Adventitious shoot mass production of hop (
Humulus lupulus
L.)
var. Eroica in liquid medium from organogenic nodule cultures
Dora Batista *, Lia Ascensa˜o, M. Joa˜o Sousa, M. Salome´ Pais
Departamento de Biologia Vegetal,Centro de Biotecnologia Vegetal,Faculdade de Cieˆncias de Lisboa,Bloco C2-1°piso,Campo Grande,
1749-016Lisboa,Portugal
Received 6 May 1999; received in revised form 27 September 1999; accepted 27 September 1999
Abstract
Two efficient methods with high potential for automation are described for mass propagation ofHumulus lupulusL. var. Eroica in liquid medium. Suspensions were initiated by culturing petioles directly in a Murashige and Skoog’s modified liquid medium (MS%) containing 0.8 mg/l indole-3-acetic acid (IAA) and 0.02 mg/l kinetin or by inoculating petiole-derived callus into MS%liquid medium supplemented with 0.01 mg/l IAA or indole-3-butyric acid (IBA) and 1 mg/l 6-benzylaminopurine (BAP). In the last case, transfer of calli to liquid medium proved essential for inducing morphogenesis. Organogenic nodules forming green organogenic nodular clusters (GONCs) were the basic regenerative structures producing large amounts of adventitious shoots. Both methods induced the formation of GONCs within 2 – 3 months. Establishment of GONCs cultures in hormone-free liquid medium favoured nodule multiplication and continuous shoot emergence and development. However, GONCs should only be separated from the suspensions when reaching a high level of differentiation. Histological studies revealed that nodules arise through a complex process that includes four developmental stages: (1) organization centers derived from neoformed xylemic cells; (2) independent nodules displaying a consistent internal cell/tissue differentiation with at least three cell types (meristematic cells, parenchymatous cells and vascular elements) and two cell regions (epidermal and cortex/vascular); (3) ‘polycenter’ nodules; and (4) organogenic nodules. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Hop;Humulus lupulusL.; Nodule culture; Liquid medium; Organogenesis
www.elsevier.com/locate/plantsci
1. Introduction
Hop cultivation is a valuable economic resource in many countries, recognized for at least 10 cen-turies due to its importance to the brewing indus-try. This dioecious, perennial, climbing plant of the Cannabinaceae family is exclusively grown for its female inflorescences. The interest of the female flowers lies in the lupulin glands which produce resins and essential oils that provide bitterness, flavour and aroma to beer and ales.
From the earliest recognition of the advanta-geous application of hops in brewing, there must have been a continuing process of selecting
im-proved hop varieties [1]. Breeding programmes were initiated in the beginning of century in UK, United States, Denmark and ex-Czechoslovakia and have been directed primarily at producing superior bitter or aromatic types combined with resistance to the major pests and diseases [1,2]. Gene transfer technologies through tissue culture are an attractive alternative to normal breeding methods since they offer ways of introducing spe-cific desirable characteristics from several different origins in established hop varieties, without alter-ing their quality profiles. However, efficient in vitro regeneration of plants is required for the application of molecular genetics to crop improvement.
Despite the great interest in hops, only a limited amount of work concerning successful plant regen-eration has been reported, in which regenregen-eration is
* Corresponding author. Tel.:+351-1-750-0165; fax:+ 351-1-757-3625.
E-mail address:[email protected] (D. Batista)
achieved on solid medium via organogenesis. Motegi [3] obtained adventitious shoots from callus cultures derived from stem explants of Shinshuwase and Italy-2 cvs. Connell and Heale [4] and Heale et al. [5] developed a green callus system for the regeneration of several cultivars including Challenger, Eastwell Golding and Ear-lybird Golding. Rakousky and Matousek [6] ob-tained direct regeneration from petioles or internodes of two Czech commercial clones. Batista et al. [7] regenerated plants of a sponta-neous Portuguese hop clone and of hop var. Brewer’s Gold from nodular green callus cultures derived from stem and petiole explants.
For most species cultured in vitro, shoot re-generation is almost always achieved on solid medium [8]. Adventitious shoot formation and development in liquid medium is not commonly reported [9 – 13], and it may present several prob-lems, mainly shoot morphological aberrations and vitrification. Any successful attempt at re-generating shoots in liquid medium is valuable because it provides an ideal regeneration system. Under these conditions mass production, devel-opment control and automation are possible, which, together with the concomitant saving in manual labor and costs, increases the system fea-sibility for commercial large-scale production in bioreactors [12].
