Changes in peroxidase activity and isoenzymes in spruce
needles after exposure to different concentrations of
cadmium
Ksenija Radotic´
a,*, Tanja Ducˇic´
a, Dragosav Mutavdzˇic´
baCentre for Multidisciplinary Studies,Uni
6ersity of Belgrade,29.No6embra142,11000Belgrade,Yugosla6ia
bFaculty of Chemistry,Uni
6ersity of Belgrade,Belgrade,Yugosla6ia
Received 17 February 1999; received in revised form 3 May 2000; accepted 3 May 2000
Abstract
We studied the guaiacol peroxidase activity, isoenzyme pattern and metal content in the needles of 2-year-old spruce grown on soils supplemented with cadmium concentrations from 1 to 21 mg kg−1. Following exposure to
cadmium, an initial increase and subsequent decrease in the activity of the soluble fraction was observed. A parallel change of their isoenzyme pattern occurred. An increase of the cell wall-bound peroxidase activity under prolonged metal treatment was evident. The results obtained show that peroxidase activity and isoenzyme pattern could be used to evaluate the capacity of one part of the defense system in spruce seedlings to withstand metal stress. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Cell walls; Heavy metals; Oxidative stress; Peroxidase;Picea abies
www.elsevier.com/locate/envexpbot
1. Introduction
Physiological processes in plants are affected by increased heavy metal contents in soils surround-ing the plant. One of the consequences of the presence of toxic metals in cells is the formation of free radical species, which can be initiated directly or indirectly by metals, and consequently cause severe damage to different cell components. Antioxidative systems, consisting of several non-enzymatic and non-enzymatic mechanisms, are
acti-vated in the cell as a response to different types of damaging conditions (Rabe and Kreeb, 1979; Hendry and Crawford, 1994; Hippeli and Elstner, 1996), and in particular to heavy metals (De Vos et al., 1992; Gallego et al., 1996; Chaoui et al., 1997; Ruc´inska et al., 1999; Sanita di Toppi and Gabrielli, 1999). Amongst them, peroxidases, both intracellular and extracellular (soluble and cell wall-bound) have an important role in the antioxidative response of plant cells (Castillo, 1986; Siegel and Siegel, 1986). Peroxidases can function as effective quenchers of reactive inter-mediary forms of oxygen and peroxy radicals induced by increased metal levels in the cell * Corresponding author.
E-mail address:[email protected] (K. Radotic´).
(Van gronsveld and Clijsters, 1994). The perox-idase reaction is unspecific towards the type of stress. Product of peroxidase-catalyzed polymer-ization of phenolic alcohols (coniferyl, synapyl, p-coumaryl alcohol) in the cell wall is a lignin polymer, the increase in lignin quantity being an indication of stress intensity (Castillo, 1986; Siegel and Siegel, 1986; Pandolfini et al., 1992; Polle and Chakrabarti, 1994). The capacity to synthesize peroxidases in tree leaves was used as a parameter for monitoring and mapping the defense of air pollution (Keller, 1974). The mechanism of perox-idase action in plants exposed to elevated concen-trations of heavy metals has not been completely elucidated, nor has the evaluation of the phyto-toxicity of metal contaminated soils been per-formed completely either.
In the published literature the effect of stress induced by heavy metals has been predominantly studied in plants either taken from the field (Polle and Chakrabarti, 1994) or grown in controlled conditions on hydroponic nutrient solutions (Brune and Dietz, 1995; Ouzounidou et al., 1995; Arduini et al., 1996; Weckx and Clijsters, 1996). Amongst them spruce grown in controlled envi-ronmental conditions on the soil has not been studied. In this work we studied the activity of intracellular and extracellular soluble peroxidases, and the activity of cell wall-bound peroxidases and the metal content in needles of spruce grown on soil supplemented with different cadmium con-centrations. More generally, the results obtained could particularly contribute to the knowledge on the effect of cadmium on the plant antioxidative enzymes, which response is activated in parallel with the synthesis of phytochelatins, another ma-jor quencher of heavy metals (Steffens, 1990; De Vos et al., 1992). It could be expected that such an approach may contribute to the under-standing of the plant response to more complex combination of external factors. An additional aim of this study was to determine whether it may be possible to use the peroxidase activity as one of the parameters for assessment of the plant antiox-idative state. Due to the presence of many modi-fying environmental factors, it is almost impossible to study the response of spruce needles to cadmium in adult plants in the field. Therefore,
we have examined the response of spruce seedlings to a range of Cd concentrations in con-trolled environmental conditions. We tried to show that under such conditions, there is a paral-lel change in general peroxidase activity and in-crease of cadmium in the needles of the spruce seedlings in response to cadmium stress.
