Explore and Expand Copyright: © 2015 Liang Ma, et al.
http://dx.doi.org/10.19104/jstb.2015.105
Open Access
Research Article
Journal of Stem Cell and Transplantation Biology
Homeodomain Transcription Factor Msx-2 Regulates Uterine
Progenitor Cell Response to Diethylstilbestrol
Yan Yin1, Congxing Lin1, Ivy Zhang1, Alexander V Fisher2, Maulik Dhandha3 and Liang Ma1*
1Division of Dermatology, Washington University School of Medicine, St. Louis, MO, USA 2Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
3Department of Dermatology, Saint Louis University School of Medicine, St. Louis, MO, USA
Abstract
The fate of mouse uterine epithelial progenitor cells is determined between postnatal days 5 to 7. Around this critical time window, exposure to an endocrine disruptor, diethylstilbestrol (DES), can profoundly alter uterine cytodifferentiation. We have shown previously that a homeo domain transcription factor MSX-2 plays an important role in DES-responsiveness in the female reproductive tract (FRT). Mutant FRTs exhibited a much more severe phenotype when treated with DES, accompanied by gene expression changes that are dependent on Msx2. To better understand the role that MSX-2 plays in uterine response to DES, we performed global gene expression profiling experiment in mice lacking Msx2. By comparing this result to our previously published microarray data performed on wild-type mice, we extracted common and differentially regulated genes in the two genotypes. In so doing, we identified potential downstream targets of MSX-2, as well as genes whose regulation by DES is modulated through MSX-2. Discovery of these genes will lead to a better understanding of how DES, and possibly other endocrine disruptors, affects reproductive organ development.
Keywords: Msx2; diethylstilbestrol (DES); Endometrium; Enviromental hormone
Introduction
Developmental exposure to endocrine disrupting chemicals (EDCs) can have catastrophic consequences in humans and animals, including malformations of various organs and tissues and increased risk for certain diseases, such as infertility, obesity and cancers [1]. For example, in humans, in utero exposure to diethylstilbestrol (DES), a synthetic estrogen commonly prescribed to pregnant women for the prevention or treatment of miscarriage in the 40’s to 70’s, led to patterning defects in the female reproductive tract (FRT) and clear cell carcinomas of the cervix and vagina in affected individuals [2]. Similar defects were found in laboratory animal models, including uterine metaplasia, loss of uterotubal junction and vaginal adenosis. The postnatal mouse DES model was established by Dr. John McLachlan’s group [3,4], and was widely adopted as the experimental model system to better understand the molecular mechanism underlying the observed DES effects. This model involves subcutaneous injection of DES from postnatal day 1 to day 5. The murine female reproductive tract develops mainly from the Müllerian duct. Classical tissue recombination experiments demonstrated that Müllerian epithelial cell fate is determined between postnatal days 5-7 in the mouse by signals
from the underlying mesenchymal cells [5]. More specifically, it was
shown recently that BMP4/Activin-A signaling is activated in the
vaginal mesenchyme to promote epithelial stratification [6]. Thus
before postnatal day 5, the Müllerian epithelial cells are pluripotent and are sensitive to EDC exposure. It was shown that DES exerts
its function mainly through estrogen receptor α, as most if not all DES effects were absent in ERα knockouts [7]. We have focused our
previous work on understanding the immediate gene expression and cellular changes induced by DES exposure [8-10]. Our rationale is that gene expression changes in early development will have a long-lasting effect on organ function in adults. We showed that neonatal DES exposure in mice caused immediate gene expression changes, especially in the uterine epithelium, and the affected genes included many transcription factors, signaling pathway components, and growth factors [9]. Intriguingly, peroxisome proliferator-activated receptor gamma (Pparγ) and a group of
genes critical to lipid metabolism, showed significant changes in
expression level in the uterine epithelium, which led to increased
lipid droplets accumulation in merely five days after DES exposure
in the same tissue layer. In addition, DES exposure changed glucose
metabolism, water/small molecule trafficking, proliferation as well as apoptosis profiles of the uterine epithelial cells.
