The role of low molecular weight organic acids from decomposing
rye in inhibiting root-knot nematode populations in soil
Robert G. McBride
a,∗, Robert L. Mikkelsen
a, Kenneth R. Barker
b aDepartment of Soil Science, North Carolina State University, Raleigh, NC 27695-7619, USA bDepartment of Plant Pathology, North Carolina State University, Raleigh, NC 27695-7616, USAReceived 26 August 1999; accepted 17 January 2000
Abstract
Organic soil amendments have been employed as an alternative to or in combination with, chemical nematicides and cultural practices to control plant-parasitic nematodes. Rye (Secale cereale L.) has been shown to be effective in minimizing the damage caused by root-knot nematodes (Meloidogyne incognita (Kofoid and White) Chitwood) when grown as a cover crop and then incorporated into the soil prior to planting. It has been suggested that the release of low molecular weight organic acids during the decomposition of rye is the cause of the nematicidal effects. This study was conducted to quantify the concentration and persistence of formic, acetic, propionic, butyric, and valeric acids in soil solution following the incorporation of fresh rye foliage. Formic and acetic acids were detected by means of ion exclusion chromatography, primarily in the first 24 h following addition of rye, and at concentrations<450mmol/l. The effect of the rye treatment on the root-knot nematode population was determined by growing tomato plants (Lycopersicon esculentum Mill.) in the rye-amended soil and assessing the nematode damage to the root systems. Despite the low concentrations of organic acids detected, the rye treatment resulted in a significant suppression of root-knot nematode activity. To determine the fate of these acids in soil, an addition of each acid was made to a field soil resulting in a soil water concentration of 1500mmol/l for each acid. Soil solution samples were collected every 2 h for 10 h and analyzed for the five added organic acids by means of ion exclusion chromatography. The concentration of all acids declined by 54–97% over the 10 h incubation. Although low molecular weight organic acids may be one of many factors that contribute to restriction in root-knot nematode damage, these acids do not appear to be solely responsible for the nematicidal effect of the rye. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Low molecular weight organic acids; Meloidogyne incognita (root-knot nematode); Secale cereale (rye); Soil amendment
1. Introduction
Plant-parasitic nematodes are invertebrate worm-like animals that require a susceptible host plant on which to feed in order to complete their life cycles. More than 1200 species of nematodes have been found to attack plants with virtually every crop being susceptible to a
∗Corresponding author. Tel.:+1-919-515-2655; fax:+1-919-515-2167.
E-mail address: [email protected] (R.G. McBride).
particular assortment. Nematode control includes the use of resistant plant cultivars, application of chemi-cal nematicides, and cultural practices, such as tillage and crop rotation. Alternative methods of control include the addition of organic materials to the soil and/or microbial antagonists (Jairajpuri et al., 1990).
It has long been observed that the addition of or-ganic materials to the soil can benefit plant growth. Organic amendments can also limit the severity of plant-parasitic nematode damage (Akhtar and Alam, 1992, 1993; Akhtar and Mahmood, 1994). Nematode
control resulting from the use of organic amendments has been attributed to several factors, including toxic effects of nitrogen, predatory fungi, nematodes, in-sects, and mites, as well as organic acids and their interactions. The contribution of organic acids from decomposing organic matter has been assumed to be the causative factor for many years (Stephenson, 1945; Johnston, 1959; Sayre et al., 1964; Badra et al., 1979). However, no research has been done to measure organic acids produced in situ and their subsequent influence on nematode infection.
Low molecular weight organic acids have been re-ported to be present in anaerobic soils following the addition of plant material (Gotoh and Onikura, 1971; Chandrasekaran and Yoshida, 1973). In well-aerated soils, these acids generally exist in low concentrations and only for relatively short periods of time, being rapidly broken down and utilized by bacteria and fungi (Schwartz et al., 1954; Hollis and Rodriguez-Kabana, 1966; Lynch, 1991). These soluble organic products are generated as metabolic by-products from soil or-ganisms, such as bacteria and fungi, and are also ex-creted from plant roots (McLaren and Peterson, 1967). The limited quantities and transient nature of low molecular weight organic acids in aerobic soils ap-pear contradictory to the nematicidal merit these acids have received. Our objective was to quantify the per-sistence and quantity of the five most cited low molec-ular weight organic acids in the soil solution after the addition of fresh rye. We also sought to determine any associated change in the root-knot nematode pop-ulation, a worldwide pest responsible for damage to nearly every food crop.
