Potential C-source utilization patterns of bacterial communities as
influenced by clearing and land use in a vertic soil of Argentina
E. Gomez
a,∗, V. Bisaro
b, M. Conti
caCátedra de Microbiolog´ıa Agr´ıcola, Facultad de Ciencias Agrarias, Universidad Nacional de Rosario,
Campo Experimental J. Villarino, 2123 Zavalla, Argentina
bCátedra de Estad´ıstica, Facultad de Ciencias Agrarias, Universidad Nacional de Rosario,
Campo Experimental J. Villarino, 2123 Zavalla, Argentina
cDepartamento de Suelos, Facultad de Agronom´ıa, Universidad de Buenos Aires, Av. San Mart´ın 4453, 1417 Buenos Aires, Argentina
Received 20 April 2000; accepted 20 April 2000
Abstract
A sole-carbon-source catabolism assay (Biolog GN microplate) was used to study whether bacterial communities from the same vertic soil, but under different management history, showed distinctive patterns of C-substrate utilization. Two sampling depths (0–7.5 and 7.5–15 cm) were also investigated. The response of microbial communities to increasing periods of time — 16, 26 and 40 years (S2, S3, S4, respectively) — since native vegetation clearing and to land use was evaluated as related to the soil in its native condition (S1).
Tenfold dilutions of soil suspensions were performed and aliquots of 10−4dilution were inoculated into each well of the
Biolog GN microplates and then incubated. Activity on C-substrates was recorded as optical density at regular time intervals. Absorbance data from the 54-h incubation time were used to calculate the average well-color development (AWCD) in each plate, richness (number of catabolized C-sources) and diversity Shannon’s index. Principal component analysis (PCA) was performed to study patterns of C-source utilization. Number of bacteria was determined by plate counts on to tryptic soy agar (TSA) and expressed as colony-forming units (CFU) g−1soil.
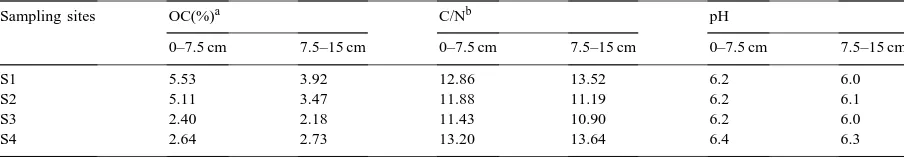
The lowest AWCD values were found in the 40 years since clearing site (S4) in both depths, despite the fact that the largest number of bacteria was found in the top 0–7.5 cm. Samples from the native condition showed the largest richness and diversity on metabolized C substrates (p<0.001) while S4 had the lowest values at a depth of 0–7.5 cm.
The locations that were investigated could be differentiated by PCA. The Biolog GN assay showed to be sensitive to distinguish soil bacterial communities from sites with different times elapsed since clearing and management history. Larger differences among samples were detected at 0–7.5 cm depth.
Distinctive patterns of ‘in vitro’ C-source utilization could be related to differences in chemical composition of soil organic matter. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Agricultural intensification; Biolog assay; Soil microbial communities
∗Corresponding author. Tel.:+54-341-456-7548; fax:+54-341-497-0199.
E-mail address: [email protected] (E. Gomez).
1. Introduction
Interest in evaluation of soil quality has been in-creasing since the critical role of soil in determining the sustainability of ecosystems became clear. Soil quality has deep effects on system health and
ductivity (Doran and Parkin, 1996). Soil biological components are fundamental to the development of ecologically relevant process such as nutrient cycling, improvement of soil structure and xenobiotic decom-position. Microbial activity dominates the degradation of soil organic substrates and it is of major concern for ecosystem functioning. Microorganisms are largely sensitive to perturbations. Therefore, changes in the communities of soil organisms or in their functions may be early signs of alterations in soil health (Dick et al., 1996; Toresani et al., 1998). Maintenance of biodiversity related to soil organisms seems to be cru-cial for agriculture to be considered sustainable (Beare et al., 1995). Nevertheless, studies on diversity have been focused on higher organisms and only in the recent years more attention is being paid to microor-ganisms (McLaughlin and Mineau, 1995; Kennedy and Gewin, 1997). Some researchers have reported variable responses of microbial communities to distur-bance. Lupwayi et al. (1998) found that conventional tillage decreased microbial diversity whereas reduced tillage enhanced it, while Hassink et al. (1991) did not find differences. Kennedy and Smith (1995) reported greater diversity indexes in cropped systems than in grasslands when they compared substrate use.