MSX-2 homeodomain transcription factor is required during organogenesis by mediating epithelial-mesenchymal interactions. Msx2 is highly expressed in a variety of tissues during embryogenesis, and is critical for the terminal differentiation of many tissue types and organs, including the limb, bone, hair follicle, nail, and tooth [11-15]. In wild type neonates, Msx2 is highly expressed in the uterine as well as vaginal epithelium, and
its expression is significantly reduced in both tissues upon DES
exposure [8,10]. Moreover, Msx2 mutant reproductive tract appears to be a sensitized background for DES exposure and the FRTs in these mice exhibited a much more severe patterning defects in response to DES compared to wild type controls [10]. These data led us to hypothesize that DES may elicit its cellular effects through down-regulation of a master regulator Msx2, which in turn led to gene expression changes of an array of down-stream targets via transcription activation/repression. To further understand the role of Msx2 in modulating FRT response to DES, here we performed
global gene expression profiling experiment in vehicle- or
DES-treated Msx2-null mice and compared this result to our previously published microarray data on wild-type animals. The differential gene regulation by DES in wild-type and Msx2-/- mice revealed many genes whose expression and/or regulation by DES depends on Msx2 in the developing uterus.
Results
Msx2
Differentially Regulates Uterine Responsiveness to
Neonatal DES Exposure
In an attempt to identify potential MSX-2 targets that mediate the DES effects, we performed cDNA microarray using uterine RNA harvested from vehicle (oil)- or DES-treated Msx2-/- neonatal mice.
Among the 46628 annotated genes included on the Affymetrix
microarray chips, 14025 were expressed in uterine tissues, with or without DES treatment. When comparing data set treated with Received Date: October 30, 2014, Accepted Date: May 04, 2015, Published Date: May 12, 2015.
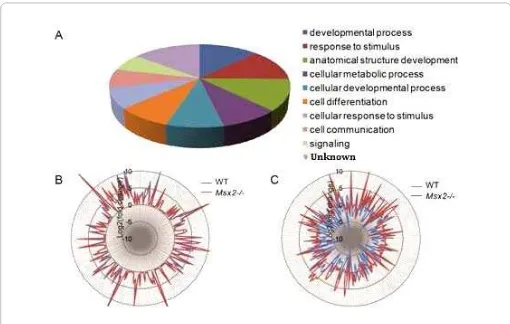
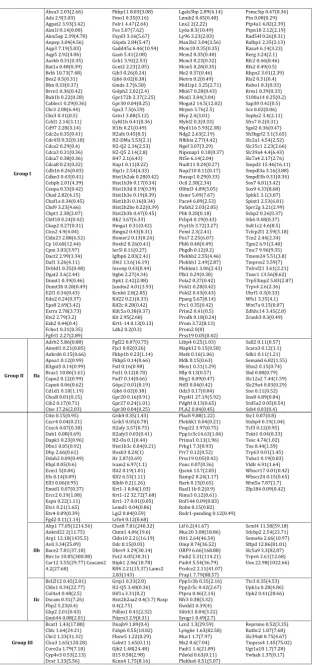
Figure 1: Differentially regulated genes by DES in Msx2-/- uterus. (A) Pie chart showing gene ontological classification of the 1459 DRGs. (B) Star glyph showing the fold changes in gene expression by DES of common DRGs between control (blue) and Msx2-/- (red). Each peak represents the Log2 value of fold change by DES of one gene, note that the peaks of wild-type and Msx2-/- for each given gene almost always overlap. The list of these common DRGs is in Table 1(Group I). (C) Star glyph showing the fold changes of the unique DRGs listed in Table 1 (Groups II-III). Note the drastic changes in the positions of wild-type peak and Msx2-/- peak for each gene.
DES to that of the vehicle-treated control group, we identified 1459
annotated genes that were differentially regulated by DES exposure in Msx2-/- uterus with a p-value less than 0.05 (data not shown).
These genes were referred to as Differentially Regulated Genes (DRGs) hereinafter. Gene ontology analysis revealed that these DRGs were involved in a plethora of cellular processes, including
development, response to stimulus, and anatomical structure development (Figure 1A). Since Msx2 is normally highly expressed in the uterine epithelium, its targets would presumably reside in the same tissue layer. To test this hypothesis, we compared
the expression of these DRGs to our previous microarray data
conducted on wild type neonatal uteri. Since the two microarrays
were performed on different platforms, only 1254 out of 1459 DRGs
overlapped between the two studies. Nevertheless, more than 80%
(1025 out of 1254) of those DRGs were detected in the wild-type
uterine epithelium, indicating that Msx2 is indeed functioning as a transcriptional regulator in the uterine epithelium. To focus on potential transcriptional targets of Msx2, we carefully excluded
DRGs that also showed mesenchymal expression from our previous tissue-specific microarray data (GEO# GSE37969, [9], and only
compared our current array data to that obtained using wild-type uterine epithelial RNA.