2. Materials and methods
In the experiments we used 2-year-old spruce seedlings (Picea abies L.) grown in the green-house. Seedlings were transferred from the nurs-ery to the greenhouse on the 5th of November and grown singly in pots of 300 ml volume, in daylight and temperature between 20 and 26°C. The seedlings were grown on a soil composed of peat and compost (4:1 v/v) mixed with quartz sand (3:1 v/v). pH value of the soil was 6.6 before treatment. Soil was analyzed for the presence of cadmium prior to the planting and no increased concentrations of cadmium were detected. The treatment of the seedlings started on the 10th of March. During 2 weeks before application of cadmium, 50 mM Na – acetate buffer pH 4.3 was supplied to the soil in the pots where plants were grown, in order to buffer the soil and lower its pH value. After this period, different cadmium con-centrations from 1 to 21 mg kg−1 were added to the soil, in the form of CdSO4in 50 mM Na – ace-tate buffer, pH 4.3. After treatment the soil was watered with the same buffer instead of water. Seedlings were harvested after 1, 2, 15, 30 and 60 days of Cd treatment. Plants used for the experi-ments were healthy, without any exogenous infec-tion detected. Needles from the whole plant were used as experimental material. The influence of metal ions on the metal content, peroxidase activ-ity and isoenzyme pattern in the needles were monitored during 2 months after treatment, using atomic absorption spectroscopy, isoelectrofocus-ing and UV – VIS spectrophotometry.
Clijsters (1996). The homogenate was squeezed through eight gauze layers and centrifuged at 12 000×g and 4°C for 10 min. The supernatant was used for peroxidase activity measurements. The pellet was washed with distilled water and 50 mM Na – acetate buffer pH 5.5. After centrifuga-tion at 200×gfor 5 min, the pellet was sonicated for 5 s in the same buffer and centrifuged at 200×g for 5 min, according to the procedure of Otter and Polle (1994), Takahama and Oniki (1994). Obtained pellet was suspended in Na – ace-tate buffer and used for the measurement of the activity of the peroxidase bound fraction.
Peroxidase activity was determined with guaia-col as the substrate in a total volume of 3 ml. The assay mixture contained 50 mM acetate buffer, pH 5.5, 92 mM guaiacol, 18 mM H2O2 and variable amounts of the enzyme preparations. The turnover of guaiacol was monitored using Shi-madzu UV-160 spectrophotometer at 470 nm. Reaction rate was calculated from the extinction coefficient for guaiacol of 25.5 mM−1cm−1. The activity was referred to the protein content of each enzymic fraction, except for the whole activ-ity of the cell wall fraction referred to the cell wall dry weight.
Protein concentration of the enzymic fractions was determined by Lowry assay (Lowry et al., 1951) with bovine serum albumin as the standard.
Soluble peroxidase isoenzymes were separated by isoelectric focusing in a pH gradient from 3 to 9 (using 3% ampholite solution) on a 5% poly-acrylamide gel by staining with 20 mM guaiacol and 5 mM H2O2in Na – acetate buffer pH 5.5 for 10 min at 25°C.
For metal determination, needles were dried at 70°C for 12 h, and ashed in a muffle furnace at 500950°C for 6 h. The ash was dissolved in 2 ml HNO3 (1:3 v/v), evaporated and dried at 5009 50°C for 1 h. For measurement the ash was dissolved in 2 ml HCl (1:3 v/v) and metal content was determined by flame atomic absorption spec-trophotometry. The calculated detection limit was 190.5 mg Cd kg−1 dry needles.
