Physicochemical and mechanical properties of extruded laminates
from native and oxidized banana starch during storage
Yunia Verónica García-Tejeda
a, Carlos López-González
a, Juan Pablo Pérez-Orozco
b,
Rodolfo Rendón-Villalobos
a, Alfredo Jiménez-Pérez
a, Emmanuel Flores-Huicochea
a,
Javier Solorza-Feria
a,*, C. Andrea Bastida
aaInstituto Politécnico Nacional, CEPROBI, Km. 6, Carretera Yautepec-Jojutla, Calle Ceprobi 8, Col. San Isidro, Z.C. 62731 Yautepec, Morelos, Mexico bInstituto Tecnológico de Zacatepec, Departamento de Ingeniería Química y Bioquímica, Calzada Tecnológico #27, Apartado postal 45, C.P. 62780 Zacatepec, Morelos, Mexico
a r t i c l e
i n f o
Article history:
Received 28 November 2012 Received in revised form 27 May 2013
Accepted 28 May 2013
Keywords: Oxidized starch Extruded laminates Physicochemical properties Modified starch
Musa paradisiaca
a b s t r a c t
Changes in some mechanical and physicochemical properties of extruded Laminates, made from native (NBS) and oxidized banana starch (OBS) with 3 g/100 g of sodium hypochlorite, as affected by storage time (0e45 days) were evaluated. Micrographs showed cracks and pores with some continuity on the surface of both OBS and native banana starch (NBS) laminates. Extruded Laminates of OBS were more transparent, soluble and homogeneous than those from NBS. Little differences were observed in water vapor permeability between NBS and OBS Laminates. Laminates solubility decreased with storage time. The X-ray diffraction from NBS and OBS Laminates, showed similar type B patterns and percent of crystallinity. Throughout storage time, an increase in temperature and enthalpy of melting was observed for all Laminates, however, the enthalpy values for OBS Laminates were lower than those of NBS. The tensile strength, percent of elongation and elasticity modulus values of OBS Laminates, were higher than those of NBS. Overall, OBS might be a suitable raw material to produce extruded Laminates with adequate functional properties.
Ó2013 Elsevier Ltd. All rights reserved.
1. Introduction
Nowadays, the indiscriminate use of plastic bags has become such an increasing environmental pollution problem, that local governments have passed laws in order to limit their use. Over the last years, the interest in biodegradablefilms made from renewable and natural polymers has increased. Water-soluble polysaccharides such as starch, cellulose derivatives, alginate and pectin can form biodegradable and ediblefilms (Garcia, Ponotti & Zaritzky, 2006). Thus, for example, the substitution of plasticfilms by short term degradation ones, mainly those from natural sources, has been strongly encouraged. The production offilms or laminates (thickness > 1 mm), based on biological materials uses fi lm-forming agents (e.g., macromolecules as polysaccharides, starch
and proteins), a solvent (usually water) and a plasticizer (e.g., pol-yols as glycerol, sorbitol, ethylene glycol).
Starch consists of two chemically distinct polysaccharides, namely; amylose and amylopectin, conforming characteristic structures called granules with both amorphous and crystalline structures. The amylose is an essentially linear macromolecule of glucose units linked mainly by
a
-D-(1-4) bond, although there may be somea-D-(1-6) bonds. It is distributed mostly in the starch
granule amorphous domains (lamella), with small amounts in the semicrystalline granule ring. The amylopectin is a branched glucose polymer, linked mainly bya
-D-(1-4) bonds (about 96 g/100 g) and the remaining 4e6 g/100 g bya-D-(1-6) bonds, conforming the
crystalline lamella (Hermansson & Svegmark, 1996). The propor-tion of these two polymers and their physical organizapropor-tion inside the granules, confer singular physicochemical characteristics and functional properties to starch (Thomas & Atwell, 1999). Starch granules are semi-crystalline polymeric systems, where the degree of crystallinity (15e45 g/100 g), is due to short-chain fraction of the amylopectin arranged as double helices and packed in small crys-tallites organized in a tri-dimensional structure (Du, MacNaughtan & Mitchell, 2011).*Corresponding author. Tel.:þ52 55 57 29 60 00x82543; fax:þ52 55 57 29 60 00x82521.
E-mail addresses:[email protected],[email protected], j.solorzaferia@ gmail.com(J. Solorza-Feria).