The small size and morphological similarity make the complete morphological and taxonomic charac-terization of soil microbial communities impossible (Garland and Mills, 1991). However, more than the analysis of taxonomic structure, biochemical and physiological properties may be suitable to a greater extent from an ecological point of view (Hassink et al., 1991; Zak et al., 1994). In this respect, some authors have pointed out the need of throwing light on the effects of disturbances at a community level (Kennedy and Smith, 1995).
Garland and Mills (1991) applied to whole environ-mental samples a redox technique based in sole car-bon source utilization profiles, originally designed for strain identification, and found that this assay could be a useful tool for classifying bacterial communities. Color development due to the reduction of tetrazolium dye is used as an indicator of catabolism of each car-bon source. Zak et al. (1994) gave this method an ecological meaning, studying functional diversity by means of indexes of richness, diversity and evenness, and patterns that emerged from the catabolized carbon sources.
Since then, and despite the successful use of car-bon substrate catabolism in terrestrial and aquatic ecosystems, recent literature refers to problems con-cerning the Biolog approach, specially as regards analysis and interpretation of results (Insam, 1997; Insam and Hitzl, 1999). Thus, the Bilog system is considered more adequate for comparisons among microbial communities than for their characteriza-tion (Glimm et al., 1997; Garland and Mills, 1999). Several findings have proved the assay to be useful to distinguish bacterial communities from differ-ent environmdiffer-ental and soil samples (Lehman et al., 1997; Goodfriend, 1998). However, only a few stud-ies have dealt with the possibility of this method to show the effects of agricultural intensification on soil.
In this work, we examined whether: (a) time since clearing and subsequent land use produced different sole carbon source patterns in the bacterial commu-nities of a vertic soil; or (b) two sampling depths showed distinctive substrate catabolism profiles. To as-sess whether relationships among bacterial communi-ties responded to years since clearing and subsequent management, patterns of potential substrate utilization were compared using the same soil, but in its native condition as reference, using the approach mentioned by Parkin et al. (1996).
2. Materials and methods
2.1. Sample collection and conditioning
This work is part of a Natural Resources Conserva-tion Project of an area of vertic soils in Entre R´ıos, Ar-gentina (31◦30′S latitude; 59◦45′W longitude). Sites for the study were selected on the basis of time elapsed since native vegetation clearing and management his-tory. Samples from four locations (S1, S2, S3, S4) on a same soil type were collected with a core (2.5-cm diameter) at two depths (0–7.5 and 7.5–15 cm) on 28 October 1998. Table 1 describes the main character-istics of sampling sites. The soil was a Vertic Argiu-doll (fine, montmorillonitic, thermal family) with clay, 236 g kg−1, and silt, 737 g kg−1, in the Horizon A.
Table 1
Description of sampling sites: the native condition, and the other sites selected on the basis of time elapsed since native vegetation clearing and management history
Location 1 (S1) Native vegetation: xerophytic bush (Prosopis sp., Celtis sp.) and herbaceous (Stipa, Setaria, Bothriochloa, Paspalum, Stenandrium, Scoparia, Trifolium)
Location 2 (S2) 16 years since clearing; cropped by the first 8 years with conventional tillage (moldboard plowing as the main labor); with naturalized prairie (Bromus sp.) since 1990
Location 3 (S3) 26 years since clearing; continual cropping with corn (Zea mays L.) and soybean (Glycine max(L.) Merr) under conventional tillage; moldboard plowed a week before sampling Location 4 (S4) 40 years since clearing; continual cropping with corn and soybean; managed with zero tillage
since 1994; soybean residue covering at sampling
In order to evaluate internal variability, plots of each location were divided into sectors (50 m2). Twenty sub-samples from each sector were composited. Three replicates from each location were sieved (<2 mm), stored at 4◦C and processed within a week.
