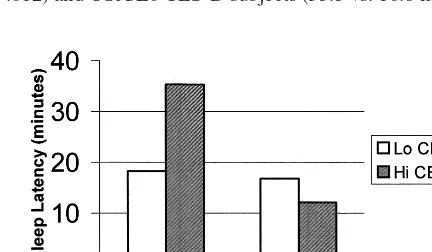Does Obstructive Sleep Apnea Confound Sleep
Architecture Findings in Subjects with
Depressive Symptoms?
Wayne A. Bardwell, Polly Moore, Sonia Ancoli-Israel, and Joel E. Dimsdale
Background:Compared with normal subjects, depressed patients have shorter rapid eye movement sleep latency (REML), increased REM and decreased slow wave sleep as a percentage of total sleep time (REM%, SWS%), and longer sleep latency (SL). Obstructive sleep apnea (OSA) patients experience longer REML, decreased REM% and SWS%, and shorter SL. We examined the interplay of depressive symptoms, OSA, and sleep architecture.
Methods:Subjects (n5106) were studied with polysom-nography. OSA was defined as a Respiratory Disturbance Index$15. Subjects were divided into Hi/Lo groups using a Center for Epidemiological Studies—Depression (CES-D) score of 16.
Results: OSA patients had shorter SL than non-OSA patients (14.5 vs. 26.8 min,p,.001); Hi CES-D subjects showed a trend toward longer SL than Lo CES-D subjects (23.7 vs. 17.5 min,p5 .079). Significant OSA3CES-D interactions emerged, however, for REM% (p5.040) and SL (p5.002): OSA/Hi CES-D subjects had higher REM% than OSA/Lo CES-D subjects (19.3% vs. 14.3%, p 5
.021); non–OSA/Hi CES-D subjects had SL (35.3 min) 2–3 times as long as other subjects (p5.002–.012).
Conclusions:Because of the high prevalence of OSA and depression, findings suggest that OSA must be considered in studies of mood and sleep architecture. Conversely, depressive symptoms must be considered in studies of OSA and sleep architecture. Biol Psychiatry 2000;48: 1001–1009 © 2000 Society of Biological Psychiatry
Key Words: Depression, mood, obstructive sleep apnea, sleep architecture, REM, sleep latency
Introduction
S
leep disruption has long been recognized as accompa-nying depression. When compared with the nonde-pressed, the most frequently reported alterations in sleeparchitecture in depressed patients include reduced rapid eye movement sleep latency (REML), increased REM as a percentage of total sleep time (REM%), reduced percent-age of slow wave sleep (Stpercent-ages 3 and 4) (SWS%), and increased time to sleep onset (also known as sleep laten-cy)(Benca et al 1992; Gillin et al 1993). During acute illness, some form of sleep abnormality is present in about 90% of depressed patients (Reynolds and Kupfer 1987). In addition, mild levels of obstructive sleep apnea (OSA) have been observed in 15% of inpatients with affective disorders (Reynolds et al 1982).
Table 1 summarizes research documenting alterations in sleep architecture in patients with varying levels of de-pressive symptoms. The mean REM% in these studies ranged from 17% to 27%, averaging 22%. REML ranged from 46 to 107 min, averaging 67 min. The mean sleep latency recorded in these studies ranged from 11 to 36 min, averaging 24 min. By comparison, normative data based on a sample of subjects free of sleep or alertness complaints and without major medical or psychiatric diagnoses (Doghramji et al 1997) reveals that REM% averaged 19.27%; REML averaged 106.6 min; SWS% averaged 16.49%; and sleep latency averaged 10.9 min. It is generally accepted that the degree of sleep disruption does not necessarily reflect the severity of the depression; even comparatively mild depressive symptomatology may produce alterations in polysomnographically recorded sleep (Buysse et al 1997; Hauri et al 1974).
An intriguing aspect that has been given little attention in the psychiatric literature is the possibility that coexist-ing sleep disorders may confound these relationships between depressive symptoms and sleep architecture. In this article, we examined how OSA affected the relation-ships between sleep architecture and depressive symp-toms. Affecting up to 9% of middle-aged adults and higher percentages in the elderly, OSA is a devastating illness (Ancoli-Israel et al 1991; Jennum and Sjol 1992; Jeong and Dimsdale 1989; Young et al 1993) that leaves patients exhausted from sleep deprivation.
The literature is mixed regarding the role played by psychological factors in OSA (for review, see Bardwell et
From the Department of Psychiatry, University of California San Diego (WAB, PM, SA-I, JED) and Veterans Affairs San Diego Healthcare System (WAB, SA-I), San Diego, California.
Address reprint requests to Wayne A. Bardwell, Ph.D., UCSD, Dept. of Psychiatry, 9500 Gilman Drive 0804, La Jolla CA 92093-0804.