In poplar, plant/organ regeneration in liquid culture was obtained from highly meristematic nodular clusters termed nodules [14]. Nodules were described as independent, spherical, dense cell clusters which form a cohesive unit and dis-play a consistent internal cell/tissue differentia-tion pattern and a high regenerative capacity [14]. Successful nodule cultures with high regen-eration potential were also described for Pinus radiata [15], Eucalyptus grandis [16], Chicorium intybus [17], Nerine sp. [12] and Ananas comosus [18].
This paper reports on the establishment of a highly productive hop regeneration system based on nodule culture in liquid medium. Two effi-cient induction methods leading to the develop-ment of organogenic nodules are described for mass production of adventitious shoots of Hu -mulus lupulus var. Eroica. The histological char-acterization of nodules and their development are also presented.
2. Materials and methods
2.1. Plant material and suspension cultures establishment
Petiole segments (6 – 8 mm) were removed from micropropagated female plants of H. lupulus var. Eroica, established under sterile conditions as described earlier [7], 3 months after subculture, and used as the primary explants. Suspension cultures were established in a Murashige and Skoog medium [19] (MS%), modified by Batista et al. [7], supplemented with 15 g/l sucrose and 30 mg/l cysteine (pH 5.7), according to two different methods: (1) inoculation on solid medium for callus induction followed by transfer to liquid medium (mixed culture); and (2) direct inoculation into liquid medium (liquid culture). All complete culture media were autoclaved at 121°C for 15 min. Suspension cultures were placed on a gyratory shaker (2492°C) at 80 – 85 rpm, under a 16/8 h photoperiod with light (73mE/m2per s) supplied by
cool white daylight fluorescent lamps (Phillips TLD 18W/33). The influence of different parameters on morphogenesis induction was evaluated for each case. Each experiment was performed using 6 flasks per treatment.
2.2. Nodule induction
2.2.1. Mixed culture
Callus induction was performed on solid MS% medium (8 g/l agar) supplemented with 0.01 mg/l indole-3-acetic acid (IAA) or indole-3-butyric acid (IBA) and 1 mg/l 6-benzylaminopurine (BAP), in 9×4.5-cm flasks (40 ml-8 petioles/flask). Solid cultures were maintained at 2292°C under a 16/8 h photoperiod (33mE/m2per s). After 5 weeks, calli
were isolated and cultured separately in MS% liq-uid medium of identical composition depleted of growth regulators for shoot emergence and devel-opment. From this point on, both callus and GONCs cultures were routinely subcultured in this medium every 4 weeks.
2.2.2. Liquid culture
Liquid cultures were established by inoculating 8 – 10 petioles into 250-ml Erlenmeyer flasks con-taining 50 ml of MS% medium with 0.8 mg/l IAA and 0.02 mg/l kinetin. Different concentrations of glucose (20, 30, 40 g/l) and sucrose (20, 25, 30, 40 g/l) were tested. After callus and/or explant mor-phogenic competence induction in this medium (3 – 4 weeks), which was considered the first cul-ture phase, culcul-tures were sequentially transferred, every 4 weeks, for up to three more culture phases in order to induce nodule formation. Cul-ture phases differed only in the hormonal supple-mentation of the medium that was as follows: (2nd culture phase) 0.1 mg/l IAA+2 mg/l BAP, (3rd) 0.1 mg/l BAP and (4th) absence of growth regulators. In the second culture phase, sugar concentrations above 30 g/l were reduced to 20 g/l. From the third culture phase onwards, all sucrose and glucose concentrations were replaced by 15 g/l sucrose (control conditions). Subcultur-ing from the last culture phase was done every 4 weeks using the same medium (hormone-free MS%
liquid medium+15 g/l sucrose). GONCs, usually detected between the third and fourth culture phase, were cultured as previously described for mixed cultures.
2.3. Shoot regeneration
GONCs produced by both methods were iden-tical and thus handled in the same way. Given the continuous production of GONCs by the original suspensions, new GONCs cultures were repeat-edly established from a single replica. Regener-ated plantlets with approximately 3 cm tall were excised from the nodular clusters surface and transferred to micropropagation medium [7]. Af-ter the first production cycle, ranging from the emergence of the first shoot through to the best moment for plantlet removal (2 – 3 months), GONCs were used for other production cycles (1 – 2 months each). Based on the high and longed regeneration capacity of GONCs, this
pro-cedure could be repeated at least five times. Experiments providing the best conditions for re-generation, for both mixed and liquid culture, were repeated three times using 12 replicates. The efficiency of the regeneration process was evalu-ated based on the regenerative capacity of GONCs, shoot regeneration rates (total and per production cycle) and average number of regener-ated shoots per GONC. Final data correspond to combined means from the three independent tri-als.