2.2. Sample analysis
The Biolog Gram negative (GN) microplates (Bi-olog Inc. Hayward CA, 1993) were used. The Bi(Bi-olog GN and Gram positive (GP) microplates are not selec-tive for either GN or GP bacteria (Zak et al., 1994). Most of the substrates in GN plates are also found in GP plates. It has been argued that several of the C-sources in Biolog plates are not present in ecosys-tems (Konopka et al., 1998). We chose working with GN plates, since some of the C-sources found in GN, but not in GP, microplates have been reported as con-stituents of root exudates (Campbell et al., 1997). Each plate consists of 96 wells (95 with a carbon source incorporated to a basal medium as sole C-source and one control without any C-source). Tetrazolium violet is used as a redox dye to colorimetrically indicate the utilization of the carbon sources. Carbon compounds
Table 2
Organic carbon (OC), carbon:nitrogen ratio (C/N) and pH in water (1:2.5) measured in soil from the sampling sites at depths of 0–7.5 and 7.5–15 cm, respectively
Sampling sites OC(%)a C/Nb pH
0–7.5 cm 7.5–15 cm 0–7.5 cm 7.5–15 cm 0–7.5 cm 7.5–15 cm
S1 5.53 3.92 12.86 13.52 6.2 6.0
S2 5.11 3.47 11.88 11.19 6.2 6.1
S3 2.40 2.18 11.43 10.90 6.2 6.0
S4 2.64 2.73 13.20 13.64 6.4 6.3
aWalkley–Black method. bKjeldahl method.
included in Biolog GN microplates and grouped into guilds by chemical structure (Zak et al., 1994) are shown in Table 3.
Soil suspensions from each bulked sample (soil 10 g; sterile saline 0.85% NaCl 100 ml) were shaken for 1 h and pre-incubated for 18 h before inoculation to allow microbial utilization of any soluble organic C derived from the soil (Dick et al., 1996). Ten-fold dilutions were performed and aliquots of 100 ml from 10−4 dilution were inoculated into each well
of the Biolog GN microplates. The plates were in-cubated at 25◦C. Color development in each well was recorded as optical density (OD) at regular time intervals with an MR700 Dynatech Plate Reader at 590 nm.
Bacterial plate counts onto tryptic soy agar (TSA) were also performed for each sample after 5 days of incubation at 25◦C, and results were expressed as colony-forming units (CFU) g−1soil.
2.3. Data analysis
deter-Table 3
Carbon compounds included in the Biolog GN microplatesa
Carbohydrates
Adonitol (8) d-Mannitol (22) i-Erythritol (12) Mono-methyl-succinate (35)
a-d-Glucose (17) d-Mannose (23) l-Arabinose (9) N-acetyl-d-galactosamine (6)
a-d-Lactose (19) d-Melibiose (24) l-Fucose (14) N-Acetyl-d-glucosamine (7)
b-Methyl-glucoside (25) d-Raffinose (27) l-Rhamnose (28) Sucrose (30)
Cellobiose (11) d-Psicose (26) Lactulose (20) Turanose (32)
d-Arabitol (10) d-Sorbitol (29) m-Inositol (18) Xylitol (33)
d-Fructose (13) d-Trehalose (31) Maltose (21)
d-Galactose (15) Gentiobiose (16) Methyl pyruvate (34) Carboxylic acids
Acetic acid (36) cis-Aconitic acid (37) d-Glucosaminic acid (43) Malonic acid (54)
a-Hydroxybutyric acid (45) Citric acid (38) d-Glucuronic acid (44) p-Hydroxy-phenylacetic acid (48)
a-Ketobutyric acid (50) d,l-Lactic acid (53) d-Saccharic acid (57) Propionic acid (55)
a-Ketoglutaric acid (51) d-Galactonic acid lactone (40) Formic acid (39) Quinic acid (56)
a-Ketovaleric acid (52) d-Galacturonic acid (41) g-Hydroxy-butyric acid (47) Sebacic acid (58)
b-Hydroxybutyric acid (46) d-Gluconic acid (42) Itaconic acid (49) Succinic acid (59) Amino acids
d,l-Carnitine (82) Glycyl-l-glutamic acid (71) l-Aspartic acid (68) l-Phenylalanine (76)
d-Alanine (64) Hydroxy-l-proline (73) l-Glutamic acid (69) l-Proline (77)
d-Serine (79) l-Alanine (65) l-Histidine (72) l-Pyroglutamic acid (78)
g-Aminobutyric acid (83) l-Alanyl-glycine (66) l-Leucine (74) l-Serine (80) Glycyl-l-aspartic acid (70) l-Asparagine (67) l-Ornithine (75) l-Threonine (81)
Amines-Amides Polymers
2-Amino-ethanol (90) Phenyl-ethylamine (88) a-Cyclodextrin Tween 40 (4)
Alaninamide (63) Putrescine (89) Dextrin (2) Tween 80 (5)
Glucuronamide (62) Succinamic acid (61) Glycogen (3) Others
2,3-Butanediol (91) Glucose-6-phosphate (95) Thymidine (87)
Bromosuccinic acid (60) Glycerol (92) Uridine (86)
d,l-a-glycerolphosphate (93) Inosine (85) urocanic acid (84)
Glucose-1-phosphate (94)
aC substrates are numbered as they are located in the GN microplates.
