Ricci G, Gresele P. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs.Blood2013;121: 5088
–97.
3 Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K,€ Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A,et al. Regula-tory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood2013;121: 679–91.
4 Langhauser F, G€ob E, Kraft P, Geis C, Schmitt J, Brede M, G€obel K, Helluy X, Pham M, Bendszus M, Jakob P, Stoll G, Meuth SG, Nieswandt B, McCrae KR, Kleinschnitz C. Kinino-gen deficiency protects from ischemic neurodeKinino-generation in mice by reducing thrombosis, blood–brain barrier damage, and inflammation.Blood2012;120: 4082–92.
5 Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renne T, Kleinschnitz C. Blockade of bradykinin recep-tor B1 but not bradykinin receprecep-tor B2 provides protection from cerebral infarction and brain edema.Stroke2009;40: 285
–93. 6 Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: a
thrombo-inflammatory disease?J Physiol2011;589: 4115 –23. 7 Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR,
Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA, Chang CWJ, Warach S, Fazekas F, Inoue M, Tipirneni A, Ham-ilton SA, Zaharchuk G, Marks MP, Bammer R, Albers GW. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study.Lancet Neurol 2012;11: 860–7.
8 Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Han-key G, Muir K, Gerraty R, Tress BM,et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial.Lancet Neurol2008;7: 299–309.
9 Delgado-Mederos R, Rovira A, Alvarez-Sabın J, Ribo M, Munuera J, Rubiera M, Santamarina E, Maisterra O, Delgado P, Montaner J, Molina CA. Speed of tPA-induced clot lysis pre-dicts DWI lesion evolution in acute stroke.Stroke2007;38: 955– 60.
10 Hossmann K. The two pathophysiologies of focal brain ische-mia: implications for translational stroke research.J Cereb Blood Flow Metab2012;32: 1310–16.
11 Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thrombo-embolic stroke and reperfusion.Stroke2007;38: 2771–8. 12 Gauberti M, Obiang P, Guedin P, Balossier A, Gakuba C,
Diep-endaele AS, Chazalviel L, Vivien D, Young AR, Agin V, Orset C. Thrombotic stroke in the anesthetized monkey (Macaca mul-atta): characterization by MRI –a pilot study.Cerebrovasc Dis 2012;33: 329
–39.
13 Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, Heeringa P, Pesquero J, Schanstra J, Bascands J. Blockade of the kinin B1 receptor ameloriates glomerulonephritis.J Am Soc Nephrol2010;21: 1157–64.
14 Gakuba C, Gauberti M, Mazighi M, Defer G, Hanouz J, Vivien D. Preclinical evidence toward the use of ketamine for recombi-nant tissue-type plasminogen activator-mediated thrombolysis under anesthesia or sedation.Stroke2011;42: 2947–9.
15 Pierce AR, Lo EH, Mandeville JB, Gonzalez RG, Rosen BR, Wolf GL. MRI measurements of water diffusion and cerebral perfusion: their relationship in a rat model of focal cerebral ischemia.J Cereb Blood Flow Metab1997;17: 183–90.
16 Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neuro-genesis in postischemic brain via protease-activated receptor 1.J Neurosci2008;28: 12788
–97.
17 Gauberti M, Montagne A, Marcos-Contreras OA, Le Behot A, Maubert E, Vivien D. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penum-bra after strokes.Stroke2013;44: 1988–96.
18 Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabın J. Timing of spontaneous recanaliza-tion and risk of hemorrhagic transformarecanaliza-tion in acute cardioem-bolic stroke.Stroke2001;32: 1079–84.