Published: August 18, 2011
r2011 American Chemical Society 2263 dx.doi.org/10.1021/jz200912a|J. Phys. Chem. Lett.2011, 2, 2263–2266
LETTER
pubs.acs.org/JPCL
Coexistence and Interconversion of Di-
σ
and
π
-Bonded Ethylene on
the Pt(111) and Pd(110) Surfaces
Tomonari Okada,
†Yousoo Kim,*
,†Yasuyuki Sainoo,
†,||Tadahiro Komeda,
†,||Michael Trenary,*
,‡and
Maki Kawai*
,†,§†
RIKEN Advanced Science Institute, Wako, Saitama, 351-0198, Japan
‡Department of Chemistry, 845 W Taylor Street, University of Illinois at Chicago, Chicago, Illinois 60607, United States
§
Department of Advanced Materials Science, The University of Tokyo, Kashiwa, Chiba, 277-8561, Japan
T
he hydrogenation of unsaturated hydrocarbons is among the most important reactions in heterogeneous catalysis. Con-sequently, the adsorption and reaction of the simplest olefin, ethylene, on transition-metal surfaces has been the subject of many fundamental surface science studies. In the mechanism of ethylene hydrogenation on metal surfaces first proposed by Horiuti and Polyani,1ethylene initially adsorbs in the so-called di-σbonded configuration in which two M C bonds are formed and the C atoms are rehybridized to sp3. Hydrogen is added sequentially to first form an ethyl intermediate, followed by ethane, which then desorbs. Although this mechanism explained early observations on ethylene hydrogen exchange and hydro-genation reactions and is appealing in its simplicity, direct information on the form of adsorbed ethylene involved in hydrogenation was lacking for many years. In 1996, Cremer et al.,2used sum frequency generation (SFG) to obtain surface vibrational spectra in the C H stretch region during ethylene hydrogenation over a Pt(111) surface under total gas pressures up to nearly 1 atm. They concluded that although di-σ bonded ethylene was present under some conditions, theπ-bonded form was the actual surface intermediate that undergoes hydrogenation. Despite the importance of understanding the nature of ethylene bonding to metal surfaces, the structure and relative stability of the di-σandπ-bonded forms of ethylene have been difficult to establish definitively. For example, a diffuse low energy electron diffraction (LEED) study3 on Pt(111) con-cluded that di-σ bonded ethylene adsorbs with the CC bond located off-center over either an fcc or hcp three-fold hollow site with the CC axis tilted by 22°from the surface plane. However, this structure is at odds with theoretical studies thatfind that a symmetric structure is favored with the CC axis parallel to thesurface.4 7An even greater discrepancy exists for the Pd(110) surface where experimental studies8 11 indicate that ethylene adsorbs in aπ-bonded form, whereas theoretical calculationsfind that di-σbonded ethylene is most stable.12 14The adsorption site ofπ-bonded ethylene has not been directly established by experiment but is assumed to occupy an on-top site by analogy to its bonding in complexes such as Zeise’s salt.15Here we use a low-temperature scanning tunneling microscope (LT-STM) to im-age individual ethylene molecules directly to determine their adsorption sites and to show that both forms can coexist at low coverages on the Pt(111) and Pd(110) surfaces. Furthermore, we show that the STM tip can be used to interconvert the two forms of ethylene under mild conditions, suggesting that they have nearly the same adsorption energy. This observation is somewhat similar to those of Ofner and Zaera,16 who found evidence of interconversion of the two forms at higher coverages under a steady flux of gas-phase ethylene molecules. This interconversion was seen as a key part of the overall ethylene hydrogenation mechanism in that the weakly adsorbedπ-bonded ethylene would need to switch to the di-σbonded form before hydrogenation could take place. The ease of interconvertability of the two forms even at low coverages, as demonstrated here, shows that this is a basic property of the ethylene metal interaction and does not require the presence of other adsorbates.