2.4. Plant transfer to soil
After development and rooting on micropropa-gation medium [7] for at least 2 months, plants obtained from both regeneration methods were potted and hardened in a growth chamber (hu-midity progressively reduced to 70%, 2292°C, 16/8 h photoperiod, 60 mE/m2 per s) over a 2 –
3-week period.
2.5. Histological study
Given the similarity of the regeneration path-way induced by both methods, histological analy-sis of the initiation and development of nodules and shoots was performed using only mixed cul-tuderived samples. Tissues were periodically re-moved from culture and fixed in formalin – glacial acetic acid – ethanol 70% (1:1:18 v/v) (FAA) for at least 3 days at 4°C or freshly sectioned at 930
mm with a Reichert freezing microtome. Sections
were stained with iodine green/Grenacher’s alum carmin [20] or directly observed on a Leitz Wet-zlar Dialux light microscope.
3. Results and discussion
3.1. Nodule induction
3.1.1. Mixed culture
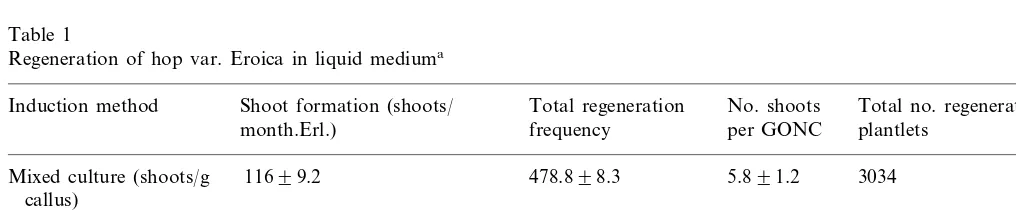
development of YGCCs into GONCs (Fig. 1B). Chlorophyll accumulation was an essential feature of YGCCs development. Further differentiation of
GONCs led to multiple shoot-bud emergence from the nodule surface (Fig. 1C – E), approximately 3 weeks after GONCs isolation.
Fig. 1. Organogenic nodule formation and shoot regeneration of hop var. Eroica in liquid medium: (A) yellow-greenish compact cell cluster (YGCC) (arrow) formed in direct contact with the phenolized calli, during the third culture phase in liquid culture. Bar, 10 mm. (B) Differentiating green organogenic nodular clusters (GONCs) 10 weeks after suspension culture establishment (mixed culture). Bar, 10 mm. (C) GONC regenerating shoot-buds 2 weeks after being transferred to hormone-free liquid medium (independent GONCs cultures). Bar, 500 mm. (D) Adventitious shoots (arrows) emerging from the GONC surface within one
Fig. 2. Effect of inoculum density on hop var. Eroica shoot regeneration from mixed culture-derived green organogenic nodular clusters (GONCs), expressed as the number of shoots regenerated per g of callus (total regeneration frequency) or per month (m) and Erlenmeyer (Erl.) (shoot formation rate). Means9S.E. (n=3). Inoculum density refer to 25 ml of liquid medium. Shoot formation was recorded monthly. Re-sults correspond to 4 months of culture after first shoot emergence.
hibitory substances of somatic embryogenesis in coffee callus cultured at high density.
A first prolonged 6 weeks subculture was re-quired for GONCs formation, but once this stage was reached medium renewal was necessary at 4-week intervals for further differentiation. In ex-periments carried out with regular subculture in-tervals, shoot regeneration from GONCs was not achieved.
Reduced agitation (80 – 85 rpm) was necessary to obtain differentiation. The temporary use (7 – 10 days) of agitations up to 105 rpm was beneficial when morphogenesis seemed blocked or when morphogenic clusters remained attached to callus, but it was inadequate for shoot-bud emergence [21]. The positive effect of a reduced agitation on nodule differentiation may also be associated with a lower oxygen level as with somatic embryogene-sis induction in Camellia japonica [24].
