251 (2000) 85–102
www.elsevier.nl / locate / jembe
The respiratory performance and survival of the bivalve
Macoma balthica (L.) at the southern limit of its distribution
area: a translocation experiment
Centre for Estuarine and Coastal Ecology, Korringaweg, Netherlands Institute of Ecology, 4401 NT Yerseke, The Netherlands
b
´ ´
Universite Bordeaux I, Laboratoire d’Oceanographie biologique, UMR 580 CNRS, 2, Rue du Professeur Jolyet, 33120 Arcachon, France
c
Hendaye Bidassoa Environnement, P.O. Box 529, 64705 Hendaye, France
d
Universidad del Pais Vasco, Facultad de Pedagogica, Departamento de Didactica y Organizacion Escolar, Avenido de Tolosa 70, 20009 Donostia, San Sebastian, Spain
e
´ ´
Universite de Nantes, Faculte de Pharmacie, Laboratoire d’Ecotoxicologie, 1, Rue Gaston-Veil, 44035 Nantes Cedex, France
Received 21 May 1999; received in revised form 9 March 2000; accepted 28 March 2000
Abstract
The hypothesis was tested that animals near their extreme Southern limits, due to high temperatures, have a high respiration rate, whereby they reach an extreme low weight-index and ultimately disappear. At estuarine stations the respiration rate of Macoma balthica (L.) (Baltic clam) did not show interseasonal changes, indicating seasonal acclimation, but within the season the respiration increased with increasing temperature, indicating the absence of short-term acclimation. In clams translocated from the Netherlands towards the Bidasoa estuary, 200 km South of their Southern distribution limit, their respiration rate was higher and weight-index lower than in specimens living in Dutch estuaries. Irrespective of an effect of the temperature, clams exposed in experiments to water from Bidasoa showed a higher respiration than clams exposed to water from the other stations. Moreover, at non-estuarine stations with a low food content, the clams showed reversed acclimation, i.e., the respiration rates in winter were much lower than summer rates, most probably a strategy to conserve energy by means of a depressed metabolism.
3
A weight index of 5 mg DW/ cm and glycogen content of 2% DW are suggested as the minimal values below which the metabolic energy balance of Baltic clams becomes more negative and the
*Corresponding author. Tel.: 131-113-577-484; fax 131-113-573-616. E-mail address: [email protected] (H. Hummel)
1
Tel.: 133-556-223-908; fax: 133-556-835-104.
2
Tel.: 134-943-448-000, ext. 5617; fax 134-943-311-056(1055).
clam population disappears. It was concluded that factors other than temperature influenced the respiration and weight-index of clams, and hence their presence or absence, e.g., food con-centration, innate seasonal cycles, and possible pollutants in the water. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Macoma balthica; Distribution limit; Performance; Survival; Respiration; Weight; Glycogen
1. Introduction
The Baltic clam Macoma balthica (L.) is in NW Europe a widespread and often dominant species in the coastal benthic zone with highest densities in muddy or silty intertidal estuarine and coastal areas. Its current Southern distribution limit is located near the Gironde (Bachelet, 1980). In the 1960s the clams were still found more to the South in Galicia, Spain (Otero and Millan, 1970). Genetically all clam populations along the Atlantic coast line from South (South-West France) to North (Murmansk in Russia),
¨ ¨ ¨
belong to one and the same race or species (Vainola and Varvio, 1989; Hummel et al., 1997).
Near the Southern distribution limit, the Baltic clam shows a poor performance. A reduced genetic diversity was observed, due to differential selection of some genotypes, as well as a reduced ecophysiological performance as was measured by means of a reduced weight, energy reserves, bio-indicators and stress resistance (Hummel et al., 1996a,b, 1997). This corresponds to the general theorem that marginal populations are living at the limits of their adaptation capacities (Conover, 1978; Hoffmann and Parsons, 1991). Yet, for the Baltic clam it is not clear which environmental and / or innate ecophysiological factors are of decisive importance.
For the Baltic clam the major ecophysiological and environmental factors involved might be summarized in two groups. The first set of factors is based on temperature interrelated effects on the energy budget in many marine species (Newell, 1979; Bayne et al., 1985). A high temperature may result in a high metabolic rate, thus high respiration, and consequently high food demand, whereas this will lead, if food is limiting, to a low weight-index and low level of energy reserves. This will minimize the chances of survival during harsh periods, e.g., periods of low food availability.
and Beukema, 1997; Honkoop and van der Meer, 1997, 1998). A higher temperature in winter results in a higher energy demand (metabolism) whereby the weight-index decreases and the gonadal tissues may partly or completely be resorbed, and subsequent spawning and recruitment may then be reduced or completely absent.