In the first experiment, the effects of three applica-tion rates of rye on the root-knot nematode infecapplica-tion and the concentration of five low molecular weight or-ganic acids in the soil solution were evaluated. A sec-ond experiment quantified the rate at which five low molecular weight organic acids were removed from the soil solution over a 10 h period.
2. Materials and methods
2.1. Experiment 1: rye addition
2.1.1. Soil
A sandy loam surface horizon was collected near Clayton, NC (Goldsboro, fine-loamy, siliceous,
thermic Aquic Paleudult). This coarse-textured, well-drained soil was selected for this experiment due to the nematode’s affinity for well-aerated soils (Van Gundy, 1985) and the necessity of collecting a leachate sample. After collection, the soil was stored moist at 25◦C for several weeks to maintain
micro-bial populations. The soil was analyzed for nematode population by the semi-automatic elutriator method (Barker, 1985a) and found to contain 590 root-knot Meloidogyne spp., 550 stunt Tylenchorhynchus spp., 10 ring Criconemella spp., and 10 spiral Helicoty-lenchus spp. nematodes/l.
2.1.2. Rye
Rye (Secale cereale L. var. Abruzzi) was grown in 15 cm diameter clay pots containing a 50/50 mixture of sterilized loamy sand soil and coarse sand. The rye was fertilized weekly with N, P, and K in the irrigation water. The rye shoots were harvested approximately 10 weeks after planting and were cut into 2.5 cm segments before being used in the experiment.
2.2. Meloidogyne egg inoculum
A mature tomato plant (Lycopersicon esculentum) infected with a North Carolina population of root-knot nematodes (Meloidogyne incognita race 3) was uti-lized as a nematode source. To increase this nema-tode, 3 cm long pieces of tomato root with observable egg masses were removed. Each piece of infected root was buried in the center of a 15 cm diameter clay pot containing a 50/50 mixture of sterilized loamy sand soil and coarse sand. Tomato seedlings (Rutgers vari-ety), were transplanted into the inoculated soil to serve as host plants. Tomatoes are generally quite suscepti-ble to root-knot nematode infestation and accordingly make an excellent host (Sasser, 1990). The plants were fertilized weekly with N, P, and K in the irrigation water. It takes approximately 30 days for the eggs to hatch and complete their life cycle (Heald and Orr, 1984). After three to four life cycles, the developing root-knot nematode eggs were extracted by means of the NaOCl-extraction method (Barker, 1985b).
2.3. Experimental set up
per pot. The drain hole in the pot was covered with pieces of woven fiberglass cloth to retain the soil in the pot, yet allow drainage.
2.4. Organic acid analysis
Three application rates (0, 23, and 34 g dry weight of rye/pot) were established by thoroughly mix-ing fresh rye shoots into the soil in the pot. These very high rye application rates (equivalent to 16 and 23 t/acre, respectively) were used to ensure that the de-sired processes would take place and that organic acid concentrations would be well above minimum analyt-ical detection limits. Root-knot nematode eggs were added to each pot at a rate of 20,000 eggs/pot. Eggs gathered by the NaOCl-extraction method (Barker, 1985b) were suspended in water. The eggs were added to the pots by removing the top 2 cm of soil, pouring the eggs suspended in 30 ml of water over the surface of the exposed soil, and then replacing the soil.
The first phase of the experiment was conducted in a temperature-controlled incubator where the temper-ature was maintained at 28◦C. Soil moisture content
was brought up to approximately 80% of saturation each day with distilled water. A leachate sample was obtained by slowly adding distilled water to the sur-face of the soil. When water was first observed drip-ping from the bottom of the pot, an additional 200 ml was added and allowed to drain into a glass jar. The pH of each sample was measured and subsequently adjusted to pH 5 with NaOH or HCl and stored at 1◦C in glass containers with Teflon-lined lids. When
drainage ceased, the pots were immediately returned to the incubator to maintain the constant environmen-tal conditions.