Each experimental variant and control were represented by three to five seedlings, which were treated as replicates. Statistical analysis of the enzyme activity data was performed using the Kruskal – Wallis ranking test, at the 0.05 level of significance.
3. Results
There were no visible symptoms of the injury of seedlings exposed to 1 – 7.5 mg kg−1
Cd. Wilting of the needles treated with 15 and 21 mg kg−1Cd was observed after 30 and 60 days of treatment.
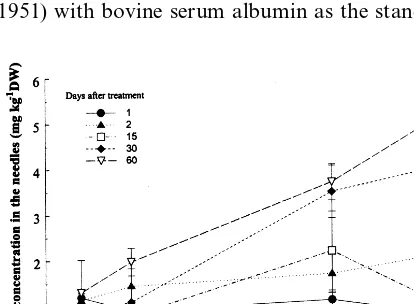
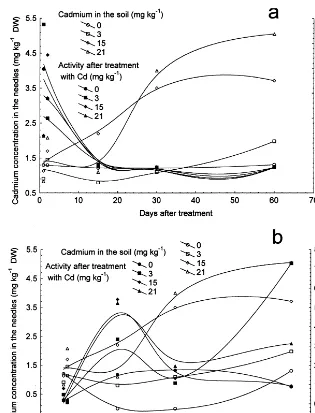
After 1 day of treatment with 7.5 – 21 mg kg−1 Cd and 2 days of treatment with 7.5 – 15 mg kg−1 Cd, 0.8 – 1.5 mg kg−1DW Cd was detected in the needles, which is within the calculated detection limit. In case of 2-day treatment with 21 mg kg−1 Cd in the soil, 2 mg kg−1Cd was detected in the needles, which is above detection limit (Fig. 1). As for long term treatments, in case of 7.5, 15 and 21 mg kg−1 Cd, 2 – 5.5 mg kg−1 DW Cd was de-tected in the needles treated 15 – 60 days, while in the case of lower metal concentrations there was no detectable amount of cadmium in the needles (Fig. 1).
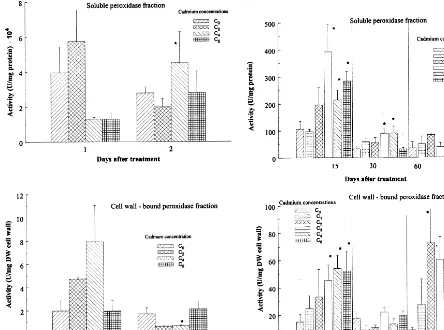
Fig. 2 presents the activity of both soluble and bound peroxidase fraction in the needles of plants exposed to different cadmium concentrations in the soil, in comparison with metal untreated plants, for various durations of treatment. Time change of activity of both peroxidase fractions, Fig. 1. Dependence of the cadmium concentration in the
Fig. 2. Specific activity of soluble and cell wall-bound peroxidase fraction from spruce leaves as a function of treatment duration, after (a) short-term and (b) long-term exposure to 1 (C1), 3 (C2), 7.5 (C3), 15 (C4) and 21 (C5) mg kg−1Cd; C0-metal untreated
leaves. * Statistically significant difference in activity in comparison with untreated plants.
for particular applied cadmium concentrations, is presented by histograms (Fig. 3). Soluble peroxi-dases, including intracellular and extracellular apoplastic enzymes, were found to have notably higher activity in comparison with cell wall-bound ones (Figs. 2 and 3).