Temperature is therefore a major factor for the occurrence or disappearance of a species near its distribution limit. Our working hypothesis was that the limiting condition for Baltic clams is related to temperature through its impact on the energy budget. We would expect that animals translocated South of their area of distribution, confronted with temperatures higher than they are able to adapt to, show a higher respiration rate, lower weight and lower level of glycogen, than within their area of distribution.
To test the above hypothesis, we carried out a translocation experiment of animals from the Netherlands to areas South of their distribution limit, where they have not been regularly observed during the last decades, i.e., the Bay of Arcachon and the Bidasoa estuary at the French–Spanish border. The respiration rate, weight index and glycogen reserves were followed in these animals.
Additionally, some animals of the same stock were translocated to the Grevelingen, a brackish lake in the Netherlands. In this lake, Baltic clams disappeared in the last two decades after some changes of the tidal regime, whereas mussels and cockles are still present in the same territory. A change of temperature and salinity in this lake did not occur, and thus the clams must have disappeared from this region due to still another reason, which may help to explain the disappearance of clams near the Southern limit. Before starting these translocation experiments a preliminary test was carried out to assess the performance of clams when translocated over longer distances between the Netherlands and France, i.e., within the distribution area. If the translocated clams showed a strongly dissimilar ecophysiological performance (weight index, glycogen reserves, etc.) from local specimens, then the translocation experiments would not be valid. These preliminary experiments showed that clams adapted within 1 month to the local situation.
In addition, two different types of laboratory experiments were performed. Firstly, each season two sub-groups of clams from the Netherlands were kept for 4 weeks at two different temperatures in the laboratory in order to assess the potential of these animals to acclimatize (their respiration rate) in a relatively short period to a changing temperature. Secondly, on the basis of the first results of the translocation experiments a second type of laboratory experiments was performed to assess the effect of local (substrate- or water-bound) factors on the respiration and weight index of clams. To this end, clams from the Netherlands (Paulina) were exposed to sediment and unfiltered water from Arcachon, Bidasoa and Grevelingen.
2. Material and methods
Fig. 1. Location of the sampling stations (open squares5used in preliminary experiment with clams from Dortsman, the Netherlands, translocated to France within their area of distribution (1991); filled circles5used in experiment with clams from Paulina, the Netherlands, translocated to France South of their distribution limit and to Grevelingen).
digestive tract, frozen in liquid nitrogen, lyophilized during 3 days, and further treated for analysis of the weight index (as explained below), the copper content (according to Amiard et al., 1987), glycogen (according to Keppler and Decker, 1974), and free amino acids content (according to Hummel et al., 1996a).
in dry cloths on top of melting ice blocks in a cooling box. The animals (1000 specimens at each location) were distributed just above mean low water level over an
2
area of 16 m at the Banc d’Arguin at the entrance of the Bay of Arcachon (44835.29N, 1814.19W; salinity526–32, and temperature512–238C, during sampling), and in the Bidasoa estuary (43822.09N, 1846.39 E; salinity59–28, and temperature510–248C, during sampling), see Fig. 1.
At intervals of 3 months some translocated specimens were collected. They were transported in water taken at the sampling location and kept at constant temperature in a Dewar isolation vessel. Within 4 h after sampling the respiration rate of ten clams was measured in respiration chambers of 0.13 l over 1–2 h at two temperatures (10 and 208C; in winter at 3 and 108C; simultaneously, one set of ten animals per temperature). For each measurement a simultaneous blank determination of oxygen consumption was made in respiration chambers with the same water at the same temperature, but without the animals. The decrease in oxygen tension was measured at intervals of 10 min by YSI 5331 Oxygen Probes (Clark type polarographic electrodes). Subsequently, the animals were frozen and lyophilized during 3 days for further analysis of the soft tissue weight, the weight index and energy reserves (glycogen). The respiration rate was calculated from the tangent of the line along the series of 6–12 measurements on the decrease of the oxygen concentration in the respiration chamber with time, and corrected for the oxygen consumption in the blank. One series of measurements with ten animals gave only one value, and thus no standard errors could be calculated.