After the clean Pt(111) surface was imaged, the sample was removed from the liquid-helium-cooled STM stage, a process that raises the sample temperature to∼50 K in the time required
Received: July 6, 2011
Accepted: August 18, 2011 ABSTRACT:A low-temperature scanning tunneling microscope operated at 4.7 K was
used to observe individual molecules of ethylene on the Pt(111) and Pd(110) surfaces following adsorption at 50 K. In both cases, two forms of the molecules were observed and were found to switch from one to the other under the influence of the tip. The form with the greater apparent height is attributed toπ-bonded ethylene, and the other form is attributed to di-σ bonded ethylene. The images provide direct evidence that on both surfacesπ-bonded ethylene occupies an on-top site and di-σbonded ethylene occupies a symmetric bridging site. The results are compared with predictions based on DFT calculations that di-σbonded ethylene should be the most stable form on both surfaces.
2264 dx.doi.org/10.1021/jz200912a |J. Phys. Chem. Lett.2011, 2,2263–2266
The Journal of Physical Chemistry Letters LETTER
for gas exposures, and exposed to an amount of C2D4to give a
coverage of∼0.03 ML. Figure 1a is a 55 nm image that shows three distinct features. The brightest two features are due to adsorbed ethylene molecules. Also apparent in the image are dim white objects with dark areas next to them. These correspond to acetylene, which was deliberately dosed onto the surface along with the ethylene. The appearance of the acetylene molecules is quite similar to what was observed on a Pd(111) surface,17and it can be assumed that the molecules occupy the same adsorption site on the two surfaces. The known acetylene adsorption site then provides a basis for determining the ethylene adsorption sites. The ethylene molecules appear the same whether or not acetylene is coadsorbed. Large scale images obtained following exposure of the Pt(111) surface to acetylene alone shows a negligible number of minority species, verifying that the acet-ylene gas did not contain any contaminants, such as acetone. On the basis of a Pt35 cluster model of the Pt(111) surface, DFT
calculations indicated that acetylene bonds in a so-called di-σ/π fashion in which the two carbon atoms formσbonds to two Pt atoms, and the molecule tilts toward a third Pt atom to interact via the π bond.4 This coordination mode places the C2H2 molecule roughly over a three-fold hollow site with a Pt atom symmetrically positioned in the middle of the dark lobe. The observation of the acetylene molecules thus establishes the posi-tion of the Pt grid, which is shown superimposed in Figure 1b onto the image of Figure 1a. The on-top sites next to the C2H2
molecules are indicated with the green dots, whereas the Pt atoms not involved in bonding to an adsorbate are represented by white dots. The image in Figure 1b shows that the brighter ethylene molecule is centered exactly above a Pt atom, whereas the dimer ethylene molecules are located between two Pt atoms. Given previous ultraviolet photoelectron spectroscopy (UPS) work18,19showing thatπ-bonded ethylene is stable on Pt(111) at these low temperatures along with the assumption that this form adsorbs at an on-top site, we assign the larger of the two pro-trusions in Figure 1 to π-bonded ethylene. With its presumed occupation of an on-top site, along with the presence of the largely unperturbed π orbital of the CdC double bond, it is reasonable that this form of ethylene should appear relatively large in the STM images. The other form can therefore be as-sumed to be di-σbonded ethylene. The various theoretical studies of the adsorption geometry of π- and di-σ bonded ethylene are entirely consistent with these conclusions. For example, the structural
parameters calculated by Jacob and Goddard imply thatπ-bonded ethylene is located 0.03 nm further than di-σbonded ethylene from the unrelaxed Pt surface.4
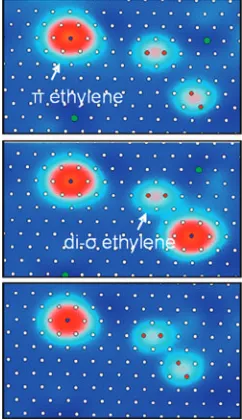
Figure 2 shows a sequence of three 2.54.1 nm STM images at 4.7 K of C2D4on Pt(111) taken one after the other in the order
from top to bottom. The sample was biased at +0.1 V, and the topographic image was obtained with a constant tunneling current of 1.0 nA. Three ethylene molecules are observed in each image. However, in the top image, there is oneπ-bonded ethylene and two di-σbonded molecules, whereas in the following image one of the di-σbonded molecules has switched to theπ -bonded form. In the bottom image, this molecule has switched back to the di-σbonded form. The observed site switching occurs only in response to the tunneling conditions used to obtain the image, indicating that the two forms are readily interconvertible under mild conditions and suggesting that they are nearly isoenergetic.