A total of 212 genes showed similar expression change trends
and fold-changes in the uterine epithelium (Table 1, Group I),
indicating that their regulation by DES is not mediated through MSX-2 transcriptional activation/suppression. We plotted fold changes of these genes in both wild-type and Msx2-/- uteri on a
star glyph, and the two sets of curves fit almost perfectly (Figure
1B). On the other hand, expression of 198 genes were regulated either in the opposite direction by DES or that their fold changes were changed dramatically (equal or greater than four-fold) in the
two different genotypes (Table 1, Groups II-III). When plotted the
log-scaled fold-changes of these genes on a star glyph, noticeable differences in the positions of peaks were observed (Figure 1C). These genes, or at least a subset of them, could be MSX-2 targets.
Validation of microarray results
To validate our microarray findings, quantitative real-time
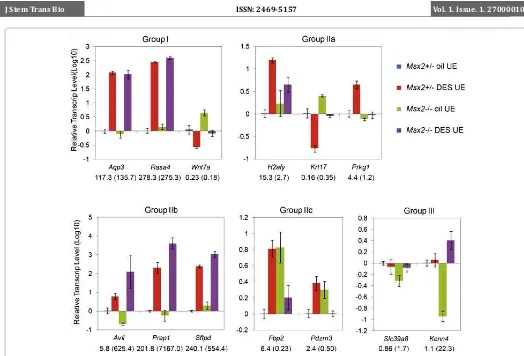
RT-PCR (qRT-PCR) was performed on RNA samples extracted from isolated control (Msx2+/-) and Msx2-/- neonatal uterine epithelia, exposed to either oil or DES. As a positive control, we
first tested some Group I DRGs that showed similar regulation by
DES in both genotypes. Wnt7a, a ligand for the canonical WNT/β-catenin pathway that is critical for many cellular processes, is highly expressed in the developing uterine epithelium and is
responsible for uterine gland formation [16,17]. We and others
have previously shown that its expression was strongly suppressed by DES administration [8,18,19]. In Msx2-/- uterus, a similar effect was observed by qRT-PCR (Figure 2). Expression of Aqp3,
a gene encoding one of the aquaporin water trafficking proteins,
was induced dramatically by DES in the wild type array. A similar regulation was observed in the Msx2-/- microarray and confirmed by qRT-PCR (Figure 2). Similar results were obtained from another group I gene, Rasa4 (Figure 2). Rpl7, a housekeeping gene whose expression level was not affected by DES in either genotype, was used in the qRT-PCR to determine relative transcript level of each
DRG, employing the comparative Ct method.
Next, we classified the 198 DRGs that showed differential
regulation by DES in Msx2-/- uterus into two major categories, as
shown in Table 1 (Group II-III). Group II contained genes whose
expression was either up-or down-regulated by more than twofold by DES in the control uterus, but showed different regulatory patterns in the absence of Msx2. Group II was further divided in
to three subgroups: Group IIa comprised of 114 DRGs whose
DES-regulation was either lost or attenuated in Msx2-/- by fourfold
or more; Group IIb included 33 DRGs whose DES-regulation
was enhanced in Msx2-/-; and Group IIc contained 24 DRGs that
showed opposite regulation by DES in the two genotypes. Group III consisted of 27 DRGs that showed expression changes by more than
twofold when exposed to DES only in Msx2-/-, but not in wild-type. We selected a few genes from each group to verify gene expression changes by qRT-PCR. As shown in Figure 2, H2afy from Group IIa, a gene encoding a histone H2A variant, was induced markedly by DES in the Msx2+/- control animals, but to a lesser extent in Msx2 -/- uterus (Figure 2). Similarly, up-regulation of Prkg1 was only detected in DES-treated control but not in mutant uterus. Another
examples of Group IIa genes was Krt17 (Krt1-17), a keratin family
member. Expression of Krt17 was decreased more than 6-fold upon DES treatment. In the Msx2-/-, however, this decrease was attenuated (Figure 2). These results indicate that MSX-2 plays an
obligatory role in the DES regulation of Group IIa genes under
normal circumstances, and in its absence, the DES-induced gene expression changes are abolished. It is noteworthy that the
majority of Group IIa DRGs (92 out of 114) were down-regulated
by DES in the wild-type, but this down-regulation was diminished in Msx2-/- mice. As clearly visualized in Fig. 1C, these genes were repressed by DES in the wild-type (blue peaks pointing inwards), while the repression was no longer detectable or much reduced in Msx2-/- uteri (corresponding inconspicuous red peaks). These results support the notion that MSX-2 acts as a transcriptional suppressor and suggest that it is involved in the DES-suppression of these genes.