The weight index was measured for all animals individually as the dry tissue weight 3
after lyophilizing per volume (volume calculated from (length) ; Hummel et al., 1996b). Glycogen was analyzed in the groups of ten animals together, according to Keppler and Decker (1974).
Additional samples were translocated from the Westerschelde (Paulina) to the brackish lake Grevelingen (51842.19N, 48 07.89E, salinity59–28). The animals were sampled from the same stock, and translocated and treated at the same dates and according the same methods as those translocated to Arcachon and Bidasoa.
To test the acclimatization potential of clams to temperature, two sub-groups of clams were kept in the laboratory in a layer of 10 cm sediment and filtered Oosterschelde water (salinity523–34; with a continuous flow of algal culture, Phaeodactylum tricornutum, into the water to give a nominal average seston concentration of 2.2 mg / l) over 4 weeks at a constant temperature of 10 and 208C (in winter 3 and 108C). After this period the respiration, weight index and glycogen was measured as described above. This experiment was repeated each season, parallel to the measurements in the translocation experiment.
A second type of laboratory experiment was performed in May and June 1997 on the basis of the first results of the translocation experiments, indicating that not only temperature caused an increased respiration. Clams from the Westerschelde (Paulina) were exposed at a temperature of 158C to unfiltered water and sediment from Arcachon, Bidasoa and Grevelingen [sediments (mix from top 2 cm) and water were transported in jerrycans of 5 l and stored at 48C]. This in order to assess the effect of local ‘substrate’-bound factors on the respiration, glycogen and weight index of clams.
translocation and exposure experiments were statistically analysed with ANOVA (Sokal and Rohlf, 1995) by the Systat programme (Wilkinson, 1988).
3. Results
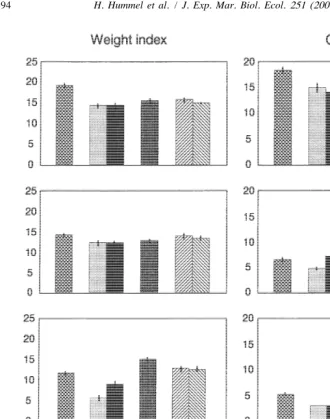
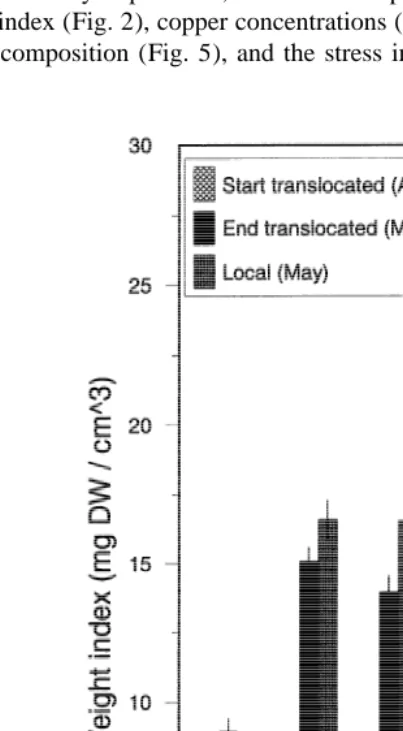
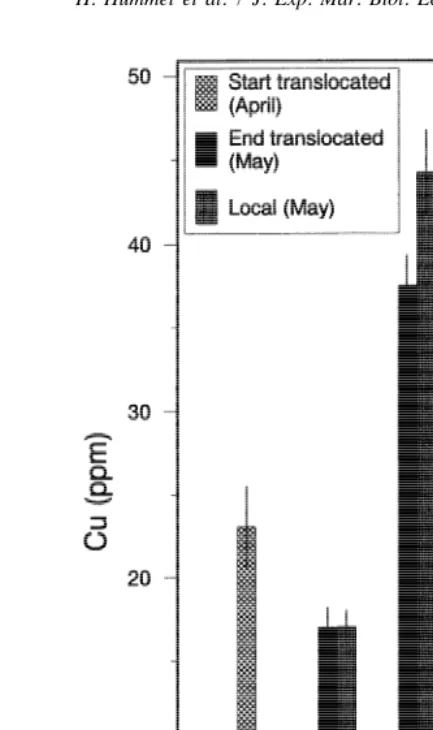
In the preliminary experiment, the measured parameters of the translocated animals, i.e., weight index (Fig. 2), copper concentrations (Fig. 3), glycogen content (Fig. 4), free amino acid composition (Fig. 5), and the stress indicator taurine / glycine ratio (Fig. 5),
3
Fig. 3. The copper concentration (mg / g DW; average and standard error, N510) of translocated clams in the preliminary experiments at the beginning of, and 1 month after, translocation in comparison to the endemic (local) specimens (dates and abbreviations as in Fig. 2).
strongly resembled the values found in the specimens from the local populations within 1 month (mid April to mid May).