2.5. Chemical analysis
Prior to analysis of the leachate, the samples were allowed to warm to room temperature, the pH mea-sured, and He was bubbled through each sample for approximately 20 min to eliminate carbonate in solu-tion. When left untreated, the carbonate in the samples resulted in a negative peak during analysis, which obscured the positive propionic and butyric acid peaks.
The samples were analyzed for formic, acetic, pro-pionic, butyric, and valeric acids by means of ion ex-clusion chromatography (Rocklin et al., 1986). Indi-vidual organic acids were separated and quantified by ion chromatography exclusion (ICE) using a Dionex model DX100 ion chromatograph equipped with a Dionex lonpac ICE-AS1 column, Dionex AMMS-ICE suppressor, and conductivity detector. The eluent was 1.0 mM heptafluorobutyric acid in HPLC-grade water at a flow rate of 2 ml/min at a column pressure of ap-proximately 4800 kPa (700 psi). The column was re-generated with 2.75% tetrabutylammonium hydroxide (TBAH) at a flow rate of 1 ml/min. Quantification of the acids was by comparison of the sample peak area with the peak area of a standard amount of the respec-tive acids. The leachate concentrations were converted to the concentration of organic acids in soil solution (at 80% of water-holding capacity) by taking into ac-count the dilution occurring during the leaching pro-cess and the amount of solution recovered from each pot.
The experiment was conducted using a randomized complete block design with blocks corresponding to the incubator’s six shelves (top two, middle two, and bottom two shelves being utilized as threee separate blocks/reps). There were a total of 15 experimen-tal units (pots) per block. The treatments (rates of rye) and sample times (0, 12, 36, 84, and 180 h af-ter rye application) were randomly assigned to the pots within each block. Each pot was leached only once. The overall statistical model corresponds to a randomized complete block design with rates of rye and sample dates as treatments, following the form Yij k=µ+Ri+Tj+Sk+(TS)j k+eij k, where Ri
repre-sents reps, Tj represents rye rates, and Sk represents
sampling dates. A statistical analysis was not con-ducted on the organic acid data because insufficient data above the detection limit precluded a meaning-ful analysis. Differences between treatments at each sample date for the pH data were determined by cal-culating the least significant difference (LSD) at the 0.05 probability level.
2.6. Nematode population assay
the blocking and randomization maintained. At this time, one 10 cm tall tomato seedling (Rutgers variety) was transplanted into the center of each pot. It was necessary to wait 2 weeks after the initial incorpora-tion of the rye in order to complete the organic acid sampling and to allow some initial decomposition of the rye to occur. Planting the tomato seedlings at the time of the rye incorporation may have re-sulted in phytotoxicity with such high rye application rates. The tomatoes were watered daily and fertil-ized weekly with a solution containing N, P, and K. The plants were watered carefully to avoid leaching the pots and to maintain aerobic conditions for the nematodes.
Five weeks after transplanting, the entire tomato plant was removed from each pot. The leaves and petioles were removed from the top two complete branches for nutrient analyses. The entire shoot was weighed after 24 h of oven drying at 60◦C. The leaves
and petioles were analyzed for N with a Perkin-Elmer 2400 CHN elemental analyzer, while P, K, and mi-cronutrients were measured by means of inductively coupled plasma spectroscopy following acid digestion. The roots were removed intact by submerging the soil and root ball in water and gently rinsing away the soil. The roots were rinsed, weighed, given a visual rating for percent root infection, and the root-knot nematode induced galls were counted.
The plant response data was analyzed as a factorial arrangement in a randomized complete block design. The three rye rates and the five sample dates were used in the model Yij k=µ+Ri+Tj+Sk+(TS)j k+eij k,
where Ri represents reps, Tj represents rye rates, and
Sk represents sampling dates. Although not a factor
in this experiment, sample dates were retained in the model due to the possibility of a relic effect from the organic acid extraction experiment. The dry shoot weight, fresh root weight, and root gall count data were transformed by taking the square root of the data to obtain homogeneity of variance. There was insufficient leaf tissue from each plant to do individ-ual analyses for each plant. For this reason, three leaf tissue samples, one from each block with the same sample date, were combined. Data were analyzed following a randomized complete block design with sampling dates as the blocking factor and rye rates as treatments following the model Yij=µ+Si+Tj+eij,
where Si represents sampling dates (replications),
and Tj represents rye treatments. The differences
between rye treatments were assessed for all the tomato plant data by performing the least signifi-cant difference (LSD) test at the 0.05 probability level.