Received January 27, 2000; revised March 15, 2000; accepted March 20, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
Reference Subjects Definition of depression Sleep latency REM latency REM% Findings Hubain et al 1998 300 (198 F, 102 M) Inpatients, drug free,
HRSD$20
NR F: 74 (60) M: 52 (37)
F: 17 (7) M: 18 (7)
Controlled for various confounding factors (such as age, weight loss, severity of depression, etc). Correlations were found with increased waking and decreased SWS, but not with REM latency. Thase et al 1998 78 (? f, ? M) Outpatients, drug free,
HRSD$14
Grp 1:26 (18) Grp 2:14 (9)
Grp 1:63 (17) Grp 2:75 (22)
Grp 1:23 (5) Grp 2:23 (5)
Two groups of depressed patients were classified on the basis of “abnormal” vs. “normal” pretreatment sleep study measures. Certain sleep abnormalities were stable across time (including REM-latency) and after treatment with cognitive behavior therapy; whereas other measures improved (such as sleep efficiency). Reynolds et al 1990 302 (151 F, 151 M) Outpatients, drug free,
HRSD$15
F: 23 (27) M: 21 (21)
F: 64 (30) M: 65 (33)
F: 23 (6) M: 22 (6)
Gender-related differences in sleep variables were found for slow-wave sleep measures but not for REM latency.
Reynolds et al 1984 25 M KDS$15 NR Grp 1:85 (46) Grp 2:55 (28)
NR Grp 15KDS$15; Grp 25KDS,15. 40% of male OSA patients met criteria for affective disorder or alcohol abuse. Kupfer et al 1981 34 (20 F, 14 M) Inpatients, drug free,
HRSD$30
36 (3)a 46 (4)a 25 (1)a Followed patients with various dosages of
tricyclic antidepressant, and found that treatment response was associated with changes in REM latency.
Coble et al 1979 12 (8 F, 4 M) Nondelusional inpatients, drug free, HRSD$
30
35 (8) 48 (7) NR Sleep parameters were examined over time in patients on placebo for 5 weeks. Sleep parameters remained fairly unchanged across time.
Gillin et al 1978 9 (5 F, 4 M) Inpatients, moderately to severely depressed
29 (16) 58 (27) 27 (2) Patients’ sleep was studied prior to treatment with a tricyclic antidepressant, during treatment and during withdrawal from treatment. Patients with clinical improvement showed a significant REM rebound effect during drug withdrawal, but the patients who failed to improve did not. Hauri et al 1974 14 patients
(10 F, 4 M) and 4 control subjects
Drug-free remitted patients
Patients: 24 (23) Control subjects: 6 (3)
Patients: 90 (25) Control subjects:
76 (20)
Patients: 21 (6) Control subjects:
21 (4)
Some sleep abnormalities found to persist in patients who had been remitted for longer than six months. NREM sleep
abnormalities (longer sleep latency, decreased SWS) seemed to persist longer than certain of the REM abnormalities.
Values given are means (SD), except wherea
indicates (SEM). Sleep latency values are in minutes. REM latency values are given in minutes, but may have been defined slightly differently in different studies. F, female; M, male; HRSD, Hamilton Rating Scale—Depression; NR, not reported; SWS, slow wave sleep; KDS, Kupfer–Detre Scale; OSA, obstructive sleep apnea; NREM, non-REM.
W.A.
Bardwell
et
al
BIOL
PSYCHIATRY
al 1999). Nonetheless, many researchers have found asso-ciations between OSA and overall psychological distress and, more specifically, clinical depression (Cheshire et al 1992; Guilleminault et al 1977; Millman et al 1989; Reynolds et al 1984) or increased levels of depressive symptoms (Borak et al 1994; Derderian et al 1988; Edinger et al 1994; Engleman et al 1994; Flemons and Tsai 1997; Kales et al 1985; Platon and Sierra 1992). For example, in a study of 25 male patients with sleep apnea, 40% met criteria for an affective disorder or alcohol abuse (Reynolds et al 1984).
Sleep architecture differences in OSA patients con-trasted with normal subjects (i.e., compared to Doghramji et al [1997] normative values) have included shorter sleep latency (Fry et al 1989; Hanzel et al 1991; Kass et al 1996; Series et al 1992; Smith et al 1985), increased REML (Mendelson 1992, 1995; Series et al 1992; Stewart et al 1992), decreased REM% (Colt et al 1991; Chervin and Aldrich 1998; Espinoza et al 1987; Hanzel et al 1991; Mendelson 1992, 1995; Oksenberg et al 1997; Roehrs et al 1989; Series et al 1992; Smith et al 1985; Stewart et al 1992), and decreased SWS% (Weitzman et al 1980; White 1992). With the exception of SWS%, these findings are opposite to those observed in depressed patients.