The animals translocated in May 1996 to Arcachon and Bidasoa were recovered in August in original densities (hardly empty shells found), and in November 1996 and February 1997 in low densities (many empty shells). In May 1997 no animals were recovered. It is not known whether the animals emigrated from the translocation plot, died or were eaten. At Grevelingen higher densities were found till the end of the experiment (May 1997).
Fig. 4. The glycogen concentration [% DW; average and standard error, N52 (two groups of ten animals)] of translocated clams in the preliminary experiments at the beginning of, and 1 month after, translocation in comparison to the endemic (local) specimens (dates and abbreviations as in Fig. 2).
to February 1997 the weight-index and glycogen decreased at the translocation stations and at the station of origin (Fig. 6). The index was always the lowest, and the decrease of the index strongest, at the Southern stations Arcachon and Bidasoa. In Grevelingen the weight-index started to increase again from February 1997; in Paulina from May 1997 (no data available on Arcachon and Bidasoa). There was no significant difference in weight between specimens kept for 1 month in the laboratory at the higher temperature (M-hi) or at the lower temperature (M-lo)(ANOVA, P50.10).
3
Fig. 6. Weight-index (dry tissue weight per volume5mg DW/ cm ; average and standard error, N520) and glycogen content (% DW; average and standard error, N52, two groups of ten animals, for M-lo and M-hi N51 group of ten animals) of clams kept in the translocation experiment (Pau5Paulina, Arc5Arcachon, Bid5Bidasoa, Gre5Grevelingen, M-lo5Mesocosm-laboratory low-temperature (108C, winter 38C), M-hi5
Mesocosm-laboratory high temperature (208C, winter 108C); the clams from the mesocosm-laboratory were kept for 1 month before measuring the respiration already at the testing temperature, and thus could acclimatize, yet with a low level of food).
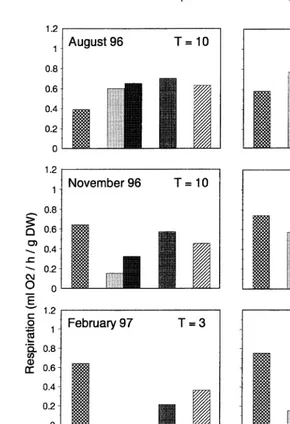
Fig. 7. Respiration rate (ml O / g DW/ h) of clams kept in the translocation experiment (in the field) or in the2
laboratory experiment (abbreviations as in Fig. 6).
tested at ambient temperatures, with slightly lower respiration (0.4 ml O / h / g DW) at2 lower temperatures, and higher respiration (0.8 ml O / h / g DW) at higher temperatures2 (Fig. 8).
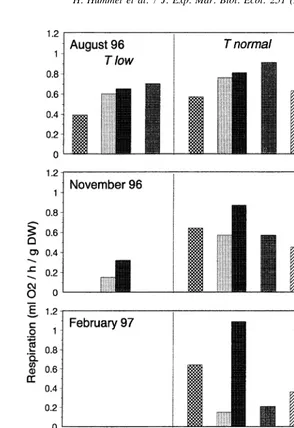
Fig. 8. Respiration rate of clams. Same data as in Fig. 7, but now arranged into groups with regard to difference between test temperature and ambient field temperature (T normal5test temperature was ,38C lower or higher than ambient temperature, T low5test temperature was .38C lower than ambient temperature, T high5test temperature was .38C higher than ambient temperature) (abbreviations as in Fig. 6).
Table 1
Significance of the differences in respiration rate (ml O / h / g DW) between clams kept at Paulina and those2
translocated to other stations (5effect of station), between seasons at a station (5effect of season), and the interaction of differences [indicating changes in the sign of differences between stations throughout the seasons
a
clams at Arcachon and Grevelingen decreased to a much lower level than the controls at Paulina (Fig. 8; November, February). Whereas at Bidasoa the respiration at ambient temperatures remained as in Paulina stable, yet at a higher level. In the laboratory, the animals that were allowed to acclimatize during 1 month, the respiration in winter decreased as did that of the translocated specimens at Arcachon and Grevelingen (Fig. 8).