3.1.2. Liquid culture
Explants established directly in liquid medium produced in 3 – 4 weeks a slow growing, thick and flat type of yellowish callus. During the second culture phase phenolic oxidation and root forma-tion were observed. Producforma-tion of YGCCs (Fig. 1A) started in the beginning of the third culture phase (98 weeks), in cultures where both pheno-lic oxidation of tissues and root formation had been previously observed. However, whenever root formation was excessive nodular clusters failed to develop. For leafy spurge cell suspension cultures, the usual pattern of development was to form shoots on cultures that rooted first [10]. GONCs were detected between the third and fourth culture phase (910 – 12 weeks), approxi-mately 2 weeks after the appearance of YGCCs as described for mixed cultures.
Increase of sugar concentration in the initial medium for morphogenic competence induction (first culture phase) favoured the formation of GONCs. GONCs differentiation in the fourth cul-ture phase was achieved after replacement of sugar, in both glucose and sucrose-containing me-dia, by 15 g/l sucrose (control conditions). The highest regeneration rates were obtained using glu-cose at 30 or 40 g/l (Fig. 3). Sucrose concentra-tions over 20 g/l became inhibitory (Fig. 3). Maintenance of the morphogenic potencial of original suspensions, by continuously producing GONCs, was also negatively affected by the use of Although morphogenic development was totally
dependent upon transfer to liquid medium, callus induction conditions on solid medium were impor-tant. Callus production in H. lupulus L. var. Eroica can be easily achieved on several media, but morphogenesis induction in liquid medium was only obtained from calli produced on MS% medium with 0.01 mg/l IAA or IBA+1 mg/l BAP [21]. In hop var. Eroica mixed cultures, morpho-genesis may be a function of the medium used for callus initiation as previously suggested for organogenic cultures of Euphorbia esula [10]. This calli conditioning suggests that morphogenic com-petence had already been acquired at the moment of transfer to liquid medium and that liquid cul-ture was crucial for the expression of the calli morphogenic competence. Among the factors con-tributing to this response in liquid medium, aera-tion, shearing forces and higher availability of BAP and nutrients were certainly very important as previously reported for other plant species [9,12,22].
in-Fig. 3. Influence of the sucrose and glucose concentration, used in the first culture phase, on shoot regeneration from liquid culture-derived green organogenic nodular clusters (GONCs), expressed as the number of shoots regenerated per inoculated petiole. Means9S.E. (n=3). At the moment of regeneration, sucrose and glucose concentrations had been replaced by 15 g/l sucrose. Results correspond to three pro-duction cycles (4 – 6 months of culture after first shoot emer-gence). Absence of bars indicates zero regeneration.
even guaranteed, shoot emergence in 2 – 3 weeks. When GONCs were subcultured together with the original calli, shoot emergence and development was arrested. However, if GONCs were isolated before reaching initial shoot-bud emergence, de-generation, reversion or inhibition occurred. The existence of endogenous substances inducing shoot regeneration which could be released by the cells participating in differentiation may explain the promoting effect of joining GONCs together. In his review, Taylor [27] claims that there is some evidence concerning the transmission of cell-fate determinant molecules from older cells to newly formed cells.
Hop var. Eroica nodules showed a higher regen-erative capacity than the nodules reported for other plant species. On average 83% of hop nod-ules regenerated shoots, regardless of their origin, whereas 41 and 70% were the best percentages of nodules regenerating shoots for Cichorium intybus and Ananas comosus respectively [17,18].
One to thirty shoot-buds formed throughout the GONCs surface (Fig. 1C – E). Shoot regeneration and elongation were obtained in hormone-free medium which seems to be the best option for several species [28]. Shoots were removed from GONCs when they reached approximately 3 cm. Smaller shoots were unable to withstand transfer to solid medium. In addition, bud elongation was more effectively achieved in liquid medium as reported forGossypium hirsutum[28] andSesbania grandiflora [29]. Regenerated shoots showed no signs of hyperhydricity. Spontaneous rooting was observed in 10% of the plantlets recovered. Regu-lar plantlet removal was necessary not only for the growth of the regenerated shoots but also for further regeneration from GONCs. Inhibition of further bud elongation caused by the persistence of buds attached to the regenerating explant was reported earlier [29].