mined as follows for the different measurement times:
AWCD=
P
ODi 95
where ODi is the optical density value from each well. Substrate richness (R) was determined as the total of reactions in each microplate, considering as ‘positive’ — that is the substrate was metabolized — those wells with OD>0.10 (Garland, 1997). Diversity Shannon’s index (H) was calculated as follows (Zak et al., 1994; Derry et al., 1998)
H = −Xpi(lnpi)
where pi is the ratio of the activity on each substrate to the sum of activities on all substrates.
Optical density values (OD) corresponding to 54 h after inoculation were used to do statistical analysis (SAS, version 6.12). The ANOVA was performed for AWCD, richness, and diversity, considering the effects from sectors, depths, locations and their interactions. Principal component analysis (PCA) was used to study patterns of C-sources catabolism.
Plate counts values were log-transformed for the analysis.
3. Results
3.1. Plate counts and microbial activity
In the analysis of CFU g−1 of soil, the
Table 4
Bacterial counts, and average color development (AWCD), richness and diversity calculated on data from the 54 h of incubation of Biolog GN microplates, for the native conditions (S1), 16-year-clearing location (S2), 26-year-clearing location (S3) and 40-year-clearing location (S4)
Sampling sites
Bacterial counts (CFU g−1soil)
AWCD Richness Diversity
(Shannon’s index) 0–7.5 cm depth
S1 1.5×107ba 0.81 a 94 a 4.39 a
S2 1.8×107b 0.49 b 81 b 4.23 b
S3 2.6×107b 0.74 a 85 b 4.28 b
S4 1.6×108a 0.37 c 61 c 4.06 c
7.5–15 cm depth
S1 5.5×107nsb 0.78 a 94 a 4.36 a
S2 5.3×107ns 0.71 ab 87 ab 4.24 b
S3 2.3×107ns 0.69 b 88 ab 4.31 a
S4 4.3×107ns 0.53 c 82 b 4.24 b
C.V. (%) 2.81 5.03 3.84 0.67
aWithin a column, means followed by the same letter are not significantly different (Duncan; p<0.01). bNot significant.
(p<0.01). When each depth was considered sepa-rately, significant differences (p<0.01) were observed only in the depth of 0–7.5 cm. The 40-years-clearing samples (S4) were different from the other locations (Table 4).
The highest AWCD values for the different mea-surement times were encountered in samples from the native condition (S1) while samples from S4 had the lowest values at both these depths (Fig. 1). All of the effects tested by ANOVA (sectors, depths, loca-tions and their interacloca-tions) were significant (p<0.01) for the AWCD from the 54-h incubation time. The highest values for both depths were encountered in samples from the native condition (S1), but it was not significantly different from the 26-year clearing sam-ples (S3) at depths of 0–7.5 cm. The AWCD value from S2 was lower than in S1 and S3 in the top layer and was between S1 and S3 at depths of 7.5–15 cm. The lowest AWCD for both these depths were found in S4 despite the fact that it had the largest number of bacteria in the top 0–7.5 cm layer (Table 4).