Contents lists available atScienceDirect
LWT - Food Science and Technology
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / l w t
Banana is a general term involving a number of species or hybrids in the genus Musaof the familyMusacea. Plantains are generally the larger, more angular starchy fruits of hybrid culti-vars in the banana family, edible raw when fully ripe, but also suitable for cooking. They are mainly produced in developing countries, being of major importance to people in the growing areas, where a lot of the produce is lost because of poor post-harvest handling methods. Nonetheless, banana starch has the potential to be a commodity starch because of its specific prop-erties and its potential production from low-cost, cull bananas (Zhang, Whistler, BeMiller &Hamaker, 2005). Various authors have attempted to produce films from starch isolated from various sources including maize, potato, cassava, tapioca, fl ax-seed and recently banana (Zhang et al., 2005). The most used techniques for film processing include casting and extrusion, being the latter a convenient technique for practical purposes. Starchfilm extrusion is more complex than that of conventional polymers due to multi-phase transitions involved during pro-cessing. There are many works done onfilms produced by cast-ing, but further investigation is needed on the physical properties of those obtained by extrusion.
Starch granules during extrusion are subjected to high shear stress and temperature, these factors produce the destruction of granules, elimination of the crystalline structure, at times the molecular weight reduction, and also, the formation of an amorphous molten mass (thermoplastic starch polymer, TSP), which is then pressurized and shaped by the die head (Su et al., 2009).
Modified starch has been used to replace native starch in various applications, because of its appropriate functional and physicochemical properties as a result of its granule modifi ca-tion (Kuakpetoon & Wang, 2008). A commonly used method of starch modification is its oxidation with chemical agents differing in their oxidation power. Oxidized starch is widely used in many industries, particularly in applications wherefilm formation and adhesion properties are desired. The major application of oxidized starches is as surface sizing agents and coating binders in the paper industry (Sangseethong, Termvejsayanon & Sriroth, 2010). Oxidized starch is commonly produced by reaction of starch with an oxidizing agent under controlled temperature and pH. Previous studies on corn starches have found that, when sodium hypochlorite (NaOCL) has been used, NaOCL concentrations of 2 g/100 g solution or higher, are enough to produce real changes on such starches physicochemical characteristics (Kuakpetoon Wang, 2008). During the oxidation process, hydroxyl groups on starch mole-cules are oxidized to carbonyl and carboxyl groups, contributing to improved stability of starch paste. The reaction also causes degradation of starch molecules resulting in a modified starch with low viscosity. This allows the use of oxidized starch in applications where high solid concentration is needed (Wurzburg, 1986) The addition of plasticizers is necessary to obtain a TSP by reduction of intermolecular forces, and increase in the mobility of polar polymer chains. In addition, plasticizers help to overcome the brittleness of starch films and improve their flexibility and extensibility. Glycerol has been one of the most used plasticizers, since it improves film extensibility (Gurgel, Vieira, da Silva, dos Santos & Beppu, 2011). To define possible uses and shelf life of these types of materials, studies on the effect of storage on the physicochemical properties are needed, but those studies are not available in the literature.
The purpose of this work was to evaluate the changes in some physical and physicochemical properties of extruded laminates, made from native and oxidized banana starches, as affected by storage time.
2. Material and methods
2.1. Materials
Unripe banana (Musa paradisiacaL.) selected with no yellow color on its peel (about 18 weeks from blossom), was obtained from the province of Veracruz, Mexico.
2.2. Starch isolation
Native banana starch was obtained as described byGonzález (2003). This consisted in peeling, cutting and immersing the ba-nana slices obtained in 0.25 g/100 g citric acid solution. The slices were blended three times with distilled water (water:banana ratio 1:1) using a Waring blender. The blended fruit pulp was sieved using an electric sieving machine (Testing Equipment, Model RNU). Sieves with mesh sizes 40, 100, 200 and 325 (0.425, 0.15, 0.075 and 0.045 mm, respectively), were used one at a time. Each paste formed in every sieve was washed with water at least three times (10 parts of water: 1 part of banana pulp) until the wash water was clean. The pulp cake was eliminated. Thefiltrate contained starch and smaller particles suspended. The starch was allowed to pre-cipitate during 24 h at room temperature (about 25C). The excess of water was decanted until getting a solid concentration within the range of 30e35 g/100 g. The slurry obtained was dried with a Niro Atomizer spray-dryer (Model 230 EA 11 No. 21, Denmark). The processing parameters were: inlet temperature of about 140C; outlet temperature of about 68C, with a feedingflow of 14 L/h. The dehydrated native starch was standardized with a 100 mesh sieve (0.15 mm) and stored at room temperature in a glass container, till use for further experiments.
2.3. Starch chemical analysis
For the sake of comparison, the total starch and also the apparent amylose content (Gilbert & Spragg, 1964), was obtained with potato starch as standard. For this last component, about 1.5 mg of starch was weighed into an aluminium pan and trans-ferred to a 50 ml volumetricflask. Then, about 0.5 ml of a solution NaOH 1 mmol equi/L was added and the mixture simmered during 3 min in a boiling water bath. It was then cooled down and neutralized with 0.5 ml of 1 mmol equi/L HCl, and 0.07 g of po-tassium bitartrate was added and diluted with distilled water until obtaining an approximate volume of 45 ml. Once the bitartrate was dissolved, 0.5 ml of a solution of iodine (2 mg/ml of Iodine and 20 mg/ml of KI) was added and the volume completed to 50 ml with distilled water. The solution was mixed and allowed to rest during 20 min at room temperature, and the absorbance was measured at 680 nm in a Genesys 5 spectrophotometer (Spectronic Instruments, USA). The amylose content in the starches was quantified by interpolation of the absorbance values in a standard curve. The amylopectin content was obtained by difference.