Adventitious shoots production in H. lupulus var. Eroica was cyclical since GONCs could be used repeatedly, after plantlet removal. Regener-ated plantlets were thus regularly obtained until the regeneration potential of GONCs cultures was lost (Fig. 1F and Fig. 4), which led to total production of hundreds to thousands of plantlets (Table 1). Mixed cultuderived GONCs re-mained productive for 9 months, whereas liquid culture-derived GONCs stopped regenerating shoots after 6 months of culture (Fig. 4). As sucrose. Lapen˜a et al. [25] suggested that the
in-hibitory effect of high sucrose concentrations on adventitious shoot-bud formation of Digitalis ob -scura was related to medium osmolarity.
Optimum development of organogenic nodules in liquid cultures depended on the application of four sequencial culture phases: (1st) obtention of morphogenic competent tissue; (2nd) nodule in-duction from that tissue; (3rd) formation and/or development of YGCCs; (4th) production and/or differentiation of GONCs. Taking into account that culture phases differed only in the hormonal supplementation of the medium, it can be sug-gested that nodule formation in liquid culture occur as a function of hormonal regulation.
3.2. Shoot regeneration
GONCs derived from both methods followed a similar pattern of development. Continuous nod-ule enlargement and fragmentation were impor-tant for shoot regeneration and GONCs multiplication, respectively. The larger the GONC, the higher the regenerative efficiency. GONCs measured frequently about 1.5 cm of diameter. Kreuger et al. [26] reported a similar development for pro-embryogenic masses of Cyclamen persicum.
Fig. 4. Shoot formation rate obtained in each production cycle, expressed as the number of shoots formed per month and Erlenmeyer (Erl.). Means9S.E. (n=5). The regenera-tion ability of both inducregenera-tion methods (MC, mixed culture; LC, liquid culture initiated with 30 or 40 g/l glucose) is compared. Results refer to the total regenerative period of mixed and liquid cultures (MC, 9 months; LC, 6 months).
Based on these results, the yearly production is estimated to be approximately 657 plantlets/g of callus for mixed culture and 139.6 plantlets per petiole for liquid culture. Plantlets could be accli-matized with 80 – 95% success, showing no sign of morphological abnormalities (Fig. 1G).
In liquid culture best results were obtained using 30 g/l of glucose, showing that higher regeneration frequencies did not necessarily lead to larger amounts of regenerated plantlets (Table 1). In hop organogenic nodule cultures the total number of regenerated shoots was influenced mostly by the capacity of GONCs cultures for proliferation and development and by the average number of shoots formed per GONC. Although the regeneration results obtained for each method are not directly comparable (due to the different duration of mixed and liquid cultures productive periods and to the particular conditions of each method), mixed culture-derived organogenic nodules have undoubtedly produced a much higher quantity of regenerated plantlets (Table 1), which also devel-oped better in vivo (data not shown). Neverthe-less, both methods proved to be efficient for adventitious shoot mass production of hop var. Eroica. This system has the potential for providing large quantities of meristematic tissue for genetic transformation purposes.
Due to its high production potential, nodule culture has been considered to be an alternative culture system for mass propagation although it has only been described in a small number of plants [12,14 – 18]. A first attempt at scaling-up hop var. Eroica organogenic nodule cultures in bioreactors is already being carried out in the laboratory.
expected, the first production cycle was longer (2 – 3 months) than the following (1 – 2 months) because emergence of the first shoots was not synchronous and bud initiation the first time round was slower. After removing plantlets for the first time, mass production was initiated (Fig. 1F). The regeneration trend up to the third cycle corre-sponded to an increasing number of shoots being obtained in a decreasing period of time (3 weeks was the minimum period of time required for regeneration). Similar results were obtained by Teng [18] with A. comosus nodule cultures. Such nodules required 6 weeks for the first visible ad-ventitious bud to be formed but after five subcul-tures only 2 weeks were necessary for regeneration.