2.7. Experiment 2: fate of added organic acids
The soil collected for Experiment 1 was utilized. The soil was maintained in a field-moist condition to sustain the pre-existing microbial population. Analyt-ical grade formic (95–97%), acetic (99.7%), butyric (99%), propionic (99.5%), and valeric (99%) acids were combined and mixed with HPLC-grade water to form a working solution. Clay pots (7.6 cm diame-ter) were filled with the equivalent of 100 g oven-dry soil. The drain hole was covered with a piece of woven fiberglass cloth to contain the soil and allow free drainage. The organic acid solution was added to the soil with each pot receiving 1500mmol of each acid.
The soil-filled pots were organized in a randomized complete block design in a temperature-controlled incubator maintained at 26.5◦C. A pan of water was
placed in the incubator to raise the humidity and reduce desiccation of the soil. Samples of the soil solution were taken by flooding the top of the pots with 35.6 ml of water and collecting the leachate. This amount of water theoretically diluted the final acid concentration to 500mmol/l. The leach-ing took place in a refrigerator at 1◦C to inhibit
further microbial decomposition of the added acids. Samples were taken by leaching the soil imme-diately after application of the acids (time zero) and new pots were leached every 2 h for 10 h. The leachate samples were adjusted to pH 5 by adding 0.1 mol NaOH/l and then analyzed by ion exclusion chromatography.
Three shelves were used to separate the samples into three blocks. The experimental design consisted of one soil treatment and six sampling periods. Six pots were randomly assigned within the shelves (blocks). At each sample period one pot was selected from each block with a pot being sampled only once. At each sampling period, organic acid analysis was performed on the leachate. The model Yij=µ+Ri+Sj+eij, was
utilized, where Ri represents reps, and Sj represents
3. Results and discussion
3.1. Experiment 1: addition of rye
3.1.1. Organic acids in leachate
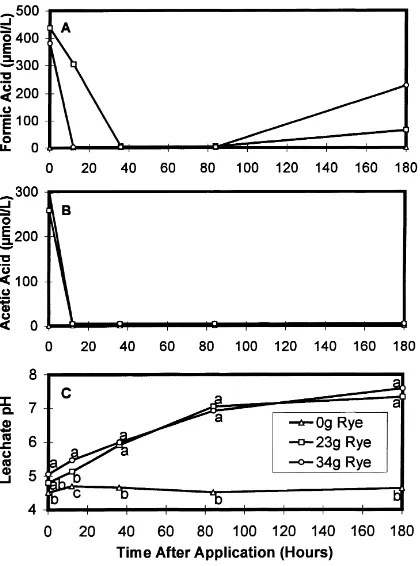
Only low concentrations (<450mmol/l) of formic and acetic acids were detected in the leachate from treatments receiving 23 and 34 g of rye (Fig. 1). These acids were primarily detectable during the first 24 h following rye application, although formic acid was detected after 180 h in one sample of each of the two rye rates. None of the other organic acids were present in detectable concentrations during the 180 h incu-bation period. These findings were consistent with a study conducted by Schwartz and Martin (1955), who observed a roughly 70% increase in the concentrations of acetic and formic acids after 24 h following incor-poration of fresh alfalfa (Medicago sativa L.).
Simi-Fig. 1. Formic acid (A) and acetic acid (B) concentration and the pH (C) of the soil leachate after the incorporation of rye. Mean separation of pH data by LSD. Means with no common letter are different at the 5% level within each sample data. All data points are averages of three pots sampled.
larly, Baziramakenga et al. (1995) showed that formic and acetic acids were the dominant acids released fol-lowing incorporation of dried quackgrass (Elytrigia repens (L.) Nevski) into soil. They reported that these two acids accounted for 75% of the total aliphatic acids present in the soil during their 8-week experiment.