3.2. Richness and diversity indexes
The interaction between locations and depths was significant in both richness and diversity indexes (p<0.001), and also locations studied at each depth of sampling were different.
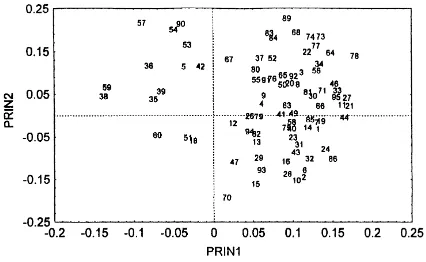
Fig. 2. Principal component analysis on the 95 C-source absorbance data from 54-h incubation time, for samples from S1, S2, S3 and S4 at the depth of 0–7.5 cm.
Richness and diversity were largest in S1 for both these depths, though S2 and S3 did not significantly differ in richness from S1, and S3 did not differ in diversity from S1 at depths of 7.5–15 cm. The lowest values in both these indexes were found in S4 at depths of 0–7.5 cm (Table 4).
3.3. Patterns of substrate utilization
At depths of 0–7.5 cm, PCA on data corresponding to 54 h of incubation allowed the separation of samples from the different locations with 63.3% of the variance explained by both the first (PC1) and second (PC2) principal components. Based on C-source usage, S2 and S4 were differentiated from S1 and S3 by PC1 (Fig. 2). The 95 C-sources were plotted in a factorial axes system and their positions indicate the correlation with PC1 and PC2 (Fig. 3).
In S2 and S4, there was a lower activity on all the carbon sources than in S1 and S3, with the exception of substrates 38, 57 and 59 (citric acid,D-saccharic acid and succinic acid, respectively), of which high con-sumption in S2 and S4 allowed the separation (Fig. 3). In respect to S1 and S3, there was a high activity on most substrates in both of them, but CP2 separated them on the basis of predominance of certain chemical structures that were metabolized by S1 or S3.
Principal components separated samples from the four locations at depths of 7.5–15 cm, but in a some-what different way, with 43.5% of the variation ex-plained by both PC1 and PC2. The first PC separated S4 from the other locations, while S2 clustered near samples from S1. As in the case of 0–7.5 cm depths,
Fig. 3. Scores of the 95 C-substrates determined by their correlation with PC1 and PC2 in the PCA on absorbance data from the 54-h incubation time, for samples S1, S2, S3 and S4 at the depth of 0–7.5 cm.
Fig. 4. Principal component analysis on the 95 C-source absorbance data from 54-h incubation time, for samples from S1, S2, S3 and S4 at the depth of 7.5–15 cm.
Table 5
Carbon substrates in the Biolog GN microplates that were not used by one or more locations at the 0–7.5 cm depth
S1 S2 S3 S4
Phenyl-ethylamine (88) + − + −
Succinamic acid (61) + + + − bIndicates OD<0.10.
PC2 differentiated S1 from S3 (Fig. 4). More sub-strates than in the surface layer of 0–7.5 cm were me-tabolized by S2 and S4 in the 7.5–15 cm layer. Mi-crobial communities from S4 assimilated sources 38, 57 and 59 with a high intensity, as they did in the top layer (Fig. 5).
Table 6
Carbon substrates in the Biolog GN microplates that were not used by one or more locations in the depth of 7.5–15 cm
S1 S2 S3 S4
Phenyl-ethylamine (88) + + − −
Others
Thymidine (87) + + + −
aIndicates OD>0.10. bIndicates OD<0.10.
The consumption in the amino acid and amine/amide guilds seemed to better explain the differences among S1 and S3. The following substrates were used by S3 at a lower intensity (0.1<OD<0.25) than by S1: glycil-l-aspartic acid, glycil-l-glutamic acid,
l-leucine, l-ornithine, l-phenilalanine, d-serine,
l-threonine, 2-amino-ethanol and glucuronamide. Communities from native condition (S1) metabo-lized all the substrates excepta-hydroxybutyric acid (OD<0.10). There were some of the C-sources that were not used by any of the other locations at both depths. In the 0–7.5 cm deep layer, the largest number of C-sources that were not assimilated was found in S4. There were less substrates that were not used in the 7.5–15 cm layer, but the response in the activity on them was more variable among the locations than in the top layer (Tables 5 and 6).