Table 1
Regeneration of hop var. Eroica in liquid mediuma
Shoot formation (shoots/ No. shoots
Induction method Total regeneration Total no. regenerated per GONC
month.Erl.) frequency plantlets
Mixed culture (shoots/g 11699.2 478.898.3 5.891.2 3034 callus)
Liquid culture(shoots/petiole)
77.6916.2 78.5913.7
30G 8.893.1 1660
50.691.8 123.895.6 6.892.1 557 40G
aComparison of the regeneration rates induced by mixed and liquid culture methods. Results refer to the total regenerative
Fig. 5. Histology of the first steps of hop var. Eroica nodule formation: (A – C) sections stained with iodine green/Grenacher’s alum carmin. (A) Neoformed tracheary elements arising in the cambial region (arrow heads) after 10 days of culture on solid medium. Bar, 40 mm. (B) Organization center (oc) on the surface of the callus tissue developing side by side with a xylemic
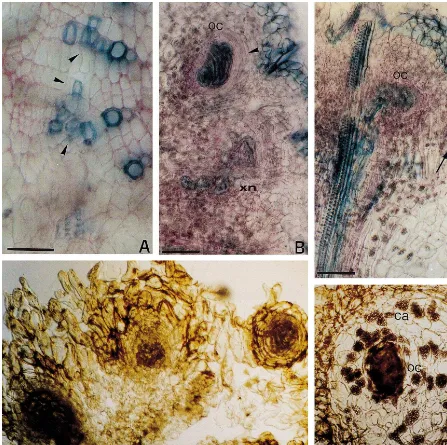
nucleous (xn), 12 days after petiole inoculation. In the organization center, meristematic cell layers are clearly developing (arrow head) around the vascularization nucleous. Note the loosely arranged cells of the peripheral area. (C) Longitudinal section showing the vascular connection maintained with a newly formed organization center (oc). Near this area another organization center is beginning to develop (arrow). (D) Formation of detaching pre-nodular structures at day 28. (E) Independent nodule showing the typical cell/tissue organization: organization center (oc), a cortical-like area of parenchymatous cells (ca) and a epidermal-like area (ea). Bar represents 100mm for all figures except (A).
3.3. Histological study
The histological study revealed a developmental sequence leading to the formation of organogenic nodules that included four main stages. In the first stage, neoformed tracheary elements seemed to interact forming circular xylemic nuclei (Fig. 5A).
Fig. 6.
petiole inoculation on solid medium. This callus tissue is delimited by three to four layers of anti-clinally elongated, loose cells arranged in an irreg-ular pattern (Fig. 5B,C). A large number of
In stage 2, the organization centers become autonomous, developing an epidermis-like layer. Such pre-nodular structures detach from the callus tissue (Fig. 5D) and acquire a more defined inter-nal structure, originating nodules (Fig. 5E). This process starts while still on the solid medium (approximately 4 weeks after petiole inoculation), but it is stimulated by callus transfer to liquid medium. Hop var. Eroica nodules consist of a central area of vascularization surrounded by ac-tively dividing meristematic cells (the organization center), a cortical-like area of parenchymatous cells and an epidermal-like area (Fig. 5E) as de-scribed by McCown et al. [14] for poplar.
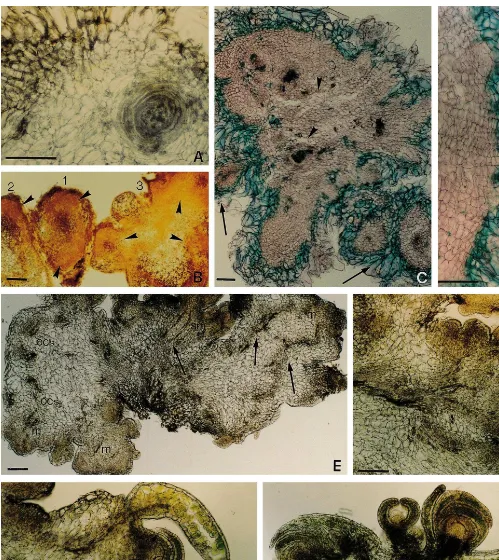
Nodules in suspension increase in diameter (Fig. 6A) and form marginal regions of procambial-like cells when reaching a critical size. Groups of such nodules (3 – 4), displaying different levels of devel-opment, formed the YGCCs (Fig. 6B). YGCCs formation was the longest event during hop var. Eroica regeneration process (approximately 6 weeks), which suggests that this may be a critical step.
About 2 weeks later, ‘polycenter nodules’ are formed (stage 3) (Fig. 6C). Although morphologi-cally similar to those reported for Populus sp.[14] and C. intybus [17], hop nodules at this stage are green. Green nodules were also observed in radi-ata pine [15]. Sections of hop var. Eroica nodules also show several regulary arranged cell layers adjacent to a peripheral area similar to that found on calli (Fig. 6C,D).