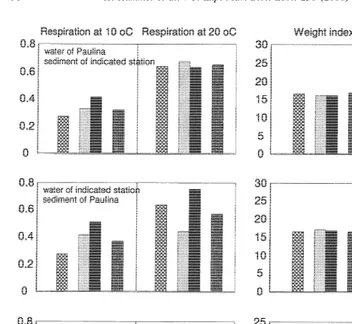
In the test with different sediment and water types, the weight index and glycogen content of the clams did not differ between treatments (Fig. 9; ANOVA, P.0.05). The respiration was higher at the higher test temperatures (ANOVA, P,0.01). A significant difference in the respiration rate occurred also in case water from the different stations was used (Fig. 9; ANOVA, P,0.05), with a consistently higher respiration in the clams exposed to water from Bidasoa.
4. Discussion
From the preliminary tests it could be concluded that translocation of M. balthica over longer distances would cause no problems, and that these clams would within a short period reflect the local situation. Thus, when translocating them South of their distribution limit, as we did in the subsequent series of translocation experiments, the clams might also reflect the conditions encountered several decades ago by the endemic specimens at extreme locations.
The higher temperatures in those Southern areas had most probably no direct lethal effect on the clams because the upper temperature tolerance of M. balthica is far above 308C, in summer even up to 408C (Kennedy and Mihursky, 1971; McMahon and Wilson, 1981; Wilson, 1981). Since the water temperature in the Bidasoa estuary ranges from 10 to 268C (Sola, 1994; 10 to 248C in this study) there would in principle be no direct reason for the clams not to be present in the Bidasoa. As the results of our experiments indicated, the adverse effect of a higher temperature and some other factors becomes only notable at the longer term, i.e., after more than half a year.
These adverse effects were clearly visible from the reduction in the weight index and glycogen content. The lowest values in the Southern translocation stations, around 5 mg
3
3
Fig. 9. Respiration rate (ml O / g DW/ h) and weight-index (mg DW/ cm , averages, N2 510, standard errors were too small to be indicated, on average for weight index 0.3 and for glycogen 0.5) of clams from Paulina kept during 4 weeks at a temperature of 158C in sediments and / or water from the translocation stations (abbreviations as in Fig. 6).
found at the end of the winter for clams from the Southern French stations Gironde and Loire (i.e., at the border of their Southern distribution; Hummel et al., 1996b; at more Northern French and Dutch stations the weight index remained higher than 8 mg
3
DW/ cm and glycogen above 5% DW, Hummel et al., 1996b; this study). These values thus most probably reflect the situation at which clams are still able to meet the most minimal metabolic requirements. Similarly, Honkoop and van der Meer (1997) reported that below a weight index of 5.6 the clams are not able to produce anymore gametes. This suggests that any further decline below the reported values would disrupt the metabolic energy balance and lead to high mortalities and local die out of the clam population.
with those reported for clams by Kennedy and Mihursky (1972; 0.05–3.6), de Wilde (1975; 0.1–1.0), Wilson (1981; 0.05–0.6), McMahon and Wilson (1981; 0.05–0.6), and Wilson and Elkaim (1991; 0.05–1.0).
The respiration rate increased at increasing temperature, as was found in all of the before mentioned studies within the normal ambient range of 5–258C. No acclimation occurred in the short term (hours, days), whereas in the longer-term (weeks, seasons) seemingly contradictory results were reported. Seasonal acclimation to changes in temperature was found (Kennedy and Mihursky, 1972), as well as the absence of seasonal acclimation (McMahon and Wilson, 1981; Wilson and Elkaim, 1991). In the last case the phenomenon of reverse acclimation was mentioned, i.e., winter respiration rates are lower than summer rates. This strategy has been found in several other bivalves (see Table 1 in Wilson and Elkaim, 1991) and is suggested to be due to low food availability in the winter when the metabolism is depressed to conserve energy.