4. Discussion
different types of vegetation. Lupwayi et al. (1998) could distinguish samples on the basis of tillage and previous crop. In this study, samples from two layers in the same type of soil, but with different manage-ments, had different potentials of utilization of the 95 C-sources in Biolog GN assay. The distinctive usage of those substrates allowed the separation of samples as related to years since clearing of native vegetation and soil management.
Samples from the native condition (S1) showed a larger richness and diversity of metabolized substrates, thus reflecting a high metabolic potential and func-tional diversity in soil bacterial communities.
The 26-year-clearing location (S3) also showed a high consumption on the C-sources, but richness and diversity indexes were lower in the 0–7.5 cm layer. This is in agreement with the results of Lupwayi et al. (1998), who reported that tillage reduced diversity. However, though diversity was diminished under con-ventional tillage with respect to the native condition, richness and diversity indexes were larger than in case of zero tillage. As regards patterns of C-sources uti-lization, S3 metabolized substrates with a high inten-sity, while differentiating from S1 by a lower activity in compounds with nitrogen in their chemical struc-ture (amino acids, amines and amides). Tillage, partic-ularly moldboard plowing that was the main labor in S3 for many years, could have enhanced the oxidative potential of microbial communities as a consequence of soil removal.
The 40-year-clearing location, managed with zero tillage since 1994, had the lower richness and diver-sity indexes and showed a distinctive pattern of sub-strate utilization. These results are in disagreement with other findings. Hassink et al. (1991), working in a silty loam soil, did not find that microbial diver-sity differed in a reduced-input system with respect to a conventional system. Lupwayi et al. (1998) re-ported more diversity under zero tillage in a sandy loam soil, with respect to conventional management. As we worked in a soil with a high montmorillonitic clay content, compaction and water retention associ-ated with zero tillage in this heavy soil could have gen-erated anaerobic conditions that restricted microbial activity. Linn and Doran (1984) reported that aerobic microbial transformation of C and N can be adversely affected by the degree of reduction of tillage. The fact that bacterial communities from S4 did not
assimi-late several substrates in the Biolog GN micropassimi-lates may be indicative of the fact that the species able to use them were absent. Thus, oxygen restrictions might have produced a shift in microbial populations, favor-ing those with less efficient anaerobic metabolism.
In the 16-year-clearing location, the more recently cleared and the less disturbed site, diversity was sig-nificantly affected and patterns of C-source utilization were distinctive from S1 in both the soil layers.
The intensity of use of C-sources has been sug-gested to be strongly influenced by cell density in the inoculum (Haack et al., 1995). In this work, it was found that samples with the largest number of bacteria per gram of soil (S4 in the 0–7.5 cm) had the lowest AWCD, richness and diversity. Aliquots from tenfold dilutions were used both to determine the number of bacteria and to inoculate the Biolog GN microplates. Hence, the activity on substrates may be related to the growth in the plate (Garland and Mills, 1991) more than to the original density in the sample. Growth, then, would be dependent on the presence of those bacterial species capable of metabolizing a particular C-source.
5. Conclusions
The sole-C-source utilization allowed a clear sep-aration between samples from a same vertic soil, but with different management history and years since na-tive vegetation clearing. Samples were classified both by richness and diversity indexes, and the patterns of substrate utilization. There were different responses of microbial communities from the four locations evalu-ated in each soil layer studied. The depth of 0–7.5 cm seemed to be more sensitive to the detection of differ-ences among samples.
possibilities of this assay as a simple and economic way for the early detection of changes in soil health.
Distinctive patterns of ‘in vitro’ C-source utilization could be related to differences in chemical composi-tion of soil organic matter. Future research is needed to shed light on the relationship between patterns of C-substrate usage and other physical and chemical variables.
Acknowledgements
We thank CONICET for the partial support of this research, to Laura Ferreras for her laboratory assis-tance, and to Patricia Torres for advice on statistical analysis.
References
Beare, M.H., Coleman, D.C., Crossley, D.A., Hendrix, P.F., Odum, E.P., 1995. A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170, 5–22.
Biolog Inc., Hayward, CA, 1993. GN MicroPlateTM. Instructions
for use.