For total starch content (Goñi, García & Saura-Calixto, 1997), a 50 mg sample was dispersed in 2 mol/equi/L KOH (30 min) and incubated in a controlled-temperature water bath (60C, 45 min, pH 4.75) with amyloglucosidase (Roché No. 102 857, Roche Di-agnostics, Indianapolis, IN, USA); the glucose hydrolyzate obtained was measured with a GOD/POD reagent (SERA-PAKÒ
Plus, Bayer de México, SA de CV). Total starch content was calculated as glucose (mg)0.9; potato starch was used as standard.
2.4. Starch oxidation
active chlorine was used; the modified method described byWang and Wang (2003)was applied. The procedure was as follows: 35e 45 g/100 g starch slurry was prepared with deionized water. This was set at 35C by using a magnetic stirrer with a heating system; the pH was adjusted to 9.5 with a 1 mol equi/L NaOH solution. Then, 50 mL of sodium hypochlorite solution were added at a constant rate, slowly and during exactly 30 min, with constant stirring of the reaction mixture into theflask, using a 1 mol equi/L H2SO4solution to maintain the pH at 9.5. The mixture was let to react 50 more min, keeping the pH at 9.5 with a 1 mol equi/L NaOH solution. At the end, the pH was set to 7.0 with 1 mol equi/L H2SO4solution and the starch separated by decantation. Finally, the remaining oxidized starch was washed four times with deionized water and dried in a convection oven at 45C for 48 h.
2.5. Laminate production
A single screw laboratory extruder (19 mm diameter; ratio length/diameter of 24) with three heating zones (Beutelspacher, Model 19-24, Mexico, D. F.) was used to produce the laminates (thickness>1.0 mm) from native and oxidized banana starch. The process consisted in two stages: 1) an optimum mixture obtained by trial and error, of 65 g/100 g starch (either NBS or OBS), 17 g/100 g glycerol (Merck, Mexico), and 18 g/100 g deionized water, were homogenized thoroughly during about 2 h and let in rest 24 h at room temperature (25C) to stabilize. This mixture was processed at a screw speed of 80 rpm. The selected temperature profile for the extrusion process was determined from trial and error experiments as shown inTable 1. For inlet (zone I), processing (zone II) and outlet (zone III) areas, respectively. A 1.0 mm diameter nuzzle was used to obtain the extruded laminates, were cut manually with scissors at room temperature, to obtain small fragments as pellets of about 5 mm long. The pellets were extruded over again at the same tem-perature conditions, at the same screw speed of 80 rpm, but using a nuzzle with a rectangular inside channel of 200 mm width per 0.5 mm thick. All extruded laminates obtained were conditioned at 25C and a relative humidity (RH) of about 57 g/100 g, in a desiccator with a saturated solution of NaBr, before testing. The color and morphology were determined in samples kept for at leastfive days at the above named conditions, while all other physical and physi-cochemical tests were undertaken through the storage time (0e45 days). All tests were done at least in triplicate.
2.6. Color evaluation
A completely random model was used to choose the surface points on the laminates to evaluate the color. A universal Milton Roy colorimeter, Color-Mate (USA) with a D65 illuminant and observation angle of 10was used to do the test. Five readings were
taken for each sample, doing each test in triplicate. The measure-ments were reported using the CIELAB (L*,a*,b*) system. Chro-maticity (C) and hue angle (h) were evaluated using the following equations:
A Scanning Electron Microscope (JEOL JSMP 100, Japan) was used to take SEM micrographs from both the surface (magnification of 500) and from the lateral (magnification of 40) sides of NBS and OBS laminates, which were those more clearly distinguished. The laminates werefixed to stainless steel stubs, dehydrated with osmium tetraoxide (OsO4) and covered with a layer of colloidal gold of about 20 nm of thickness in the ionizer of metals JEOL. Samples were observed with 5 kV of voltage.
2.8. Water vapor permeability
The water vapor permeability (WVP) was evaluated using the modified method E 96-80, also known as “the test cell” (ASTM, 1989). The laminates were previously equilibrated for at leastfive days in a desiccator at 57 g/100 g RH using a saturated NaBr solu-tion. A circular opening with an area of 0.005439 m2on the test or permeation cell was covered thoroughly with a laminate of the same shape and area. Dried silica was placed into the cell, making it almost exempt of moisture (about 0 g/100 g RH). An atmosphere of 75 g/100 g RH was obtained with a saturated solution of NaCl out of the permeation cell. Every hour, the gain in weight was monitored until no further variations were observed. Once the permeation tests were done, the laminate thicknesses were measured at ten different points using a digital micrometer (Mitutoyo, Tokyo, Japan) and the WVP in g Pa 1s 1m 1was calculated using the following equation.