In this study both phenomena, normal acclimation and reverse acclimation of respiration, occurred, depending on the season and the character of the station. At the estuarine stations Paulina and Bidasoa, with relatively turbid water (seston concentration at Paulina is on average 46 mg / l, Rijkswaterstaat, 1998; in Bidasoa 13.1 mg / l, Borja et al., 1996), the clams showed all year round acclimation to seasonal changes in temperature. At the stations with low levels of suspended matter, Arcachon and Grevelingen (seston concentrations on an average 5 and 3 mg / l; Manaud et al., 1997; Rijkswaterstaat, 1998), as holds also for the animals kept in the experimental mesocosms (2.2 mg / l), reverse acclimation occurred in winter. Since the previous researchers may have been testing clams coming from different environmental conditions, the results may have seemed to be contradictory.
The resemblance of the seasonal changes in respiration (reverse acclimation), weight-index and glycogen between clams translocated to Arcachon and Grevelingen, and those acclimated for 1 month in the laboratory, is thus probably connected to low food levels. Obviously a seasonal change in the total ecophysiology of the clams occurs in such a way that during spring and summer the clams will compensate for low levels of food by vigorous pumping (and thus respiring), whereas during autumn and winter they will be in a more dormant stage adapted to low food levels with a low pumping activity. Such a seasonal adjustment of the activities in clams was indeed shown before (Hummel, 1985), with the lower food concentrations high clearance rates (up to 1.2 ml / h / g) during summer and low clearance rates (around 0.1 ml / h / g) during Autumn / Winter, whereas at the higher food concentrations intermediate pumping rates (0.3 to 0.5 ml / h / g) were found all year round. Thus, the changes in clearance rates, and thereby the higher respiration in summer and decreasing respiration in Autumn and Winter for the clams from Arcachon, Grevelingen and the mesocosm experiments, can now be explained from an innate fixed seasonal pattern of changes in the gills and palps in reaction to food concentrations.
more so than in the Grevelingen, explaining the lower weight-index at Arcachon. Such is in agreement with our hypotheses.
The continuously higher respiration rates at Bidasosa, a territory with higher food concentrations, can then still not be understood; a similar stable intermediate respiration rate as found for Paulina should have been expected.
As shown in the sediment / water test, a higher respiration rate was found for clams kept in water from Bidasoa. Presumably a stressing factor existed in the water from Bidasoa. The character of this stressing factor is not known, salinity may be excluded since the salinity of the Bidasoa and Paulina station fell in the same range. Contaminants as an influencing factor can not be excluded. Rather high levels of heavy metals in sediments, e.g., copper and lead at 71 and 120 ppm, respectively, were measured in the Bidasoa, leading to high mortalities in oyster larvae tests when the sediment was resuspended in the water (Geffard, 1997). Sola et al. (1991) reported that the Bidasoa in 1987 belonged to the estuaries in North Spain with a relatively lower degree of pollution; the other surrounding estuaries had often two orders higher concentration of metals (metal concentrations in the order of permilles). Thus, the northern Spanish estuaries are heavily contaminated. Further studies are necessary to resolve this matter. These studies should then also include measurements of the respiration rate and weight-index in translocated Baltic clams from unpolluted embayments (if possible to find) nearby Bidasoa.
Once the clams disappear from an estuary like Bidasoa, resettlement with clams might be difficult since other resource areas are not nearby, because the South-West coastal zone of France mainly consists of, for clams, unsuitable sandy beaches, and as discussed above the silty and muddy tidal flats of estuaries and embayments are often heavily contaminated.
In summary, as hypothesized, in the Bidasoa estuary, 200 km South of the current Southern distribution limit of the clam, the respiration of the translocated clams was indeed all year round relatively high (30–50% higher than in Paulina), and the weight-index and glycogen reserve content decreased to extremely low levels, resulting ultimately in the disappearance of clams from this estuary. However, temperature is not the only influencing factor, as was also found for the clams translocated to other stations. It may be concluded that the coastal zone of South-West France (Bay of Biscay) does not promote the presence of Baltic clams, because of: (1) a relatively high temperature, and consequently a higher respiration and low weight-index and glycogen content in clams; (2) (outside the estuaries) clear water, and low food availability, which (because of innate ecophysiological processes) induce in summer higher and in winter lower respiration rates in clams (5reverse acclimation); (3) a stressing factor, probably contaminants, that may occur in the water of some estuaries towards Spain, as e.g., for the Bidasoa, inducing higher respiration rates; (4) a couple of hundred kilometers of sandy beaches along the South-West coast, without considerable embayments or estuaries that might be a refuge and source of clams.