Table 2 summarizes research documenting sleep archi-tecture in OSA patients. The mean REML in these studies ranged from 63 to 175 min, averaging 121 min. OSA patients also tend to experience a lower REM% than normal subjects do. This may simply be a consequence of the impaired sleep continuity and fragmentation of sleep resulting from repetitive airway obstruction. The mean REM% in these studies ranged from 6% to 20%, averaging 14% of total sleep time. In addition, sleep latency in OSA patients tends to be shorter than in normal subjects— probably because of the accumulated sleep debt. The mean sleep latency recorded in these studies ranged from 4 to 24 min, averaging 10 min.
Because the presence of OSA may not be routinely determined in studies of sleep and mood, findings in this area of research may be confounded. Conversely, depres-sive symptoms may complicate studies of sleep architec-ture in OSA patients. To our knowledge, no previous studies have examined the potential interaction effect of OSA and depressive symptoms on sleep architecture. We wondered, for example, if the contrasting sleep effects seen in OSA and in depressive symptoms would offset each other or if some other pattern might emerge in patients exhibiting both high levels of depressive symp-toms and OSA.
Most previous research into affective disorders and sleep architecture has focused on patients with diagnosed mood disorders. Because of the growing interest in the impact of subclinical levels of depressive symptoms, we
wondered if these previously reported findings would hold when dividing patients into high and low depressive symptom groups, regardless of diagnosis. In this study we examined the relationships between sleep latency, REML, and REM% versus OSA diagnosis and level of depressive symptoms. We wondered if a diagnosis of OSA would impact these sleep architecture variables in subjects with high levels of depressive symptoms, and if depressive symptoms would impact them in subjects with OSA.
Methods and Materials
Subjects (n5106) included 57 men and 10 women with OSA and 27 men and 12 women without OSA. None of the subjects had any other sleep disorder. Subjects ranged from 32 to 64 years of age (Table 3) and were recruited by advertising and word of mouth to participate in our research on sympathetic nervous system physiology in subjects with and without OSA. To qualify, subjects had to be between 100% and 150% of ideal body weight as determined by Metropolitan Life Insurance tables (Metropol-itan Life Foundation 1983). Although OSA is more common among the obese, subjects with greater than 150% of ideal body weight were excluded because of the possibility of confounding by other conditions associated with obesity. Subjects were also excluded if they had major medical illnesses other than OSA. Subjects were studied after we had obtained written informed consent. The protocol was approved by the University of California, San Diego, Human Subjects Committee.
Polysomnographic sleep was monitored all night in the Clin-ical Research Center, and included standard central and occipital electroencephalogram (EEG); bilateral electrooculogram (EOG); submental electromyogram (EMG); airflow, thoracic, and ab-dominal excursions with Respitrace respiratory inductive pleth-ysmography (Non-Invasive Monitoring Systems, Miami Beach, FL); and bilateral tibialis EMG. Tracings were scored according to the criteria of Rechtshaffen and Kales (1968) and the number of apnea/hypopnea events was recorded. The majority of subjects had solely obstructive type events; only a few subjects showed evidence of central apneas.
Subjects were classified as having sleep apnea if their Respi-ratory Disturbance Index (RDI) was 15 or more: in OSA subjects (n567), RDI ranged from 15 to 142 (mean550.7, SD528.7); for non-OSA subjects (n 5 39), RDI ranged from 1 to 14 (mean55.6, SD53.7). Sleep latency was defined as the time from lights out to the first epoch of stage 2 sleep. REM latency was defined as time from the first epoch of stage 2 sleep to the first epoch of REM sleep. REM% and SWS% were calculated by dividing min of REM by total sleep time, and min of stage 3 plus stage 4 sleep by total sleep time, respectively.
Subjects completed the Center for Epidemiological Studies— Depression (CES-D) scale (Radloff 1997), a frequently used 20-item self-report measure of depressive symptoms. When used with medically ill patients, depression rating scales are some-times influenced by symptoms of their illness. For example, mood-related observations in OSA patients could be confounded by the fatigue/sleepiness they experience; however, the CES-D primarily taps the cognitive/affective symptoms of depression Depression, Sleep Architecture, OSA BIOL PSYCHIATRY 1003
Reference Subjects criterion nighttime REM latency REM% Findings
Espinoza et al 1987 10 M NR 11.2 (2.3) NR 9.6 (2.1) Compared a 1-night infusion of aminophylline to a placebo infusion night. Aminophylline caused a decrease in central events, but no change in obstructive events and also disturbed sleep efficiency.
Hanzel et al 1991 7 M, 5 F NR 4.1 (1.1) NR 17 (2) Compared the effect of fluoxetine to protryptiline. Both drugs decreased REM time and number of REM-related respiratory events.
Fry et al 1989 30 M, 3 F RDI$10 7.5 (6.2) 62.8 (20.2) 19.8 (6.0) Periodic limb movements in OSA were measured at baseline, and with two CPAP recordings. Smith et al 1985 13 M, 2 F RDI$10 6.9 (1.5) NR Approx. 10a The therapeutic effects of weight loss on OSA
were examined.