Similar behavior is seen for ethylene on the Pd(110) surface after dosing ethylene at a surface temperature of∼50 K, followed by imaging at 4.7 K. The STM image in Figure 3a shows that two Figure 1. (a) 55 nm STM image obtained withVs= 0.1 V, 1.0 nA at
4.7 K showing two forms of ethylene on the Pt(111) surface following exposure at a surface temperature of∼50 K to C2D4. Coadsorbed
acetylene molecules are also observed as the faint white objects. (b) Same 55 nm image as shown in part a but with a superimposed grid of Pt atom positions based on the known adsorption sites for acetylene. The Pt atoms next to the acetylene molecules are indicated with the green dots.
Figure 2. Series of three 2.54.1 nm images taken atVs= 0.1 V and
1.0 nA in the order from top to bottom showing that a di-σmolecule can
switch to theπ-bonded form and then back to the di-σbonded form in
response to the scanning conditions used to acquire the images.
Figure 3. (a) 55 nm STM image obtained with a sample bias of 50 mV and a tunneling current of 1.0 nA taken at 4.7 K following exposure of the Pd(110) surface to C2H4with the sample at a temperature of ∼50 K. (b) Image shown in part a with a superimposed grid indicating
2265 dx.doi.org/10.1021/jz200912a |J. Phys. Chem. Lett.2011, 2,2263–2266
The Journal of Physical Chemistry Letters LETTER
forms of ethylene are present on the surface, one brighter than the other. Figure 3b is a smaller scale image with a superimposed grid showing the positions of the Pd atoms, which reveals that the brighter molecule is centered at an on-top site and the dimmer molecule is off-center above a two-fold bridge site. The grid is based on images obtained under different tunneling conditions in which individual Pd atoms are clearly resolved, as previously reported.20Again, based on theoretical calculations,12we assign the on-top site molecule to π-bonded ethylene and the other molecule to di-σ bonded ethylene. Although the azimuthal orientation of the ethylene molecules is not apparent in the image, the calculations indicate that the most stable orientation at both sites is with the CdC axis along the [110] direction.
Calculations also indicate thatπ-bonded ethylene is 4 pm higher above the surface than di-σbonded ethylene.14
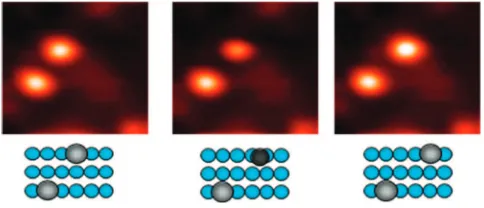
As for the two forms of ethylene on Pt(111), the two forms on Pd(110) can be interconverted under the influence of the STM tip, as shown in Figure 4. In the left panel, twoπ-bonded ethylene molecules are observed. The STM tip was placed over the upper molecule, and a voltage pulse of 350 mV was applied for 2 s. The subsequent STM image shown in the middle panel reveals that the molecule has moved to a bridge site. The tip was then placed over the di-σbonded molecule observed in the middle image, and another 350 mV pulse was applied again for 2 s, and the image shown in the right panel was obtained revealing that the molecule moved back to an on-top site. As in the case of ethylene on Pt(111), the STM results imply that the two forms have similar stabilities on the Pd(110) surface and are therefore readily interconverted. Presumably, a similar voltage pulse would have caused site switching for ethylene on Pt(111), although this was not explicitly tried.