An additional effect of the rye treatment was a sig-nificant increase (F-test of rye×time interaction effect, p<0.01) in the pH of the soil solution at each sam-ple date after time zero, compared with the control (Fig. 1). At this higher solution pH (7.0–7.5), acetic acid (pKa=4.76) and formic acid (pKa=3.75) are
al-most exclusively in the dissociated, anionic form. The dissociated form of these acids does not have a toxic effect on root-knot nematodes, even at high concen-trations (Djian et al., 1991). It is unclear what mech-anism was responsible for the rise in pH; the addition of Ca and Mg in the rye would not explain such a large increase.
3.2. Nematode population
The failure to detect high concentrations of low molecular weight organic acids in the rye-treated soils did not correspond to a failure to alter the root-knot nematode population in the treatments. The total num-ber of nematodes found in the soil following rye ad-ditions might not be the best measurement of the rye-treatment effect. For example, nematodes in the soil may be observed to be alive following rye addi-tions, but may no longer be able to infect a host plant. For this reason, tomato plants were planted into the treated soil to serve as direct bio-indicators of tode activity as measured by the ability of the nema-todes to infect the plants.
concen-Fig. 2. Transformed weight of tomato shoots and roots grown in soil amended with different rates of fresh rye. Weights represent an average of 15 reps. Means with no common letter within a row are significantly different at the 5% level. LSD (0.05)=0.316 for dry shoot weight, and LSD (0.05)=0.621 for fresh root weight.
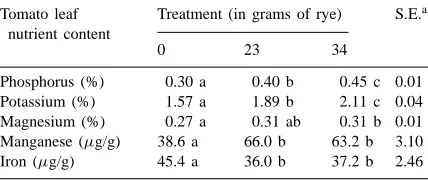
tration of Fe compared with the unamended control. There were highly significant increases (p<0.01) in the concentrations of P, K, and Mn, and a signifi-cant increase in Mg concentration (p<0.05) over the plants grown in the soil receiving no rye (Table 1). The observed rise in solution pH might contribute to in-creased P availability, but K and Mg availability would remain relatively unchanged, and Mn solubility would have been expected to decline. The differences in plant size were possibly due to damage by the nematode populations.
The root-knot nematode populations on the toma-toes were significantly (p<0.001) suppressed by
Table 1
Nutrient concentration of tomato leaves from plants grown in a non-amended soil and a soil amended with fresh rye shoots
Tomato leaf nutrient content
Treatment (in grams of rye) S.E.a
0 23 34
aS.E. of the mean=square root (mean square error/reps); mean square error computed from the model Yij=µ+Ti+Sj+eij, where
Ti represents rye treatments, and Sj represents sampling dates (replications); means with no common letter within a row are significantly different at the 5% level (LSD).
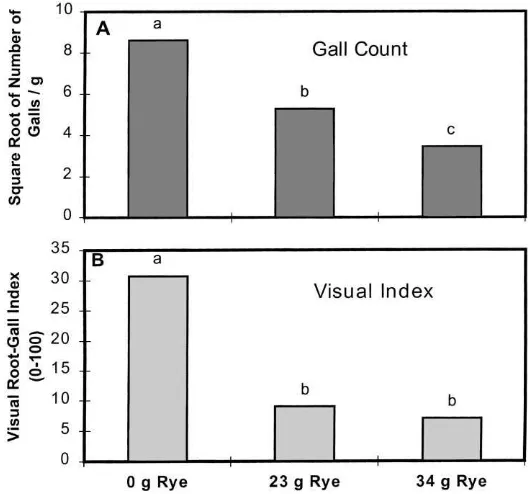
the addition of the rye, as measured by the visual root-gall rating of the root system (Fig. 3). When nematode-induced root galls were counted, damage was reduced from a mean of 8.3 galls/g of root in the 0 g rye treatment to a mean of 4.8 galls/g in the 23 g rye treatment and 3.0 galls/g in the 34 g rye treatment with a standard error of the difference of 0.78 (Fig. 3). The root damage reduction is an indication that the addition of rye to the soil adversely affected the abil-ity of root-knot nematodes to parasitize the tomato plants in the study, with the high rye addition rate being the most effective. Similar results have been found with cotton (Gossypium hirsutum L.) following rye additions (McBride et al., 1999).