Mendelson 1992 169 M, 23 F RDI$5 11.7 (1.5) 134 (6.5) 13.8b(unknown) OSA patients were compared to patients with
central sleep apnea and pathologic snorers (only the OSA data are reported in this table). OSA patients were subjectively sleepier. Hypertension was strongly associated with weight and also with RDI.
Mendelson 1995 430 M, 88 F RDI$5 13.8 (4.6) 131.0 (84.1) 14.4b(unknown) Compared OSA patients to patients with
subclinical sleep-disordered breathing. Oksenberg et al 1997 574 patients in 2 groups
(G1, G2)
RDI$10 G1: 12.9 (4.2) G2: 11.7 (3.7)
G1: 89.6 (46.6) G2: 104 (63.7)
G1: 19 (7.4) G2: 17 (8.5)
OSA patients divided into two groups based on whether or not the RDI was worsened32 when positioned supine.
Series et al 1992 8 M NR 8.7 (3.0) 149.6 (24.9) 13.4 (1.7) Administered a short-acting SWS-enhancing drug,
g-hydroxy-butyrate, to see if overall RDI could be improved.
Stewart et al 1992 8 M NR 24 (3.3) 175 (32) 13.4b(unknown) Apnea patients were treated for one week with a
nonsteroidal antiandrogen.
Kass et al 1996 34 (15 M, 19 F) RDI,10 8.3 (3.4) NR ns REM RDI correlated with sleepiness measures in patients with mild OSA but worsening during REM.
Colt et al 1991 7 M NR NR NR 6 (5) No statistically significant differences in MSLT scores after CPAP treatment under hypoxemic or nonhypoxemic conditions.
Roehrs et al 1989 415 M, 51 F NR NR NR 12.1 (6.4) Found that the best predictor of MSLT score was the degree of sleep fragmentation during the previous night (i.e., the respiratory arousal index).
Chervin and Aldrich 1998
814 M, 332 F RDI$10 NR NR 14.2 (6.2) Concluded that apneic events during both REM and non-REM sleep contribute to sleepiness.
Values given are means (SEM).
Sleep latency values are in minutes. REM latency values are given in minutes, but may have been defined slightly differently in different studies.
OSA, obstructive sleep apnea; M, male; NR, not reported; F, female; RDI, Respiratory Disturbance Index; CPAP, continuous positive airway pressure; SWS, slow wave sleep; MSLT, multiple sleep latency test. aValue not specified but estimated from figure.
bValue was calculated from given information of total sleep time, or TST (min), and REM sleep (min): (REM min/TST min)3100 to give % REM.
W.A.
Bardwell
et
al
BIOL
PSYCHIATRY
and has been shown to be useful in chronically ill groups experiencing fatigue (e.g., human immunodeficiency virus [HIV], cancer) (Cockram et al 1999; Hann et al 1999). In a variety of populations, a large percentage of subjects with a score of$16 have been shown to meet diagnostic criteria for dysthy-mia or major depression (Murphy 1982; Schulberg et al 1985). Using this commonly accepted cut-off score, our subjects were divided into Hi/Lo depressive symptom groups. For the Lo CES-D group, scores ranged from 0 to 15 (mean56.2, SD5 4.2); for the Hi CES-D group, scores ranged from 16 to 49 (mean525.0, SD57.2).
Data were analyzed using SPSS 9.0 software (SPSS, Chicago) multiple analysis of covariance (MANCOVA) routines (using age as a covariate): REML, REM%, SWS%, and sleep latency as the dependent variables, and OSA/non-OSA and Hi/Lo CES-D as the independent variables. Stepwise regression analysis was also employed, using the independent variables in their contin-uous form. An interaction term was created by first standardizing and then multiplying RDI and CES-D scores. In step one, age was controlled via forced entry; in step two, RDI and CES-D were entered; in step three, the interaction term was entered.
Results
Table 3 shows values for demographic, sleep, and mood variables by Apnea/CES-D group. OSA subjects were slightly older (49.3 vs. 45.1 years,p5 .007), so age was used as a covariate in subsequent analyses. As expected, OSA subjects were heavier (29.7 vs. 27.4 body mass index,p5 .004), and had significantly greater RDI (50.7 vs. 5.6,p, .001) than non-OSA subjects. Also, subjects in the Hi CES-D group reported significantly more depres-sive symptoms than Lo CES-D subjects (25.0 vs. 6.2,p,
.001). OSA and non-OSA subjects did not differ signifi-cantly on the CES-D (16.3 vs. 14.8,p5.190); and Hi and Lo CES-D subjects did not differ significantly in terms of RDI (25.8 vs. 29.0,p5 .509).