The results presented here can now be considered in the context of the previous studies, both experimental and theore-tical, on these systems. In the case of ethylene on Pt(111), all of the DFT calculations predict that di-σbonded ethylene is more stable by 36 68 kJ/mol.4 7 However, whereas Jacob and Goddard4predicted that there is essentially no barrier separating π-bonded from di-σbonded ethylene, Watson et al.6considered this issue in detail and calculated a barrier of 12 kJ/mol. They then argued that this barrier could explain the conclusion reached based on angle-resolved UPS data18,19that aflat-lyingπ-bonded ethylene is present below 52 K, whereas it converts to di-σ bonded ethylene above this temperature. It was assumed6that steric factors in the adsorption process led to the formation of
metastableπ-bonded ethylene, which requires thermal energy to overcome the barrier to the more stable form. The UPS spectra18,19do not actually exclude the possibility that bothπ
-bonded and di-σ bonded ethylene are present below 52 K. Therefore, our observation of both forms following adsorption at a temperature of∼50 K is not incompatible with the UPS results.18,19The one experimental result that is at odds with our STM images on Pt(111) is the LEED study3reporting that di-σ bonded ethylene is centered above a three-fold hollow site in a tilted geometry. The observation of the C C stretching mode in a RAIRS study of di-σbonded ethylene on Pt(111) might seem to present evidence of a tilted geometry, but it was argued that on the basis of symmetry, the CC stretch would be surface-IR-allowed, even for the CC bond oriented strictly parallel to the surface.21Although the STM images do not necessarily reveal if the molecule is titled or not, the images do indicate a symmetric adsorption site bridging two Pt atoms.
There is a greater discrepancy between calculations and experi-ments in the case of ethylene on Pd(110), where all previously used experimental techniques8 11 revealed only the π-bonded form, but all calculations12 14predict that the di-σbonded form is more stable. For example, Pichierri et al.12 calculated a higher binding energy of 21 kJ/mol for di-σbonded ethylene compared with theπ-bonded form. Our results are the only experimental observation of a di-σbonded ethylene on Pd(110). The explana-tion is likely similar to the case on Pt(111) but with a barrier separating di-σbonded ethylene from the more stableπ-bonded form so that when adsorbed at 50 K the molecules do not have enough thermal energy to attain the most stable configuration, which isπ-bonded ethylene based on the fact that it is the form observed in previous experimental studies.8 11 Although the images were quite a bit noisier than the ones reported here, a previous STM study8 detected onlyπ-bonded ethylene on Pd-(110) at a temperature of 112 K, which was apparently high enough to overcome the conversion barrier. Pichierri et al.12
considered in detail the apparent deficiencies in the DFT calcula-tions for ethylene on the Pd(110) surface. They note that it is similar to the well-known case of DFT calculations failing to predict the higher stability of on-top adsorption at low coverages for CO on Pt(111).22Pichierri et al.12concluded that the inability of DFT calculations to predict the correct adsorption mode for ethylene on Pd(110) represents a deficiency in the theory that remains poorly understood. Although there is probably a similar underestimation of the stability ofπ-bonded ethylene relative to di-σbonded ethylene on Pt(111), the larger difference in energy enables DFT to correctly determine the relative stabilities of the two moieties.