Campbell, C.D., Grayston, S.J., Hirst, D.J., 1997. Use of rhizosphere carbon sources in sole-carbon-source tests to discriminate soil microbial communities. J. Microbiol. Methods 30, 33–41.
Derry, A.M., Staddon, W.J., Trevors, J.T., 1998. Functional diversity and community structure of microorganisms in uncontaminated and creosote-contaminated soils as determined by sole-carbon-source utilization. World J. Microbiol. Biotechnol. 14, 571–578.
Dick, R., Breakwell, D., Turco, R., 1996. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran, J., Jones, A. (Eds.), Methods for Assessing Soil Quality, SSSA Special Publication 49, SSSA, Madison, WI, pp. 247–271.
Doran, J., Parkin, T., 1996. Quantitative indicators of soil quality: a minimum data set. defining and assessing soil quality. In: Doran, J., Jones, A. (Eds.), Methods for Assessing Soil Quality, SSSA Special Publication 49, SSSA, Madison, WI, pp. 25–37. Garland, J.L., 1997. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 24, 289–300.
Garland, J., Mills, A., 1991. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359.
Garland, J., Mills, A., 1999. Further comments on the interpretation of community-level physiological profiles. Soil Biol. Biochem. 31, 1203.
Glimm, E., Heuer, H., Engelen, B., Smalla, K., Backhaus, H., 1997. Statistical comparisons of community catabolic profiles. J. Microbiol. Methods 30, 71–80.
Goodfriend, W.L., 1998. Microbial community patterns of potential substrate utilization: a comparison of salt marsh, sand dune, and seawater-irrigated agronomic systems. Soil Biol. Biochem. 30, 1169–1176.
Haack, S.K., Garchow, H., Klug, M.J., Forney, L.J., 1995. Analysis of factors affecting the accuracy, reproducibility, and interpretation of microbial community carbon source utilization patterns. Appl. Environ. Microbiol. 61, 1458–1468.
Hassink, J., Oude Voshaar, J.H., Nijhuis, E.H., van Veen, J.A., 1991. Dynamics of the microbial populations of a reclaimed-polder soil under a conventional and a reduced-input farming system. Soil Biol. Biochem. 23, 515–524.
Insam, H., 1997. Substrate utilization tests in microbial ecology. Preface to the special issue of the Journal of Microbiological Methods. J. Microbiol. Methods 30, 1–2.
Insam, H., Hitzl, W., 1999. Data evaluation of community-level physiological profiles: a reply to the letter of P.J.A. Howard. Soil Biol. Biochem. 31, 1198–1200.
Kennedy, A.C., Smith, K.L., 1995. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 170, 75–86. Kennedy, A.C., Gewin, V.L., 1997. Soil microbial diversity: present
and future considerations. Soil Sci. 162, 607–617.
Konopka, A., Oliver, L., Turco Jr., R.F., 1998. The use of carbon substrate utilization patterns in environmental and ecological microbiology. Microbial Ecol. 35, 102–115.
Lehman, R.M., Colwell, F.S., Garland, J.L., 1997. Physiological profiling of indigenous aquatic microbial communities to determine toxic effects of metals. Environ. Toxicol. Chem. 16, 2232–2241.
Linn, D.M., Doran, J.W., 1984. Aerobic and anaerobic microbial populations in no-till and plowed soils. Soil Sci. Soc. Am. J. 48, 794–799.
Lupwayi, N.Z., Rice, W.A., Clayton, G.W., 1998. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 30, 1733–1741. McLaughlin, A., Mineau, P., 1995. The impact of agricultural
practices on biodiversity. Agric. Ecosys. Environ. 55, 201–212. Parkin, T., Doran, J., Franco-Vizca´ıno, E., 1996. Field and laboratory tests of soil respiration. In: Doran, J., Jones, A. (Eds.), Methods for Assessing Soil Quality. SSSA Special Publication 49, SSSA Madison, WI, pp. 231–245.
Toresani, S., Gomez, E., Bonel, B., Bisaro, V., Montico, S., 1998. Cellulolytic population dynamics in a vertic soil under three tillage systems in the Humid Pampa of Argentina. Soil Till. Res. 49, 79–83.