Comparing short and long term treatments, it is worth noting a considerable decrease in the activity of soluble, and an increase in the activity of the bound peroxidase fraction, in both metal treated plants and metal untreated ones but grown on the acidified soil, during 15, 30 and 60 days, in comparison with 1- and 2-day treated ones (Figs. 2 and 3). The increase in soluble peroxidase activity with exposure time was
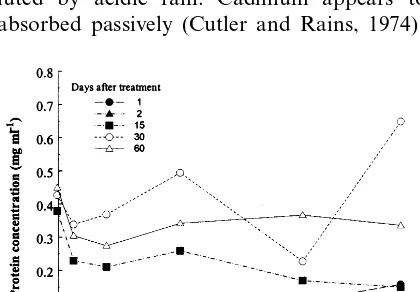
con-siderably more pronounced than the decrease of the bound fraction activity. There was an in-crease of the total protein concentration in the soluble fraction of the long-term treated plants in comparison with the short term treated ones, independent of the metal exposure (Fig. 5).
The change of peroxidase activity of both solu-ble and cell wall-bound fraction (Figs. 2 and 3) occurred in parallel with the raise of cadmium content in the needles exposed to long-term treat-ments with increasing cadmium concentrations in the soil (Figs. 1 and 4). A significant change in the activity of soluble peroxidase fraction was de-tected in time after treatment with 7.5, 15 and 21 mg kg−1 Cd, in comparison with untreated trees (Fig. 2). Lower metal concentrations did not in-duce significant activity changes in time. In the case of addition of 7.5 – 21 mg kg−1 Cd to the soil, an increase of soluble peroxidase activity was found in comparison to the control 15 days after treatment. In 30-day treated plants significant ac-tivity increase of the soluble fraction was observed in the case of 7.5 and 15 mg kg−1Cd in the soil.
After 60 days of treatment there was no signifi-cant difference in the soluble peroxidase activity in treated seedlings from the control plants. The effect was more pronounced 15 days after treat-ment (Fig. 2b). In case of cell wall-bound perox-idase fraction, a significant activity change in time occurred after treatment with 7.5 and 15 mg kg−1 Cd in the soil (Fig. 2b). There was no significant effect in case of lower metal concentrations, as well as after addition 21 mg kg−1Cd (Fig. 2b and Fig. 3). The increase in the activity of cell wall-bound peroxidase fraction, in comparison with the control, was statistically significant after treat-ment with 7.5, 15 and 21 mg kg−1
applied cad-mium 15 days after treatment, as well as in case of 3, 7.5 and 21 mg kg−1 Cd 60 days after treat-ment. After 30 days of treatment there was no significant difference from the control (Fig. 3b).
All detected soluble peroxidase isoenzymes were anionic ones, with pIvalues between 5 and 7 (Fig. 6). There was no change in the isoenzyme pattern of soluble peroxidases, in comparison with the control, in the seedlings treated with 1 and 3 mg kg−1 Cd, 1 and 2 days after treatment. In the plants treated with 15 and 21 mg kg−1
Cd, the isoenzyme with pI about 5 disappeared after 1 day of treatment and reappeared after 2 days of treatment, while an isoenzyme with pI about 6 disappeared after 2 days of treatment (Fig. 6a). Similarly to 1- and 2-day treated plants, there was no change in the isoenzyme pattern of soluble peroxidases, in comparison with the control, in the seedlings treated with 1 and 3 mg kg−1Cd 15, 30 and 60 days after treatment. In the case of 7.5 mg kg−1 Cd, an isoenzyme with pI 5.5 disap-peared 15 days after treatment, and a new anionic isoenzyme with pIabout 6 appeared 30 days after treatment, in comparison with the control. In case of 15 and 21 mg kg−1
Cd treatment, a new anionic isoenzyme with pI about 6 appeared 15 days after treatment, while an additional isoen-zyme with pI7 appeared 60 days after treatment. This change of isoenzyme pattern was more pro-nounced in the case of seedlings treated with 21 mg kg−1 Cd (Fig. 6b and c). The results of the isoenzyme pattern change were in accordance with the activity change in time in the plants treated with the higher metal concentrations. Fig. 3. Specific activity of soluble (a) and cell wall-bound (b)
Fig. 4. Parallel increase of cadmium concentration in the needles and change in peroxidase activity of soluble (a) and cell wall-bound (b) fraction with increasing cadmium concentration in the soil.