Finally, we can speculate on the implications of our results for ethylene hydrogenation. As the barrier height for interconversion between the two forms of ethylene is quite low on Pt(111) and certainly less than the thermal energy available at room tempera-ture, under hydrogenation conditions it can be assumed that ethylene molecules are freely exchanging between the di-σ bonded and π-bonded forms. Thus, under actual reaction conditions, there is probably equilibrium between the two forms, and as the di-σethylene molecules are converted to ethane and removed from the surface, they would be continually replaced from the supply ofπ-bonded ethylene molecules on the surface. Because the removal of di-σ ethylene through hydrogenation would be fast under high pressures of gas-phase hydrogen, it is likely that the surface concentration of this moiety would be too low to detect spectroscopically. This could then explain why only Figure 4. 2.52.5 nm STM images (Vs= 50 mV, 1.0 nA) showing two
2266 dx.doi.org/10.1021/jz200912a |J. Phys. Chem. Lett.2011, 2,2263–2266
The Journal of Physical Chemistry Letters LETTER
π-bonded ethylene was detected in the SFG studies of Cremer et al.2 under hydrogenation conditions, even though such a relatively unperturbed form of ethylene is not much more likely to undergo H atom addition than would ethylene in the absence of a catalyst.
’EXPERIMENTAL METHODS
The experiments were performed with a LT-STM (Omicron GmbH) housed in an ultrahigh vacuum chamber (base pressure: 310 11Torr). Acetylene and ethylene were purchased from Taiyo Nippon Sanso with quoted purities of >99.9% by volume and were used without further purification. The Pt(111) and Pd(110) surfaces were cleaned by repeated cycles of Ar+ sputtering, followed by annealing to 1100 K, which resulted in clean, atomically resolved STM images. All STM results were obtained with electrochemically etched tungsten tips while the surfaces were maintained at 4.7 K. A positive sample bias (Vs)
with respect to the tip was used for all images.
’AUTHOR INFORMATION
Corresponding Author
*E-mail: mtrenary@uic.edu, Tel: 312 0777, Fax: 312 996-0431 (M.T.). E-mail: maki@riken.jp, Tel: +81-48-467-9407, Fax: 462-4663 (M.K.). E-mail: ykim@riken.jp, Tel: +81-48-467-4073, Fax: +81-48-467-1945 (Y.K.).
Present Addresses
)
Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Aoba-ku, Sendai 980 8577, Japan.
’ACKNOWLEDGMENT
We thank Mr. Hiroyuki Miyashita for assistance with the experiments. M.T. acknowledges support from the U.S. National Science Foundation under grant CHE-1012201.
’REFERENCES
(1) Horiuti, I.; Polanyi, M. Exchange Reactions of Hydrogen on Metallic Catalysts.Trans. Faraday Soc.1934,30, 1164–1172.
(2) Cremer, P. S.; Su, X.; Shen, Y. R.; Somorjai, G. A. Ethylene Hydrogenation on Pt(111) Monitored in Situ at High Pressures Using Sum Frequency Generation.J. Am. Chem. Soc.1996,118, 2942–2949.
(3) Doll, R.; Gerken, C. A.; VanHove, M. A.; Somorjai, G. A. Structure of Disordered Ethylene Adsorbed on Pt(111) Analyzed by Diffuse LEED: Asymmetrical Di-σBonding Favored.Surf. Sci. 1997, 374, 151–161.
(4) Jacob, T.; Goddard, W. A. Chemisorption of (CHxand C2Hy)
Hydrocarbons on Pt(111) Clusters and Surfaces from DFT Studies
J. Phys. Chem. B2005,109, 297–311.
(5) Ge, Q.; King, D. A. The Chemisorption and Dissociation of Ethylene on Pt{111} from First Principles. J. Chem. Phys. 1999,
110, 4699–4702.
(6) Watson, G. W.; Wells, R. P. K.; Willock, D. J.; Hutchings, G. J. Density Functional Theory Calculations on the Interaction of Ethene with the{111}Surface of Platinum.J. Phys. Chem. B2000,104, 6439– 6446.
(7) Zhao, Z.-J.; Moskaleva, L. V.; Aleksandrov, H. A.; Basaran, D.; R€osch, N. Ethylidyne Formation from Ethylene over Pt(111): A Mechanistic Study from First-Principle Calculations.J. Phys. Chem. C
2010,114, 12190–12201.