An example of Group IIb genes was Avil, encoding the protein
Group I Fgfrl1 2.27(2.89)
Fkbp11 8.03(3.08) Car12 3.55(29.77) Ceacam2 4.2(27.68) Klf4 2.21(15.37) Lamc2 2.83(143) Hist2h2aa2 0.4(3.7) Nasp 0.4(2.75)
Comparison of DRGs by DES between wild-type and Msx2-/- uterus.
antagonizes transcriptional activation of Group IIb genes by DES,
and in its absence, this effect is augmented. Group IIc genes showed
a more complicated regulation, where DES exhibited opposite regulation on gene expression in the control and Msx2-/- uterus. For example, Fbp2, a gene encoding a gluconeogenesis regulatory enzyme, was up-regulated in the control uterus upon DES exposure, but was down-regulated by DES in Msx2-/- uterus (Figure 2). Similar results were obtained for Pdzrn3, a gene encoding a PDZ domain containing E3 ubiquitin ligase. It is likely that Msx2 is involved in the DES regulation of these genes, but the exact mechanisms remains
unclear at present. Group III genes showed no apparent expression
changes in the control uterus by DES, but were either up- or down-regulated by more than twofold in the Msx2 -/- when exposed to DES. Expression of two such genes, Slc39a8 and Kcnn4, were
validated by qRT-PCR (Figure 2). These genes are similar to Group
IIb genes in the sense that MSX-2 antagonizes DES-regulation, and DES effect on gene expression changes was only observed in Msx2 -/- uterus.
Msx2
Is Dispensable for the DES-induced Changes in
Ad-ipogenesis and Lipid Metabolism in the Uterine
Epithe-lium
As mentioned in the introduction, we have previously reported that neonatal DES exposure altered lipid metabolism in the uterine epithelium through upregulation of a master transcription factor
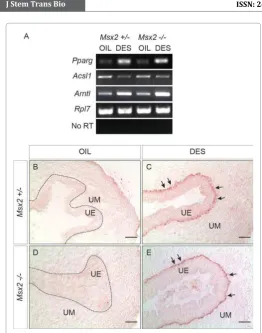
of adipogenesis, PPARγ [9]. To investigate whether Msx2 plays a
role in this process, we analyzed expression of Pparγ in the control
and Msx2-/- uterus. As shown in Figure 3A, Pparγ is upregulated by DES, regardless of Msx2 status, indicating that this process is not
mediated through Msx2. Many Pparγ downstream targets including Acsl1 and Arntl, and genes involved in lipid metabolism and
trafficking (e.g. Agpat2, Slc2a1), also showed similar regulation by
DES in Msx2-/- uterus (Figure 3A and Table 1). We next examined frozen uterine sections stained with Oil red O, a lysochrome diazo dye that binds neutral lipids, for signs of lipid metabolism changes. As we have previously reported, administration of DES caused increased lipid deposition exclusively in the uterine epithelium (Figure 3B, C). Similar results were obtained in Msx2-/- uteri (Figure 3D, E). These results collectively demonstrated that the DES-induced lipid metabolism changes in the uterine epithelium
through Pparγ upregulation is Msx2-independent.