In the MANCOVA, REML, REM%, SWS%, and sleep
latency were the dependent variables, and OSA (yes/no) and CES-D (Hi/Lo) were the independent variables. No significant main effects emerged for OSA (yes/no) for REML (109.6 vs. 109.8 min,p5 .991), REM% (17.1% vs. 16.6%, p 5 .784), or SWS% (7.0% vs. 9.6%, p 5
.148). OSA subjects showed significantly shorter sleep latency than non-OSA subjects, however (14.5 vs. 26.8 min, p 5 .001). Similarly, no significant main effects emerged for CES-D Hi/Lo for REML (119.8 vs. 99.6 min, p5.203), REM% (17.5% vs. 16.3%,p5.495), or SWS% (9.6% vs. 7.0%,p5 .133); however, Hi CES-D subjects showed a trend toward longer sleep latency than Lo CES-D subjects, (23.7 vs. 17.5 min,p5 .079).
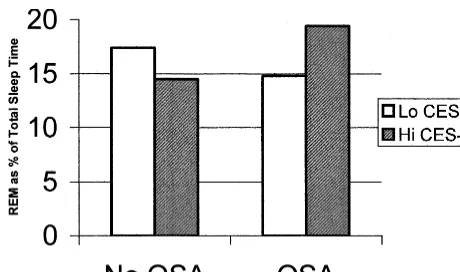
The OSA3CES-D interaction was not significant for REML (p 5 .066) or SWS% (p 5 .141); however, significant OSA3 CES-D interactions emerged for both sleep latency (p5.002; Figure 1) and REM% (p5.040; Figure 2). Post hoc analyses revealed that the sleep latency in non–OSA/Hi CES-D subjects was twice as long as non–OSA/Lo CES-D subjects (35.3 vs. 18.3 min, p 5
.012) and OSA/Lo CES-D subjects (35.3 vs. 16.8 min,p5
Figure 1. Sleep latency vs. obstructive sleep apnea (OSA)3
Center for Epidemiological Studies—Depression (CES-D) inter-action (p5.002).
Table 3. Demographic Variables (Mean6SD)
No apnea Lo CES-D (n 5 23)
No apnea Hi CES-D (n 5 16)
Apnea Lo CES-D (n5 45)
Apnea Hi CES-D (n 5 22)
Agea,e 4666.4 4365.6 5067.7 4868.0
Body mass indexa,e 27.063.6 28.163.5 28.963.7 31.165.4
REM latency 80.7615.7 120.0619.5 120.7611.2 106.0615.7
REM as % of total sleep timeb,d 1867.6 1668.2 1567.7 1969.4
SWS as % of total sleep time 10.069.4 9.568.0 4.666.0 9.669.5
Sleep latency (min)a,b,e 18.3614.0 35.3631.7 16.8612.3 12.1613.1
Respiratory Disturbance Indexa,e 5.763.6 5.563.9 52.0629.6 48.0627.8
CES-D Scorec,e 7.064.1 23.366.2 6.064.2 26.368.2
n5106. CES-D, Center for Epidemiological Studies—Depression; REM, rapid eye movement sleep; SWS, slow wave sleep (stages 314).
aSignificant main effect for apnea diagnosis.
bSignificant apnea diagnosis3CES-D Hi/Lo interaction. cSignificant main effect for CES-D.
d
p,.05.
e
p,.01.
Depression, Sleep Architecture, OSA BIOL PSYCHIATRY 1005
.010), and nearly three times as long as OSA/Hi CES-D subjects (35.3 vs. 12.1 min, p 5 .002). Sleep latency differences between OSA/Lo CES-D and OSA/Hi CES-D and between OSA/Lo CES-D and non–OSA/Lo CES-D subjects were not significant.
Regarding the significant interaction for REM%, post-hoc analyses revealed that OSA/Hi CES-D subjects expe-rienced higher REM% than OSA/Lo CES-D subjects (19.4% vs. 14.8%,p5.021); however, non-OSA subjects did not differ in terms of REM% regardless of CES-D level; Hi CES-D subjects did not differ in terms of REM% regardless of apnea diagnosis; and Lo CES-D subjects did not differ in terms of REM% regardless of apnea diagnosis.
Data were also examined in stepwise regression analy-ses using continuous RDI and CES-D scores and the RDI3 CES-D interaction. Age was controlled by forced entry. Using sleep latency as the dependent variable, only the interaction term entered the model (b5 24.462,b 5 2.260,p5.008). Using SWS% as the dependent variable, RDI (b5 2.001,b 5 2.352, p,.001) and CES-D (b5
.002, b 5 .265, p 5 .004) entered the model. Using REM% or REML as the dependent variables, none of the independent variables entered the model.
Discussion
It is well documented that mood disorders can have a detrimental effect on quality and quantity of sleep. Several researchers have observed sleep architecture differences between patients with varying levels of depressive symp-toms. These differences have generally, but not always, included a cluster of abnormalities, such as shorter REML, increased REM%, diminished SWS%, and prolonged sleep latency; however, no single aspect of sleep architec-ture can be considered a definitive biological marker for depressive disorders.