Acknowledgements
This study, CEMO communication no. 2627, was made possible by several grants for French–Dutch cooperation from the former Netherlands Marine Research Foundation (SOZ) and the Geosciences Foundation (GOA) (presently Council for Earth and Life sciences, ALW). Special thanks are due the owner Philippe Berra of the hotel Santiago, Hendaye (France), who allowed us several times to transform his dining hall into a laboratory, Xavier de Montaudouin for helping us in the field, and Edouard His for environmental data. [RW]
References
Amiard, J.C., Amiard-Triquet, C., Berthet, B., Metayer, C., 1987. Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms. J. Exp. Mar. Biol. Ecol. 106, 73–89.
Bachelet, G., 1980. Growth and recruitment of the tellinid bivalve Macoma balthica at the Southern limit of its geographical distribution, the Gironde estuary (SW France). Mar. Biol. 59, 105–117.
Bayne, B.L., Brown, D.A., Burns, K., Dixon, D.R., Ivanovici, A., Livingstone, D.R., Lowe, D.M., Moore, M.N., Stebbing, A.R.D., Widdows, J., 1985. In: The Effects of Stress and Pollution On Marine Animals, Preager Publishers, New York, p. 384.
Beukema, J.J., Meehan, B.W., 1985. Latitudinal variation in linear growth and other shell characteristics of Macoma balthica. Mar. Biol. 90, 27–33.
´ Borja, A., Valencia, V., Uriarte, A., 1996. La Calidad De Aguas Para Cultivo De Moluscos En El Paıs Vasco,
˜ ´
Seis Anos De Seguimento (1990–1995), Informes Tecnicos, Vol. No. 74, Servicio Central de Piublicaciones del Gobierno Vasco, Vitoria.
Caddy, J.F., 1967. Maturation of gametes and spawning in Macoma balthica (L.). Can. J. Zool. 45, 955–965. Conover, R.J., 1978. Transformation of organic matter. In: Kinne, O. (Ed.), Marine Ecology, Dynamics, Vol.
IV, John Wiley and Sons, Chichester, pp. 221–499.
´ ´ ´ `
Geffard, O., 1997. Mise en evidence de la toxicite potentielle des sediments marins a l’aide des embryons et des larves de Crassostrea gigas et de Paracentrotus lividus. D.E.A. In: Toxicologie de L’environnement,
´
Universite de Metz-Rouen-Strasbourg, France, p. 48.
Hoffmann, A.A., Parsons, P.A., 1991. In: Evolutionary Genetics and Environmental Stress, Oxford University Press, New York, p. 284.
Honkoop, P.J.C., Beukema, J.J., 1997. Loss of body mass in winter in three intertidal bivalve species: an experimental and observational study of the interacting effects between water temperature, feeding time and feeding behaviour. J. Exp. Mar. Biol. Ecol. 212, 277–297.
Honkoop, P.J.C., van der Meer, J., 1997. Reproductive output of Macoma balthica populations in relation to winter-temperature and intertidal-height mediated changes of body mass. Mar. Ecol. Prog. Ser. 149, 155–162.
Honkoop, P.J.C., van der Meer, J., 1998. Experimentally induced effects of water temperature and immersion time on reproductive output of bivalves in the Wadden Sea. J. Exp. Mar. Biol. Ecol. 220, 227–246. Hummel, H., 1985. Food intake and growth in Macoma balthica (Mollusca) in the laboratory. Neth. J. Sea
Res. 19, 77–83.
Hummel, H., Amiard-Triquet, C., Bachelet, G., Desprez, M., Marchand, J., Sylvand, B., Amiard, J.C., Rybarczyk, H., Bogaards, R.H., Sinke, J., de Wolf, L., 1996a. Free amino acids as a biochemical indicator of stress in the estuarine bivalve Macoma balthica. Sc. Total Environ. 188, 233–241.
Hummel, H., Bogaards, R., Bek, T., Polishchuk, L., Amiard-Triquet, C., Bachelet, G., Desprez, M., Strelkov, P., Sukhotin, A., Naumov, A., Dahle, S., Denisenko, S., Gantsevich, M., Sokolov, K., de Wolf, L., 1997. Sensitivity to stress in the bivalve Macoma balthica from the most northern (Arctic) to the most Southern (French) populations: low sensitivity in Arctic populations because of genetic adaptations? Hydrobiologia 355, 127–138.