4. Discussion
Toxicity of metals in the soils depends on vari-ous physical and chemical soil parameters (pH, cation exchange capacity, chemical form of the metals, organic matter content etc.) and biological parameters, such as the presence of microorgan-isms that determine the metal availability to the plant. The fact that there was no effect of 1- and 2-day treatment with most of the used cadmium
activity significantly changed in the needles 2 days treated with 15 mg kg−1
Cd (Fig. 2a) and metal detected in the needles treated with 21 mg kg−1 Cd was above detection limit (Fig. 1), shows that the plants initiated changes of antioxidative de-fense system to long-term stress. Since the perox-idase activity (U) of the soluble fraction in case of long-term treatments was of the same order of magnitude in comparison with short-term treat-ments, considerable decrease of corresponding specific activity (U mg−1 protein) with treatment duration (Fig. 2) was mainly due to the increase of protein concentration in this fraction (Fig. 5). The effect of soil acidification itself, without metal treatment, on the activity of both peroxidase frac-tions, could be also explained through the in-creased protein content after long-term treatments. It was shown that soil acidification can influence the exchange of mineral nutrients between plant and soil (Schulze, 1989). Under natural conditions, soil acidification increases metal uptake by plants, and as such may be a precondition of their pronounced effect on plants. The effect of stress on biological systems depends on the ratio between stress intensity and buffer capacity of the soil. We used Na – acetate buffer to decrease pH of the soil. This buffer has a pK value of 4.7, which means that its highest buffer capacity falls within a pH range of the soil pol-luted by acidic rain. Cadmium appears to be absorbed passively (Cutler and Rains, 1974) and
translocated freely (Jarvis et al., 1976) in plants. Even if there is some complexation of acetate ions with cadmium, this should not influence the availability of cadmium ions in the reactions, since these complexes are not stable.
The results of long-term treatments showed that increasing cadmium concentration induced an increase of the capacity of the antioxidative defense system in the seedlings, alleviating the toxic effects of metal ions. The increase of perox-idase activity in treated seedlings is probably re-lated to oxidative reactions corresponding to an increase in peroxides and free radicals in the plant cells. Activation of oxygen has been proposed as a general reaction on several stress factors including metals, and reactive oxygen forms could be quenched by the induction of specific enzymes, one of them being peroxidase (Vangronsveld and Clijsters, 1991, 1994; Hippeli and Elstner, 1996). The induction of the antioxidative enzymes is one of the processes implicated in the regulation of the metal ion concentration in plants. Synthesis of phytochelatins is a parallel process that removes metal ions intracellularly (Steffens, 1990; De Vos et al., 1992). Cadmium complexed by chelating proteins was found in many higher plants (Steffens, 1990; Das et al., 1997). However, many metal-tolerant plants have not been found to ac-cumulate phytochelatins as a response to metal stress (Steffens, 1990). This was explained by the fact that overproduction of phytochelatins seems to be an unlikely mechanism for metal tolerance, owing to the energy required for sulfate reduction to support phytochelatin synthesis.
A cadmium concentration of 3 mg kg−1in the soil is considered to be a threshold value for plant toxicity, according to the criteria of the Economi-cal board of the United Nations for Europe and International Union of Biological Sciences (Inter-national Union of Biological Sciences, 1994). Our results show that, in case of spruce seedlings, soil cadmium concentrations up to 3 mg kg−1 do not cause any significant changes in the part of oxida-tive metabolism, as measured by induced changes in guaiacol peroxidase activity and isoenzyme pat-tern. Higher cadmium concentrations caused an increase in oxidative metabolism, and this can be caused by an increased quantity of free radicals Fig. 5. Total protein concentration in the soluble fraction from
Fig. 6. Isoelectrofocusing of soluble peroxidases in a pH gradient from 3 to 9. (a) Isoenzyme pattern 1 and 2 days after treatment with three cadmium concentrations; (b) Isoenzyme pattern 60 days after cadmium treatment, for all of the ana-lyzed cadmium concentrations; (c) isoenzyme pattern at three sampling dates, for the cadmium concentrations that were most effective in changing the peroxidase activity. Labeling is as in Fig. 2. The figure is composed from representative lanes of several gels.