OSA is a relatively common sleep disorder and patients with OSA often experience higher levels of depressive symptoms than people without a sleep disorder. Beyond such accepted markers as RDI, arousals, and oxygen desaturation, researchers have also documented longer REML, decreased REM%, diminished SWS%, and shorter sleep latency in OSA patients. As in the depression research, findings have not been consistent across studies for these variables.
Because depression and OSA have often been shown to have opposite effects on REML, REM%, and sleep latency, we were interested in examining how the depressive symp-toms 3 OSA interaction would impact sleep architecture. Also, because of the burgeoning interest in the impact of subclinical levels of depressive symptoms on health and behavior, we wondered if these sleep architecture differences would hold when comparing subjects reporting a range of depressive symptoms—regardless of the presence of a diag-nosed mood disorder. We hypothesized that OSA may confound sleep architecture observations when comparing groups of subjects with comparatively high and low levels of depressive symptoms. Conversely, we also hypothesized that high levels of depressive symptoms may confound sleep architecture differences found when comparing subjects with and without OSA.
In our sample of 106 subjects, we were able to replicate some of the findings from the sleep-in-depression litera-ture as well as the sleep-in-OSA literalitera-ture. Our OSA subjects experienced significantly shorter sleep latency— about half as long as that in subjects without OSA. There was also a trend for subjects who reported high levels of depressive symptoms to experience sleep latency one third longer than subjects reporting low levels of depressive symptoms. We did not observe significant differences between OSA and non-OSA subjects nor between Hi CES-D and Lo CES-D subjects for REML, REM%, or SWS%; however, the interaction of depressive symptoms and OSA diagnosis produced results that were statistically and clinically significant.
We had speculated that the REML-reducing effects of depression and the REML-increasing effects of OSA might offset each other in OSA patients who had depres-sive symptoms. Reynolds et al (1982, 1984) have reported REML findings that differ from our results. In that study, the REML in patients with OSA and depression was significantly longer than in patients having only OSA. They postulated that the association between the REM sleep abnormalities of depression and OSA might be due to the fact that both disorders increase with age. Our larger sample size allowed us to test the depression 3 OSA interaction and to control for the effects of age. These factors, along with the use of different scales for rating depression, may help explain our divergent findings.
Depressive symptoms and OSA diagnosis interacted to produce SL and REM% patterns that were not consistent with previously reported findings. Non-OSA subjects who endorsed high levels of depressive symptoms experienced the longest sleep latency—two to three times as long as all other subjects. Apparently, in non-OSA subjects, the presence of high levels of depressive symptoms signifi-cantly prolongs sleep latency. In OSA subjects, however, sleep latency seems to be resistant to the effects of depressive symptoms—remaining comparatively low re-gardless of the level of depressive symptoms reported. In OSA subjects reporting high levels of depressive symp-toms, the fatigue associated with OSA may override the longer sleep latency that often accompanies high levels of depressive symptoms.
Regarding REM%, OSA subjects reporting high levels of depressive symptoms experienced REM% nearly one third higher than those OSA subjects with low levels of depressive symptoms. Thus, the presence of compara-tively high levels of depressive symptoms may override the diminution of REM sleep often associated with OSA. In subjects without OSA, however, REM% did not differ in terms of level of depressive symptoms.
We did not find significant main effects or interaction effects with REML as the dependent variable. Some readers may ask about first-night effects, particularly in terms of REM sleep variables. Although this may have had an impact on our data, it is typical of the studies reported in our review to rely on single-night recordings.
We wondered if subjects in our sample might have experienced only minor depression rather than more seri-ous levels of dysphoric mood and, if so, if this might help explain our results. To test this hypothesis, we increased the CES-D cutoff to 20; however, this change in cutoff did not significantly affect the findings reported here.
Our findings suggest that the presence of comparatively high levels of depressive symptoms—not simply the presence or absence of a diagnosed clinical mood disor-der—must be considered in studies of sleep architecture in OSA patients. Conversely, the presence of OSA must be considered in studies of sleep architecture in subjects with mood disorders or with high levels of depressive symp-toms. The interaction effects we observed may account for some of the differences in findings reported separately in previous studies of sleep architecture in depressed patients and OSA patients.
Limitations
There are certain limitations of this study that could impact the generalizability of our findings. Regarding potential bias in recruitment, we sought both subjects with and without symptoms suggestive of sleep apnea. It is
possible that persons with particularly prominent apnea symptoms were more likely to seek inclusion in the study, thus skewing our sample toward those with more severe illness; however, the RDI in our participants ranged from 0 to 152, averaging 50 in the apneic group.
Some of our findings varied depending on the statistical methodology employed. Using sleep latency as the depen-dent variable, our findings regarding the RDI3 CES-D interaction were consistent when using either dichotomous or continuous forms of this independent variable. Our findings regarding REM% could not be duplicated, how-ever, when using continuous independent variables. Rep-lication in another data set is required.