Discussion
Since the discovery of the adverse effects of DES on human health, numerous studies in model animals have been conducted trying to uncover the underlying molecular mechanisms. The neonatal DES mouse model is widely used, because DES-treated animals exhibited a variety of developmental abnormalities and pathologies recapitulating the human syndrome including uterine metaplasia and vaginal adenosis. We have previously
conducted global gene profiling studies, and found that neonatal
oil- or DES-treated Msx2-/- uteri, and compare this result to that of our previous array data performed on wild-type animals. By comparing the two data sets, we uncovered three groups of genes in general: group I contains genes that are similarly regulated by DES both in wild type and in Msx2-/- uteri. Thus Msx2 is dispensable
for the regulation of this group of 212 genes by DES. Group II
consists of 171 genes whose regulation by DES is altered (in either direction) in Msx2 mutant uteri. Thus Msx2 plays important roles in
modulating their regulation by DES. Group III consists of 27 genes
that are not regulated by DES in wild type uterus, but are now DES targets in Msx2 mutant uteri. These genes are likely not MSX-2 transcription targets but rather exhibit altered DES responsiveness due to secondary changes, such as cell fate change. We further subdivided group II genes into three categories: genes whose regulations by DES are either lost or attenuated in Msx2 mutant uterus; those whose regulations are enhanced by Msx2 mutation; and those oppositely regulated by DES in the two genotypes. RT-PCR was used to validate representative genes from each subgroup. Interestingly, we observed that most DES-induced genes showed a greater response in Msx2-/- uterus, evidenced by the red peaks in the star glyph (Figure 1C). On the other hand, DES-repressed genes lost this repressive regulation in Msx2 mutant uterus, evidenced by the blue peaks (Figure 1C). These results are in agreement with previous studies suggesting that Msx2 mainly functions as a transcriptional repressor [20]. In this scenario, DES can induce target gene expression to a greater degree due to the removal of
a repressor that normally counteracts this induction. On the other hand, repression of certain gene expression by DES may require MSX-2 participation, as its absence leads to failure of repression by DES.
We previously showed that the regulation of Aqp3 by DES was abolished in Msx2 mutant vaginal epithelium [10]. Surprisingly, here we found that Aqp3 was similarly induced in both wild-type and Msx2 mutant uterus. These seemingly contradictory results can be reconciled by the fact that the intermediate cell layers expressing Aqp3 in the developing vagina are missing in Msx2 mutants, and thus failure of induction by DES in Msx2 mutant vagina is secondary to a defective patterning event. In this sense, Aqp3 is likely not a target of Msx2 either in the uterus or in the vagina.
We showed recently that lipid metabolism in the neonatal uterus was dramatically affected by DES exposure [9]. This change is mediated through the upregulation of a transcription factor
PPARγ, which in turn led to gene expression changes in an array of genes involved in lipid trafficking and metabolism. By analyzing the
expression of those genes in DES-treated Msx2-/- uterus, we found that Msx2 is not involved in the DES-regulation of this process.
Pparγ was similarly induced in Msx2-/- as in controls, so were many
Pparγ downstream genes. Consistently, lipid droplet accumulation
in the uterine epithelium was also observed in the Msx2-/- uterus upon DES exposure. Interestingly, it was previously reported that
in myofibroblasts, Msx2 stimulates osteogenesis but suppress
adipogenesis by activating Osx transcription and concomitantly
suppressing Pparγ expression [21]. Our results that Pparγ showed
no change in basal level in the absence of Msx2 indicate that Msx2
is unlikely to be a transcriptional suppressor of Pparγ, at least
in the uterus. In addition, DES increases Pparγ, and alters lipid metabolism in the uterus in an Msx2-independent way.
Future investigations on the function of these MSX-2 target candidates are needed to further evaluate their function(s) during normal uterine development as well as during DES-induced pathogenesis of the female reproductive tracts. Nevertheless,
identification of these genes may shed light on how Msx2 regulate
terminal differentiation of the uterus, and possibly other Msx2 -expressing tissues.
Materials and Methods
Mice
All mice were housed in the animal facility at Washington University with controlled light/dark cycles and handled in accordance with National Institutes of Health guidelines. All procedures were approved by the Washington University Institutional Animal and Use Committee. Msx2 mutants were generated and described previously [11], and breeding between female Msx2+/- and male Msx2-/- were used to obtain control and homozygous pups. DES was prepared and injected from P1 to P5 as described previously [8]. Uteri from female pups were harvested
for fixation or RNA extraction 24 hours after the last injection.