change at higher cadmium concentration was fol-lowed by a change of the soluble peroxidase isoenzyme pattern, as well as with the accumula-tion of cadmium in the needles. Wilting of the needles was an exogenous symptom of toxicity in the plants treated with higher cadmium concen-trations. Our results showed that the initial in-crease of one part of antioxidative defense system capacity is related to de novo synthesis of the new soluble peroxidase isoenzymes, having a role in elimination of the reactive molecules. The new soluble isoenzymes (Fig. 6) probably do not pos-ses sufficient capacity for maintenance of protec-tive level of oxidaprotec-tive metabolism. On the other hand, the increased response of the bound peroxi-dases in case of prolonged metal stress (Figs. 3 and 4) may be linked to their role in lignin synthesis, one of the important protective reac-tions in the plant cell under the stress condireac-tions (Imberty et al., 1985; Pandolfini et al., 1992). It would be important to define the physiological threshold of different plants towards different kinds of stress, that is the stress intensity causing a decrease of oxidative metabolism and physiolog-ical functions. It has been shown that the perox-idase activity can be used as a potential biomarker for sublethal metal toxicity in examined plant species (Vangronsveld and Clijsters, 1991, 1994; Mocquot et al., 1996). Our results showed that in the spruce seedlings, even in case of 21 mg kg−1 Cd in the soil and corresponding 4 – 6 mg kg−1 Cd in the needles after 60-day treatment, mecha-nisms of antioxidative defense were active. Al-though studies on the physiology of metal toxicity are difficult, our data support the concept that monitoring of the oxidative metabolism parame-ters in different plant species should be an integral part of the evaluation and quantification of the effect of metal stress on plants.
Acknowledgements
This work was supported by the Ministry of Science and Technology of the Republic of Serbia. The authors are grateful to Prof Miodrag Jakovljevic´, Faculty of Agriculture of the Bel-grade University, for assistance in determining metal content in the needles.
References
Arduini, I., Godbold, D.L., Onnis, A., 1996. Cadmium and copper uptake and distribution in mediterranean tree seedlings. Physiol. Plant. 97, 111 – 117.
Brune, A., Dietz, K.-J., 1995. A comparative analysis of element composition of roots and leaves of barley seedlings grown in the presence of toxic cadmium, molybdenum, nickel, and zinc concentrations. J. Plant Nutr. 18, 853 – 868.
Castillo, F.J., 1986. Extracellular peroxidases as markers of stress? In: Grepin, H., Penel, C., Gaspar, T. (Eds.), Molec-ular and Physiological Aspects of Plant Peroxidases. Uni-versity of Geneva, Geneva, pp. 419 – 426.
Chaoui, A., Mazhoudi, S., Ghorbal, M.H., El Ferjani, E., 1997. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus6ulgarisL.). Plant Sci. 127, 139 – 147.
Cutler, J.M., Rains, D.W., 1974. Characterization of cadmium uptake by plant tissue. Plant Physiol. 54, 67 – 71. Das, P., Samantaray, S., Rout, G.R., 1997. Studies on
cad-mium toxicity in plants: a review. Environ. Pollut. 98, 29 – 36.
De Vos, C.H.R., Vonk, M.J., Vooijs, R., Schat, H., 1992. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress inSilene cucublaus. Plant Physiol. 98, 853 – 858.
Gallego, S.M., Benavides, M.P., Tomaro, M.L., 1996. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 121, 151 – 159. Hendry, G.A.F., Crawford, R.M.M., 1994. Oxygen and
envi-ronmental stress in plants — an overview. Proc. R. Soc. Edinburgh 102B, 1 – 10.
Hippeli, S., Elstner, E.E., 1996. Mechanisms of oxygen activa-tion during plant stress: biochemical effects of air pollu-tants. J. Plant Physiol. 48, 249 – 257.