Researchers have suggested that the addition of a labile C source to the soil results in an increase in bacteria and fungi that utilize the added C for energy (Linford et al., 1938). Populations of nematodes that feed on the bacteria and fungi would in turn increase. With the overall nematode population increasing, it is likely that organisms such as predatory nematodes, mites, and nematode trapping fungi, which prey on nematodes, would also increase. The plant-parasitic nematodes, having received no direct benefit from the C source, would suffer from the increase in predator populations.
3.3. Experiment 2: fate of added organic acids
The concentrations of acetic, propionic, butyric, and valeric acids in the soils declined significantly (p<0.01) from a maximum of 630mmol/l (formic acid) to a minimum of 10mmol/l (acetic acid) dur-ing the 10 h incubation (Fig. 4). This finddur-ing clearly shows the rapid disappearance of these low molecular weight organic acids from the soil solution.
Fig. 3. Nematode-induced root damage to tomato roots grown in soil amended with different rates of fresh rye. The gall count (A) is expressed as nematode induced galls/g. The visual index (B) is expressed as percent of the root mass damaged. The gall counts were transformed by taking the square root of the data to stabilize variance. Means with no common letter with are significantly different at the 5% level. Data represent an average of 15 plants. LSD (0.05)=1.6 for gall counts, and LSD (0.05)=3.9 for visual index.
(pKa 3.8–4.9) at a pH>4.3 and therefore are likely
to participate in this type of reaction. Aliphatic acids are known to have chelating characteristics, forming complexes with metals in the soil (Stevenson and Ardakani, 1972; Brynhildsen and Rosswall, 1997).
Fig. 4. Decline in five low molecular weight organic acids in soil water over time. The five acids declined significantly over time as measured by analysis of variance at the 0.05 level, with data points representing averages of three pots. Standard errors were 59 for formic, 32 for acetic, 52 for propionic, 61 for butyric, and 106 for valeric acid.
4. Conclusions
In these experiments, it was not possible to mea-sure the concentration at which acetic and formic acids might become toxic to root-knot nematodes in the soil. The concentrations of the acids detected in the soil leachate were lower than that which has been reported to be necessary for a nematicidal effect. Low molec-ular weight organic acids proved to be short lived in soil solution. Acids produced from decomposing or-ganic materials were readily removed from solution and would not be expected to accumulate under aero-bic conditions. This is in agreement with the findings of researchers who have examined these acids in soil (Schwartz et al., 1954; Lynch, 1991).
Even with the high rates of rye used, evidence for a direct link between low molecular weight organic acids and root-knot nematodes was not found. Given the low concentrations detected and the short time these acids remain in the soil, their direct effect as a nematicide seems unlikely. It is possible that these acids may have contributed to nematode suppression in association with other factors, resulting in the ob-served reduction in root-knot nematode populations. Factors contributing to the reduction in nematode dam-age may include other organic acids not investigated in this study, other compounds released from the rye or microbes during decomposition, and stimulation of competitive organisms that utilize the root-knot nema-todes as a food source.
References
Akhtar, M., Alam, M.M., 1992. Effect of crop residues amendments to soil for the control of plant-parasitic nematodes. Biores. Technol. 41, 81–87.
Akhtar, M., Alam, M.M., 1993. Utilization of waste materials in nematode control: a review. Biores. Technol. 45, 1–7. Akhtar, M., Mahmood, I., 1994. Potentiality of phytochemicals in
nematode control: a review. Biores. Technol. 48, 189–201. Badra, T., Mahmound, S.A., Bakir, O.A., 1979. Nematicidal
activity and composition of some organic fertilizers and amendments. Rev. Nematol. 2, 29–36.
Barker, K.R., 1985a. Nematode extraction and bioassays. In: Barker, K.R., Carter, C.C., Sasser, J.N. (Eds.), An Advanced Treatise on Meloidogyne: Vol. 2. Methodology. North Carolina State University Graphics, Raleigh, NC, pp. 26–27.
Barker, K.R., 1985b. Nematode extraction and bioassays. In: Barker, K.R., Carter, C.C., Sasser, J.N. (Eds.), An Advanced
Treatise on Meloidogyne: Vol. 2. Methodology. North Carolina State University Graphics, Raleigh, NC, 28 pp.