It is possible that the fatigue/sleepiness experienced by OSA patients confounded our evaluation of mood symp-toms. As mentioned previously, the CES-D tends to assess cognitive/affective domains of depression and has been used successfully in other chronically ill groups experi-encing fatigue. Nevertheless, we examined the relation-ship between CES-D and Epworth Sleepiness Scale (Johns 1991) scores in our OSA subjects. Results were not significant, suggesting that CES-D scores are not con-founded by sleepiness/fatigue.
This work was supported by National Institutes of Health Grants Nos. HL44915, AG02711, and RR00827.
References
Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O (1991): Sleep disordered breathing in community-dwelling elderly.Sleep14:486 – 495.
Bardwell WA, Berry CC, Ancoli-Israel S, Dimsdale JE (1999): Psychological correlates of sleep apnea. J Psychosom Res
47:583–596.
Benca RM, Obermeyer WH, Thisted RA, Gillin JC (1992): Sleep and psychiatric disorders: A meta-analysis.Arch Gen Psychi-atry49:651– 668.
Borak J, Cieslicki J, Szelenberger W, Wilczak-Szadkowska H, Koziej M, Zielinski J (1994): Psychopathological character-istics of the consequences of obstructive sleep apnea prior to and three months after CPAP.Psychiatr Pol28:33– 44. Buysse DJ, Frank E, Lowe KK, Cherry CR, Kupfer DJ (1997):
Electroencephalographic sleep correlates of episode and vul-nerability to recurrence in depression. Biol Psychiatry 41: 406 – 418.
Chervin RD, Aldrich MS (1998): The relation between multiple sleep latency test findings and the frequency of apneic events in REM and non-REM sleep.Chest113:980 –984.
Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ (1992): Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome.Arch Intern Med152: 538 –541.
Coble PA, Kupfer DJ, Spiker DG, Neil JF, McPartland RJ Depression, Sleep Architecture, OSA BIOL PSYCHIATRY 1007
(1979): EEG sleep in primary depression: A longitudinal placebo study.J Affect Disord1:131–138.
Cockram A, Judd FK, Mijch A, Norman T (1999): The evalua-tion of depression in inpatients with HIV disease.Aust N Z
J Psychiatry33:344 –352.
Colt HG, Haas H, Rich GB (1991) Hypoxemia vs. sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea.Chest100:1542–1548.
Derderian SS, Bridenbaugh RH, Rajagopal KR (1998): Neuro-psychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pres-sure.Chest94:1023–1027.
Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, et al (1997): A normative study of the mainte-nance of wakefulness test (MWT).Electroencephalogr Clin
Neurophysiol103:554 –562.
Edinger J, Carwile S, Miller P, Hope V, Mayti C (1994): Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea.Percept Mot Skills
78:1116 –1118.
Engleman HM, Martin SE, Deary IJ, Douglas NJ (1994): Effect of continuous positive airway pressure treatment on daytime functioning in sleep apnea/hypopnea syndrome.Lancet343: 572–575.
Espinoza H, Antic R, Thornton AT, McEvoy RD (1987): The effects of aminophylline on sleep and sleep-disordered breathing in patients with obstructive sleep apnea syndrome.
Am Rev Respir Dis136:80 – 84.
Flemons WW, Tsai W (1997): Quality of life consequences of sleep-disordered breathing.J Allergy Clin Immunol99:S750 – S756.
Fry JM, DiPhillipo MA, Pressman MR (1989): Periodic leg movements in sleep following treatment of obstructive sleep apnea with nasal continuous positive airway pressure.Chest
96:89 –91.
Gillin JC, Dow BM, Thompson P, Parry B, Tandon R, Benca R (1993): Sleep in depression and other psychiatric disorders.
Clin Neurosci1:90 –96.
Gillin JC, Wyatt RJ, Fram D, Snyder F (1978): The relationship between changes in REM sleep and clinical improvement in depressed patients treated with amitryptiline.
Psychopharma-cology59:267–272.
Guilleminault C, Eldridge FL, Tilkian A, Simmons FB, Dement WC (1977): Sleep apnea syndrome due to upper airway obstruc-tion: A review of 25 cases.Arch Intern Med137:296 –300. Hann D, Winter K, Jacobsen P (1999): Measurement of
depres-sive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D).
J Psychosom Res46:437– 443.
Hanzel DA, Proia NG, Hudgel DW (1991): Response of obstruc-tive sleep apnea to fluoxetine and protryptiline.Chest 100: 416 – 421.
Hauri P, Chernik D, Hawkins D, Mendels J (1974): Sleep of depressed patients in remission. Arch Gen Psychiatry 31: 386 –391.
Hubain PP, Staner L, Dramaix M, Kerkhofs M, Papadimitriou G, Mendlewicz J, et al (1998): The dexamethasone suppression test and sleep electroencephalogram in nonbipolar major depressed inpatients: A multivariate analysis.Biol Psychiatry
43:220 –229.