This nodular phase corresponds to the initial development of GONCs. Recently isolated GONCs contain three to four developing polycen-ter nodules, loosely attached to each other (Fig. 6C). Expansion of polycenter nodules in diameter is accompanied by the formation of cracks in the parenchymatous tissue (Fig. 6C). According to
McCown et al. [14], poplar polycenter nodules can break up along the surfaces of voids where the dividing cell layers from different centers meet, multiplying the number of independent nodules. Although cracks were frequently detected on hop var. Eroica polycenter nodules, nodule break-up was never observed.
Natural division of hop’s polycenter nodules occurs prior to further differentiation by budding off of small distinct ‘daughter’ nodules (Fig. 6C). A similar process was reported for other nodule cultures [14,16 – 18]. This event together with polycenter nodule detachment along the external loosely arranged cells, contributes to the high propagation potential of GONCs cultures. Nodule multiplication and differentiation seem to be in-compatible. It appears that organization centers may participate either in the formation of new nodules (Fig. 6C) or in the vascularization of shoot meristems (Fig. 6E), depending on the polycenter nodule determination (for multiplica-tion or differentiamultiplica-tion).
Finally, highly organogenic nodules (stage 4) forming typical regenerating GONCs were ob-served (Fig. 6E). Such nodules lost the external loosely arranged cells and formed a typical epider-mal layer. Multiple meristemoids appeared throughout the nodule surface apparently con-nected with one another and with the nodule centre through a vascularization net (Fig. 6F). Shoot-buds developed in a few days (Fig. 6G,H). Although stages 2 and 3 appeared to correspond to those described by McCown et al. for poplar nodules [14], the overall nodular process occurring in hop var. Eroica cultures appeared to be more complex than the general developmental sequence proposed by these authors. The system developed is of tremendous potential for both hop mass propagation and transformation purposes.
Acknowledgements
We thank the Foundation for Science and Tech-nology (Programme Praxis XXI) for supporting this work (Master grant BM/1456/94).
References
[1] R.A. Neve, Centenary review: Hop breeding worldwide — its aims and achievements, J. Inst. Brew. 92 (1986) 21 – 24. [2] M. Pillay, S.T Kenny, Random amplified polymorphic DNA (RAPD) markers in hop,Humulus lupulus: level of genetic variability and segregation in F1progeny, Theor.
Appl. Genet. 92 (1996) 334 – 339.
[3] T. Motegi, Differentiation of shoots from hop stem callus culture, Kyoyubo Kenkyu Neupo Iwate Ika Daigaku 14 (1979) 15 – 17 in: Chem. Abstr., 94, p. 12901.
[4] S.A. Connell, J.B. Heale, Development of an in vitro selection system for novel sources of resistance toVerticil
-lium wiltin hops, in: L.A. Withers, P.G. Alderson (Eds.), Plant Tissue Culture and its Agricultural Applications, Butterworths, University Press, Cambridge, 1986, pp. 451 – 458.
[5] J.B. Heale, T. Legg, S. Connell,Humulus lupulusL. (Hop): In vitro culture; attempted production of bittering compo-nents and novel disease resistance, in: Y.P.S. Bajaj (Ed.), Biotechnology in Agriculture and Forestry, Medicinal and Aromatic Plants II, vol. 7, Springer-Verlag, Berlin, 1989, pp. 264 – 285.
[6] S. Rakousky, J. Matousek, Direct organogenesis in hop — a prerequisite for an application ofA.tumefaciens -me-diated transformation, Biol. Plant. 36 (1994) 191 – 200. [7] D. Batista, M.J. Sousa, M.S. Pais, Plant regeneration from
stem and petiole-derived callus of Humulus lupulus L. (Hop) clone Braganc¸a and var. Brewer’s Gold, In Vitro Cell. Dev. Biol. 32 (1996) 37 – 41.
[8] C.E. Flick, D.A. Evans, W.R. Sharp, Organogenesis, in: D.A. Evans, W.R. Sharp, P.V. Ammirato, Y. Yamada (Eds.), Handbook of Plant Cell Culture, Techniques for Propagation and Breeding, vol. 1, Macmillan, New York, 1983, pp. 13 – 81.
[9] G. Hussey, Problems and prospects in the in vitro prop-agation of herbaceous plants, in: L.A. Lyndsey, A. With-eers, P.G. Alderson (Eds.), Plant Tissue Culture and its Agricultural Applications, Butterworths, London, 1986, pp. 69 – 84.
[10] D.G. Davis, P.A. Olson, R.L. Stolzenberg, Organogenesis in cell cultures of leafy spurge (Euphorbiaceae) accessions from Europe and North America, Plant Cell Rep. 7 (1988) 253 – 256.