(8) Ichihara, S.; Yoshinobu, J.; Ogasawara, H.; Nantoh, M.; Kawai, M.; Domen, K. Adsorption of π-Bonded Ethylene and Ethynyl on
Pd(110): An STM Study. J. Electron Spectrosc. Relat. Phenom.1998,
88 91, 1003–1007.
(9) Okuyama, H.; Ichihara, S.; Kato, H.; Yoshinobu, J.; Kawai, M. Molecular Rearrangement Induced by Hydrogen Co-adsorption: C2H4
on Pd(110).Chem. Phys. Lett.1999,310, 451–458.
(10) Okuyama, H.; Ichihara, S.; Ogasawara, H.; Kato, H.; Komeda, T.; Kawai, M.; Yoshinobu, J. Orientation and Symmetry of Ethylene on Pd(110): A Combined HREELS and NEXAFS Study.J. Chem. Phys.
2000,112, 5948–5956.
(11) Ogasawara, H.; Ichihara, S.; Okuyama, H.; Domen, K.; Kawai, M. Orientation of Unsaturated Hydrocarbons on Pd(110).J. Electron Spectrosc. Relat. Phenom.2001,114 116, 339–343.
(12) Pichierri, F.; Iitaka, T.; Ebisuzaki, T.; Kawai, M.; Bird, D. M. First-Principles Pseudo-Potential Study of the Pd(110)-c(2 2)-Ethylene Adsorption System.J. Phys. Chem. B2001,105, 8149–8154.
(13) Filhol, J. S.; Simon, D.; Sautet, P. Ethylene Adsorption and Coadsorption with H on Pd(110) from First Principles.J. Phys. Chem. B
2003,107, 1604–1615.
(14) Ge, Q.; Neurock, M. Correlation of Adsorption Energy with Surface Structure: Ethylene Adsorption on Pd Surfaces.Chem. Phys. Lett.
2002,358, 377–382.
(15) Grogan, M. J.; Nakamoto, K. Infrared Spectra and Normal Coordinate Analysis of Metal-Olefin Complexes. I. Zeise’s Salt Potas-sium Trichloro(ethylene)platinate(II) Monohydrate.J. Am. Chem. Soc.
1966,88, 5454–5460.
(16) Ofner, H.; Zaera, F. Ethylene Adsorption on Platinum: Kinetics, Bonding, And Relevance to Catalysis.J. Am. Chem. Soc.2002,124, 10982– 10983.
(17) Matsumoto, C.; Kim, Y.; Okawa, T.; Sainoo, Y.; Kawai, M. Low-Temperature STM Investigation of Acetylene on Pd(111). Surf. Sci.
2005,587, 19–24.
(18) Hugenschmidt, M. B.; Dolle, P.; Jupille, J.; Cassuto, A. Ethylene
πSpecies on Bare and Cesiated Pt(111) Surfaces.J. Vac. Sci. Technol., A
1989,7, 3312–3316.
(19) Cassuto, A.; Kiss, J.; White, J. M. On the Orientation of Low Temperature π-Bonded Ethylene on Pt(111). Surf. Sci.1991, 255,
289–294.
(20) Komeda, T.; Kim, Y.; Kawai, M. Lateral Motion of Adsorbate Induced by Vibrational Mode Excitation with Inelastic Tunneling Electron.Surf. Sci.2002,502 503, 12–17.
(21) Fan, J.; Trenary, M. Symmetry and the Surface Infrared Selec-tion Rule for the DeterminaSelec-tion of the Structure of Molecules on Metal Surfaces.Langmuir1994,10, 3649–3657.
(22) Feibelman, P. J.; Hammer, B.; Norskov, J. K.; Wagner, F.; Scheffler, M.; Stumpf, R.; Watwe, R.; Dumesic, J. The CO/Pt(111)