Jennum P, Sjol A (1992): Epidemiology of snoring and obstruc-tive sleep apnea in a Danish population, age 30-60.J Sleep Res1:240 –244.
Jeong DU, Dimsdale JE (1989): Sleep apnea and essential hypertension: A critical review of the epidemiological evi-dence for co-morbidity.Clin Exp Hypertens11:1301–1323. Johns MW (1991): A new method for measuring daytime
sleepiness: The Epworth sleepiness scale.Sleep14:540 –545. Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD (1985): Severe obstructive sleep apnea—II: Asso-ciated psychopathology and psychosocial consequences.
J Chronic Dis38:427– 434.
Kass JE, Akers SM, Bartter TC, Pratter MR (1996): Rapid-eye-movement-specific sleep-disordered breathing: A possible cause of excessive daytime sleepiness.Am J Respir Crit Care Med154:167–169.
Kupfer DJ, Spiker DG, Coble PA, Neil JF, Ulrich R, Shaw DH (1981): Sleep and treatment prediction in endogenous depres-sion.Am J Psychiatry138:429 – 434.
Mendelson WB (1992): Sleepiness and hypertension in obstruc-tive sleep apnea.Chest101:903–909.
Mendelson WB (1995): The relationship of sleepiness and blood pressure to respiratory variables in obstructive sleep apnea.
Chest108:966 –972.
Metropolitan Life Foundation (1983): Metropolitan height and weight tables.Stat Bull (Metropolitan Life Insurance)64:1. Millman RP, Fogel BS, McNamara ME, Carlisle CC (1989):
Depression as a manifestation of obstructive sleep apnea: Reversal with nasal continuous positive airway pressure.
J Clin Psychiatry50:348 –351.
Murphy J (1982):Psychiatric Instrument Development for Pri-mary Care Research: Patient Self-Report Questionnaire,
Report on Contract 80M01428101D.Bethesda, MD: National
Institute of Mental Health.
Oksenberg A, Silverberg DS, Arons E, Radwan H (1997): Positional vs. nonpositional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic and multiple sleep latency test data.Chest112:629 – 639.
Platon MJ, Sierra JE (1992): Changes in psychopathological symptoms in sleep apnea patients after treatment with nasal continuous positive airway pressure.Int J Neurosci62:173– 195.
Radloff LS (1997): The CES-D Scale: A self-report depression scale for research in the general population.Appl Psychol Meas1:385– 401.
Rechtshaffen A, Kales A (1968): A Manual of Standardized Terminology: Techniques and Scoring System for Sleep
Stages of Human Subjects.Los Angeles: UCLA Brain
Infor-mation Service/Brain Research Institute.
Reynolds CF, Coble PA, Spiker DG, Neil JF, Holzer BC, Kupfer DJ (1982): Prevalence of sleep apnea and nocturnal myoclo-nus in major affective disorders: Clinical and polysomno-graphic findings.J Nerv Ment Dis170:565–567.
Reynolds CF, Kupfer D, McEachran A, Taska L, Sewitch D, Coble P (1984): Depressive psychopathology in male sleep apneics.J Clin Psychiatry45:287–289.
PA, et al (1990): Sleep, gender, and depression: An analysis of gender effects on the electroencephalographic sleep of 302 depressed outpatients.Biol Psychiatry28:673– 684. Roehrs T, Zorick F, Wittig R, Conway W, Roth T (1989):
Predictors of objective level of daytime sleepiness with sleep-related breathing disorders.Chest95:1202–1206. Schulberg HC, Saul M, McClelland M, Ganguli M, Christy W,
Frank R (1985): Assessing depression in primary medical and psychiatric practices.Arch Gen Psychiatry42:1164 –1170. Series F, Series I, Cormier Y (1992): Effects of enhancing
slow-wave sleep by gamma-hydroxybutyrate on obstructive sleep apnea.Am Rev Respir Dis145:1378 –1383.
Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER (1985): Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med 103:850 – 855.
Stewart DA, Grunstein RR, Berthon-Jones M, Handelsman
DJ, Sullivan CE (1992): Androgen blockade does not affect sleep-disordered breathing or chemosensitivity in men with obstructive sleep apnea. Am Rev Respir Dis
146:1389 –1393.
Thase ME, Fasiczka AL, Berman SR, Simons AD, Reynolds CF (1998): Electroencephalographic sleep profiles before and after cognitive behavior therapy of depression. Arch Gen
Psychiatry55:138 –144.
Weitzman E, Kahn E, Pollack CP (1980): Quantitative analysis of sleep and sleep apnea before and after tracheostomy in patients with hypersomnia-sleep apnea syndrome. Sleep
3:407– 423.
White DP (1992): Obstructive sleep apnea.Hosp Pract (Off Ed)
27(5A):57– 84.
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993): The occurrence of sleep-disordered breathing among middle-aged adults.N Engl J Med328:1230 –1235. Depression, Sleep Architecture, OSA BIOL PSYCHIATRY 1009