[11] L. Lapen˜a, P. Pe´rez-Bermu´dez, J. Segura, Factors affect-ing shoot proliferation and vitrification inDigitalis obscura
cultures, In Vitro Cell. Dev. Biol. 28P (1992) 121 – 124. [12] M. Ziv, S. Kahany, H. Lilien-Kipnis, Scaled-up
prolifera-tion and regeneraprolifera-tion of Nerine in liquid cultures. Part I. The induction and maintenance of proliferating meristem-atic clusters by paclobutrazol in bioreactors, Plant Cell Tissue Org. Cult. 39 (1994) 109 – 115.
[13] W.L. Teng, Regeneration of Anthurium adventitious shoots using liquid or raft culture, Plant Cell Tissue Org. Cult. 49 (1997) 153 – 156.
[14] B.H. McCown, E.L. Zeldin, H.A. Pinkalla, R.R. Dedolph, Nodule culture: a developmental pathway with high po-tencial for regeneration, automated micropropagation, and plant metabolite production from woody plants, in: J.W. Hanover, D.E. Keathley (Eds.), Genetic Manipula-tion of Woody Plants, Plenum, New York, 1988, pp. 149 – 166.
[15] J. Aitken-Christie, A.P. Singh, H. Davies, Multiplication of meristematic tissue: a new tissue culture system for radiata pine, in: J.W. Hanover, D.E. Keathley (Eds.), Genetic Manipulation of Woody Plants, Plenum, New York, 1988, pp. 413 – 432.
[16] E. Warrag, M.S. Lesney, D.J. Rockwood, Nodule culture and regeneration ofEucalyptus grandishybrids, Plant Cell Rep. 9 (1991) 586 – 589.
[17] S. Pie´ron, M. Belaizi, Ph. Boxus, Nodule culture, a possible morphogenetic pathway inCichorium intybusL. propagation, Scientia Hortic. 53 (1993) 1 – 11.
[18] W.L. Teng, An alternative propagation method ofAnanas
through nodule culture, Plant Cell Rep. 16 (1997) 454 – 457.
[19] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiol. Plant. 15 (1962) 473 – 497.
[20] D.A. Johansen, Plant Microtechnique, McGraw-Hill, New York, 1940, p. 59, 70, 71.
[21] D. Batista, Regenerac¸a˜o de Plantas de Humulus lupulus L. (lu´pulo) var. Eroica em Meio Lı´quido: Cultura de No´dulos Organoge´nicos (English abstract), Faculdade de Cieˆncias da Universidade de Lisboa, Lisboa, 1998, pp. 23 – 105 (Thesis).
[22] C.H. Bornman, T.C. Vogelman, Effect of rigidity of gel medium on benzyladenine-induced adventitious bud for-mation and vitrification in vitro in Picea abies, Physiol. Plant. 61 (1984) 505 – 512.
[23] J.V. Boxtel, M. Berthouly, High frequency somatic em-bryogenesis from coffee leaves, Plant Cell Tissue Org. Cult. 44 (1996) 7 – 17.
[24] M.C. Pedroso, M.S. Pais, Plant regeneration from em-bryogenic suspension cultures of Camellia japonica, In Vitro Cell. Dev. Biol. 31 (1995) 31 – 35.
[25] L. Lapen˜a, P. Pe´rez-Bermu´dez, J. Segura, Morphogenesis in hypocotyl cultures of Digitalis obscura: influence of carbohydrate levels and sources, Plant Sci. 57 (1988) 247 – 252.
[26] M. Kreuger, E. Postma, Y. Brouwer, G.J. van Holst, Somatic embryogenesis of Cyclamen persicum in liquid medium, Physiol. Plant. 94 (1995) 605 – 612.
[27] C.B. Taylor, Plant vegetative development: from seed and embryo to shoot and root, Plant Cell 9 (1997) 981 – 988. [28] D.C. Agrawal, A.K. Banerjee, R.R. Kolala, A.B. Dhage, A.V. Kulkarni, S.M. Nalawade, S. Hazra, K.V. Krishna-murthy, In vitro induction of multiple shoots and plant regeneration in cotton (Gossypium hirsutumL.), Plant Cell Rep. 16 (1997) 647 – 652.
[29] C. Detrez, S. Ndiaye, B. Dreyfus, In vitro regeneration of the tropical multipurpose leguminous treeSesbania gran