cDNA Microarray and Data Analysis
Oil- or DES-treated P5 Msx2-/- mice were harvested for RNA preparation. Uterine tissues from 3-4 animals of the same treatment group were pooled together and RNA isolated to make one sample. Total RNA was cleaned using RNeasy Kit (Qiagen,
Valencia, CA) and submitted to the Genome Technology Access Center (GTAC) at Washington University School of Medicine for microarray analysis on Affymetrix Genechip. A total of three
biological replicates were used for microarray analysis. Normalized
data were provided by the GTAC and detailed analysis protocol was
Figure 3: DES alters uterine lipid metabolism in an Msx2-independent manner. (A) RT-PCR showing that activation of the adipogenic marker,
Pparγ, as well as its downstream targets, is independent of Msx2. No
available upon request. Subsequent analyses were performed and
star glyphs generated in Microsoft Excel 2007. DRGs that showed
high expression in the wild-type uterine mesenchymal tissue from our previous microarray data [9], either with or without DES treatment, were excluded from the current analysis to better focus on Msx2-dependent gene regulation.
Uterine Epithelium Isolation, RNA Extraction and
Quan-titative Reverse Transcription-Polymerase Chain
Reac-tion (qRT-PCR)
Uterine epithelium isolation was performed as described
previously [9]. Briefly, dissected neonatal uterine horns were cut
into 3-4 mm long segments and incubated on a rotating platform in calcium-free, magnesium-free Hank’s balanced salt solution containing 1% trypsin for 1 hour at 4°C. Enzymatic digestion was stopped by adding an equal volume of 5 mg/ml bovine serum albumin after the incubation, and epithelial tissues were extruded from the uterine horns by applying gentle pressure along each segment. Epithelial tissues from individual animals of the same genotype/treatment were transferred to fresh tubes and pooled together. Low speed centrifugation was applied to collect tissue pellets [22]. Total RNA was isolated from the cell/tissue pellets
with RNA Stat-60 (Tel-Test, Inc., Friendswood, TX) following
manufacture’s instruction. Primer design, reverse transcription and qRT-PCR were performed as previously described [23,24]. Primer sequences used were as follows:
Aqp3: 5’CCTTGGCATCTTGGTGGCT3’, 5’AGGAAGCACATTGCGAAGGT3’; Rasa4: 5’CAGGATCCTTGTCCCAGTC3’, 5’GAGCAGTTGGTTCTCCCAAG3’; Wnt7a: 5’GAACTTACACAATAACGAGGCG3’, 5’GTGGTCCAGCACGTCTTAGT3’; H2afy: 5’CGGTGGTGAAGTAGGAAACAC3’, 5’GCTGCCAATGGATGGGAAG3’; Krt17: 5’ACCATCCGCCAGTTTACCTC3’, 5’CTACCCAGGCCACTAGCTGA3’; Prkg1: 5’CCCCTCAACAAAACAGGATG3’, 5’GAAGGAGGAGGGCTAGCAAC3’; Avil: 5’GAGTGCTCACGGCAACTTCTA3’, 5’GGGAGGAGTCCTTCCCGAT3’; Prap1: 5’CCCTAACCACTCTTCCACTCC3’, 5’TTCTCCCACCAATTTTCAGG3’; Sftpd: 5’GGACAATATTTGGCCAGGAG3’; 5’AGCTATACACCTTTTATTAGGATGTTG3’; Fbp2: 5’CGCTTCCCTTTGTCTTTGTC3’, 5’TTCTGACCGTGACCTGTGTC3’; Pdzrn3: 5’CTGCGCTACCAGAAGAAGTTC3’, 5’TCCATCTTGATTGTCCACACAG3’; Slc39a8: 5’GCCAAGCTCATGTACCTGTCT3’, 5’AAGATGCCCCAATCGCCAA3’; Kcnn4: 5’CAAGCCCCACGATAAATCAC3’, 5’GTGGGAGGTCCAATTCAGTG3’; Arntl: 5’TGACCCTCATGGAAGGTTAGAA3’, 5’GGACATTGCATTGCATGTTGG3’; Ascl1: 5’GCAACCGGGTCAAGTTGGT3’, 5’GTCGTTGGAGTAGTTGGGGG3’; Pparg: 5’GGAAGACCACTCGCATTCCTT3’, 5’GTAATCAGCAACCATTGGGTCA3’; Rp17: 5’AGCCCAAAGGTTCGTAAGGT3’, 5’AGCCTCGCTTGTAGATGAGC3’.