Baziramakenga, R., Simard, R.R., Leroux, G.D., 1995. Determination of organic acids in soil extracts by ion chromatography. Soil Biol. Biochem. 27, 349–356.
Brynhildsen, L., Rosswall, T., 1997. Effects of metals on the microbial mineralization of organic acids. Water Air Soil Pollut. 94, 45–57.
Chandrasekaran, S., Yoshida, T., 1973. Effect of organic acid transformations in submerged soils on growth of the rice plant. Soil Sci. Plant Nutr. 19, 39–45.
Djian, C., Pijarowski, L., Ponchet, M., Arpin, N., Favre-Bonvin, J., 1991. Acetic acid: a selective nematicidal metabolite from culture filtrates of Paecilomyces lilacinus (Thom) Samson and
Trichoderma longibrachiatum Rifai. Nematologica 37, 101–
112.
Fox, T.R., Comerford, N.B., 1990. Low molecular-weight organic acids in selected forest soils of the Southeastern USA. Soil Sci. Soc. Am. J. 54, 1139–1144.
Gotoh, S., Onikura, Y., 1971. Organic acids in a flooded soil receiving added rice straw and their effect on the growth of rice. Soil Sci. Plant Nutr. 17, 1–8.
Gould, W.A., 1992. Tomato production, processing & technology, 3rd Edition. CTI Publications Inc., Baltimore, MD, 536 pp. Heald, C.M., Orr, C.C., 1984. Nematode parasites of cotton. In:
Nickle, W.R. (Ed.), Plant and Insect Nematodes. Marcel Dekker, New York (Basel), pp. 147–166.
Hollis, J.P., Rodriguez-Kabana, R., 1966. Rapid kill of nematodes in flooded soil. Phytopathology 56, 1015–1019.
Jairajpuri, M.S., Alam, M.M., Ahmad, I., 1990. Nematode Bio-Control: Aspects and Prospects. CBS Publishers & Distributors, Delhi, India, 152 pp.
Johnston, T.M., 1959. Effect of fatty acid mixtures on the rice styles nematode (Tylenchorhynchus martini Fielding, 1956). Nature 183, 1392.
Linford, M.B., Yap, F., Oliveria, J.M., 1938. Reduction of soil populations of the root-knot nematode during decomposition of organic matter. Soil Sci. 45, 127–147.
Lynch, J.M., 1991. Sources and fate of soil organic matter. In: Wilson, W.S. (Ed.), Advances in Soil Organic Matter Research: The Impact on Agriculture and the Environment. Royal Society of Chemistry, Cambridge, UK, pp. 231–237.
McBride, R.G., Mikkelsen, R.L., Barker, K.R., 1999. Survival and infection of root-knot nematodes added to soil amended with rye at different stages of decomposition and cropped with cotton. Appl. Soil Ecol. 13, 231–235.
McLaren, A.D., Peterson, G.H., 1967. Soil Biochemistry. Marcel Dekker, New York (Basel).
Rocklin, R.D., Slingsby, R.W., Pohl, C.A., 1986. Separation and detection of carboxylic acids by ion chromatography. J. Liquid Chromatogr. 9, 757–775.
Schwartz, S.M., Varner, J.E., Martin, W.P., 1954. Separation of organic acids from several dormant and incubated Ohio soils. Soil Sci. Soc. Am. Proc. 1954, 174–177.
Schwartz, S.M., Martin, W.P., 1955. Influence of soil organic acids on soluble phosphorus in Miami and Wooster silt loam soils. Soil Sci. Soc. Am. Proc. 1954, 185–188.
Stephenson, W., 1945. The effects of acid on a soil nematode. Parasitology 36, 158–164.
Stevenson, F.J., Ardakani, M.S., 1972. Organic matter reactions
involving micronutrients in soils. In: Mortvedt, J.J., Giordano, P.M., Lindsay, W.L. (Eds.), Micronutrients in Agriculture. Soil Science Society of America, Madison, WI, pp. 79– 114.
Van Gundy, S.D., 1985. Ecology of Meloidogyne spp. — emphasis on environmental factors affecting survival and pathogenicity. In: Sasser, J.N., Carter, C.C. (Eds.), An Advanced Treatise on
Meloidogyne: Vol. 1. Biology and Control. North Carolina State