Kennedy, V.S., Mihursky, J.A., 1971. Upper temperature tolerances of some estuarine bivalves. Chesapeake Sci. 12, 193–204.
Kennedy, V.S., Mihursky, J.A., 1972. Effects of temperature on the respiratory metabolism of three Chesapeake Bay bivalves. Chesapeake Sci. 13, 1–22.
Keppler, D., Decker, K., 1974. Glycogen determination with amyloglucosidase. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis, Vol. Part 3, Academic Press, New York, pp. 1127–1131.
Lammens, J.J., 1967. Growth and reproduction in a tidal flat population of Macoma balthica (L.). Neth. J. Sea Res. 3, 315–382.
Manaud, F., Bouchet, J.M., Deltreil, J.P., Maurer, D., Trut, G., Auby, I., Dreno, J.P., Masson, N., Pellier, C., ´ ´
L’Yavanc, J., 1997. Etude Integree du Bassin d’Arcachon, Ifremer Report DEL /AR / RDN / 1997-09. McMahon, R.F., Wilson, J.G., 1981. Seasonal respiratory responses to temperature and hypoxia in relation to
burrowing depth in three intertidal bivalves. J. Therm. Biol. 6, 267–277.
Newell, R.C., 1979. In: Biology of Intertidal Animals, Marine Ecological Surveys Ltd, Faversham, p. 781. Otero, J.H., Millan, F.J., 1970. Distribucion de los moluscos: gasteropodos y pelecipodos, marinos, de las
costas de Galicia. Cuad. Biol. 1, 79–93.
Rijkswaterstaat, 1998. In: Jaarboek Monitoring Rijkswateren 1996 / 7, Lakerveld, Den Haag, p. 237. Sokal, R.F., Rohlf, F.J., 1995. In: Biometry, Freeman and Co, San Francisco, p. 887.
Sola, J.C., 1994. In: Estudio de la Communidad Reducidad de macoma en el Estuario del Bidasoa, Universidad de Navarra, Pamplona, p. 526, PhD thesis.
Sola, M.J., Millan, E., Legonburu, I., Canton, L., 1991. Heavy metal levels in superficial river sediments, from Guipuzcoa (Spain). Environ. Technol. 12, 441–445.
¨ ¨ ¨
Vainola, R., Varvio, S.-L., 1989. Biosystematics of Macoma balthica in Northwestern Europe. In: Ryland, J.S., Tyler, P.A. (Eds.), Reproduction, Genetics and Distributions of Marine Organisms, Olsen and Olsen, Fredensborg, pp. 309–316.
de Wilde, P.A.W.J., 1975. Influence of temperature on behaviour, energy metabolism, and growth of Macoma balthica (L.). In: Barnes, H. (Ed.), Proc. 9th Europ. Mar. Biol. Symp., Aberdeen Univ. Press, Aberdeen, pp. 239–256.
de Wilde, P.A.W.J., Berghuis, E.M., 1978. Laboratory experiments on the spawning of Macoma balthica; its implication for production research. In: McLusky, D.S., Berry, A.J. (Eds.), Physiology and Behaviour of Marine Organisms, Pergamon Press, Oxford, pp. 375–384.
Wilkinson, L., 1988. Systat: the System For Statistics, Systat Inc, Evanston, IL.
Wilson, J.G., 1981. Temperature tolerance of circatidal bivalves in relation to their distribution. J. Therm. Biol. 6, 279–286.
![Fig. 4. The glycogen concentration [% DW; average and standard error, N 5 2 (two groups of ten animals)] oftranslocated clams in the preliminary experiments at the beginning of, and 1 month after, translocation incomparison to the endemic (local) specimens](https://thumb-ap.123doks.com/thumbv2/123dok/3098785.1375707/8.612.66.405.73.376/concentration-oftranslocated-preliminary-experiments-beginning-translocation-incomparison-specimens.webp)
![Fig. 5. The free amino acid concentration (% DW), and taurine/glycine ratio (used as a stress indicator)[averages and standard error, N 5 3 (three groups of ten animals)] of translocated clams in the preliminaryexperiments at the beginning of, and 1 month](https://thumb-ap.123doks.com/thumbv2/123dok/3098785.1375707/9.612.67.407.92.566/concentration-taurine-indicator-averages-standard-translocated-preliminaryexperiments-beginning.webp)