Oil-red O Staining
For Oil red O staining, uterine tissues were embedded in O.C.T. and snap frozen in liquid nitrogen. Slides of 10 µm cryosections
were allowed to dry briefly at room temperature, washed in water
once, twice in 100% propylene glycol (Sigma), then incubated in 0.7% Oil red O (Sigma) in propylene glycol for 7 minutes with
agitation at 60°C. Slides were then washed in 85% propylene glycol briefly, rinsed in water, and mounted with glycerin jelly.
Acknowledgements
This grant is supported by the National Institutes of Health
(ES014482 to LM). We thank the Genome Technology Access Center in the Department of Genetics at Washington University
School of Medicine for help with genomic analysis. The Center is
partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant#
UL1RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. This publication is solely the responsibility of the authors and does not necessarily represent the
official view of NCRR or NIH.
References
1. Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nature reviews. Endocrinology. 2010; 6: 363-370. doi:10.1038/nrendo.2010.87
2. Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995; 51(6): 435-445
3. McLachlan JA. Prenatal exposure to diethylstilbestrol in mice: toxicological studies. Journal of toxicology and environmental health. 1977; 2(3): 527-537. DOI:10.1080/15287397709529453
4. McLachlan JA, Newbold RR, Shah HC, Hogan MD., Dixon, R. L. Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES). Fertil Steril. 1982; 38(3):364-371
5. Kurita T., Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol. 2001; 240(1):194-211
6. Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, et al. Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Mullerian duct epithelium. Dev Biol. 2013; 381(1): 5-16. doi: 10.1016/j.ydbio.2013.06.024
7. Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, and Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology. 1998; 139(10): 4092-4101
8. Huang WW, Yin Y, Bi Q, Chiang TC, Garner N, Vuoristo J, et al. Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol. 2005; 19(3): 669-682 9. Yin Y, Lin C, Veith GM, Chen H, Dhandha M, Ma L. Neonatal diethylstilbestrol
exposure alters the metabolic profile of uterine epithelial cells. Dis Model Mech. 2012; 5(6):870-880. doi: 10.1242/dmm.009076.
10. Yin Y, Lin C, Ma L. MSX-2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol Endocrinol. 2006; 20(7): 1535-1546
11. Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000; 24(4): 391-395
12. Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, et al. Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003; 130(2): 379-389
13. Bei M, Stowell S, Maas R. Msx2 controls ameloblast terminal differentiation. Developmental dynamics. 2004; 231(4):758-765. DOI: 10.1002/dvdy.20182
14. Cai J, Ma L. Msx2 and Foxn1 regulate nail homeostasis. Genesis. 2011; 49(6): 449-459. doi: 10.1002/dvg.20744
15. Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, et al. Conservation of early odontogenic signaling pathways in Aves. Proceedings of the National Academy of Sciences of the United States of America. 2000; 97(18): 10044-10049. doi: 10.1073/pnas.160245097
16. Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002; 296(5573): 1644-1646
during the development of the mouse female reproductive tract. Development. 1998; 125(16): 3201-3211.
18. Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nature genetics. 1998; 20(3): 228-230
19. Couse JF, Dixon D, Yates M, Moore AB, Ma L, et al. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001; 238(2): 224-238
20. Newberry EP, Boudreaux JM, Towler DA. Stimulus-selective inhibition of rat osteocalcin promoter induction and protein-DNA interactions by the homeodomain repressor Msx2. J Biol Chem. 1997 ; 272(47): 29607-29613
21. Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX-2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003; 278(46): 45969-45977
22. Bigsby RM, Cooke PS, Cunha GR. A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol. 1986; 251, E630-636
23. Yin Y, Huang WW, Lin C, Chen H, MacKenzie A, Ma L. Estrogen suppresses uterine epithelial apoptosis by inducing birc1 expression. Mol Endocrinol. 2008; 22(1): 113-125
24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4): 402-408
*Corresponding Author: Liang Ma, Department of Medicine, Box 8123, Washington University 660 S. uclid Ave. St. Louis, MO 63110, USA, Fax: 314-454-5626; Email: [email protected]
Received Date: Received Date: October 30, 2014, Accepted Date: May 04, 2015, Published Date: May 12, 2015.
Copyright: © 2015 Liang Ma, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.