Imberty, A., Goldberg, R., Catesson, A.-M., 1985. Isolation and characterization ofPopulusisoperoxidases involved in the last step of lignin formation. Planta 164, 221 – 226. International Union of Biological Sciences, 1994. Element
concentration cadasters in ecosystems, Progress report, 25th General Assembly, Paris
Jarvis, S.C., Jones, L.H.P., Hopper, M.J., 1976. Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil 44, 179 – 191.
Keller, T., 1974. The use of peroxidase activity for monitoring and mapping air pollution areas. Eur. J. For. Pathol. 4, 11 – 19.
Lowry, O., Rosebrough, W., Farr, A., Randall, R., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265 – 275.
Mocquot, B., Vangrosveld, J., Clijsters, H., Mench, M., 1996. Copper toxicity in young maize (Zea mays L.) plants:
effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 182, 287 – 300.
Otter, T., Polle, A., 1994. The influence of apoplastic ascor-bate on the activities of cell wall-associated peroxidase and NADH oxidase in needles of norway spruce (Picea abies L.). Plant Cell Physiol. 35, 1231 – 1238.
Ouzounidou, G., C&iaporova, M., Moustakas, M., Karataglis, S., 1995. Responses of maize (Zea mays L.) plants to copper stress — I. Growth, mineral content and ultra-structure of roots. Environ. Exp. Bot. 35, 167 – 176. Pandolfini, T., Gabrielli, R., Comparini, C., 1992. Nickel
toxicity and peroxidase activity in seedlings of Triticum aesti6umL. Plant Cell Environ. 15, 719 – 725.
Polle, A., Chakrabarti, K., 1994. Effects of manganese defi-ciency on soluble apoplastic peroxidase activities and lignin content in needles of Norway spruce (Picea abies). Tree Physiol. 14, 1191 – 1200.
Rabe, R., Kreeb, K.H., 1979. Enzyme activities and chloro-phyll and protein content in plants as indicators of air pollution. Environ. Pollut. 19, 119 – 136.
Ruc´inska, R., Waplak, S., Gwozdz, E.A., 1999. Free radical formation and activity of antioxidant enzymes in lupin roots to lead. Plant Physiol. 37, 187 – 194.
Sanita di Toppi, L., Gabrielli, R., 1999. Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105 – 130. Schulze, E.D., 1989. Air pollution and forest decline in a
spruce (Picea abies) forest. Science 244, 776 – 783. Siegel, S.M., Siegel, B.Z., 1986. Peroxidase activity and stress:
a complex relationship. In: Grepin, H., Penel, C., Gaspar, T. (Eds.), Molecular and Physiological Aspects of Plant Peroxidases. University of Geneva, Geneva, pp. 427 – 431. Steffens, J.C., 1990. The heavy metal-binding peptides of plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 553 – 575.
Takahama, U., Oniki, T., 1994. Effects of ascorbate on the oxidation of derivatives of hydroxycinnamic acid and the mechanism of oxidation of sinapic acid by cell wall-bound peroxidases. Plant Cell Physiol. 35, 593 – 600.
Vangronsveld, J., Clijsters, H., 1991. A biological test system for the evaluation of metal phytotoxicity and immobiliza-tion by additives in metal contaminated soils. In: Merian, E., Haerdi, W. (Eds.), Metal Compounds in Environment and Life (Interrelation Between Chemistry and Biology), vol. 4. Science and Technology Letters, Northwood, pp. 117 – 125.
Vangronsveld, J., Clijsters, H., 1994. Toxic effects of metals. In: Farago, M.E. (Ed.), Plants and the Chemical Elements — Biochemistry, Uptake, Tolerance and Toxicity. VCH, Weinheim, pp. 149 – 177.
Weckx, J.E., Clijsters, H.M.M., 1996. Oxidative damage and defense mechanisms in primary leaves ofPhaseolus6ulgaris
as a result of root assimilation of toxic amounts of copper. Physiol. Plant. 96, 506 – 512.