Where: WVTR is the water vapor transmission rate, calculated from the slope of the straight line (g/s) divided by the cell area (m2).
S is the saturated water vapor pressure at the test temperature (25C),R
1is the relative humidity (RH) of the desiccator,R2is the relative humidity of the permeation cell, and d the laminate thickness (m).
2.9. Laminates solubility
Laminates of 20 30 mm were stored one week into a dry desiccator with silica gel, until the g/100 g RH of equilibrium was almost exempted of moisture. The laminate samples were weighed (initial dry weight) and immersed in 250 mL beakers containing 80 mL of deionized water. Laminates were kept under constant stirring for 1 h, at either 25 or 80C. This last temperature was tried,
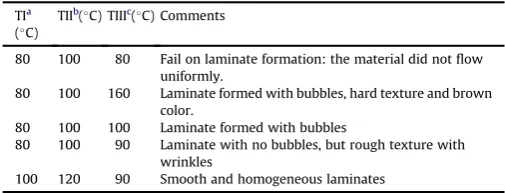
Table 1
Temperature program assayed for the laminates extrusion from banana starch.
TIa (C)
TIIb(C) TIIIc(C) Comments
80 100 80 Fail on laminate formation: the material did notflow uniformly.
80 100 160 Laminate formed with bubbles, hard texture and brown color.
80 100 100 Laminate formed with bubbles
80 100 90 Laminate with no bubbles, but rough texture with wrinkles
100 120 90 Smooth and homogeneous laminates
since previous studies have shown that, to dissolve any remains of oxidized amylose and its complex species, temperatures higher than 65C are needed (Lawal, 2004). After soaking, the samples
were dried in an oven at 60C to constant weight (final weight).
The tests were run in triplicate. The percent of solubility was calculated as follows:
2.10. X-ray diffraction
Samples of laminates at different storage times were cut into pieces of 1520 mm and analyzed with a Siemens diffractometer (Model D5000, Germany), working at a voltage of 30 kV, 20 mA, CueK radiation, wave length of 1.54A. The tests were done with the following parameters: angle interval 3e40(2
q
), chart speed was 10 mm 2q
with a running rate of 2q
min 1and a measuring time of 5 s. The crystallinity degree was calculated as the ratio of the crystalline area respect to the total area. The software Win PLOTR (Full Prof Team); a windows tool for powder diffraction patterns analysis, was employed to draw the graphics.2.11. Differential scanning calorimetry
With the aid of a differential scanning calorimeter (DSC) (TA Instruments, Model 2010; New Castle, DE), the melting tempera-ture (Tf), and the melting enthalpy (
D
H, evaluated by integrating the peak area, corresponding to such transition) of the laminates were measured. The calibration was done with Indium (point of fusion of 156.4C; enthalpy of 6.8 cal/g). The base line was achievedby running a heating program from room temperature (about 25C) to 250C. About 2.5 mg of laminate sample was weighed
with a micro balance (Model AD2Z, Perking-Elmer Corp., St Louis, MO, USA; 0.01 mg of precision). The above mentioned heating program was run three times.
2.12. Texture evaluation
A texture meter (Stable Micro Systems, Haslernere, UK and Texture Technologies Corp., Scarsdale, NY., USA), was employed to measure the tensile strength or tensile fracture (maximum force/ cross sectional area; TF, MPa), percent of elongation at break (percent of change of the initial length of the sample; %E), and elastic modulus (EM, MPa), evaluated as the slope of the forcee deformation plot of the laminates. A cell load of 25.0 kg was used for testing. A laminate height of 7 cm and a speed of deformation of 0.9 mm/min were set. All samples were prepared according to the official methodASTM (1995)D-888-95 and were placedfive days into an atmosphere of 57 g/100 g of RH (desiccator with saturated NaBr) at 25C before the texture evaluation. Laminate thicknesses
were measured randomly in at least ten different positions of the specimens with a digital micrometer (thickness gauge Mitutoyo, Tokyo, Japan).
2.13. Statistical analysis
A one way analysis of variance (ANOVA) with a significance level of 5% (*P<0.05), was applied (Montgomery, 1991) to evaluate the effect of storage on Laminate characteristics. The SigmaStat ver. 2.01 (Systat Software Inc., San José Calif., USA) statistical program was used.
3. Results and discussion
3.1. Laminates production condition and appearance
Films produced from starches are usually regarded as hydro-philic materials because of its glycerol content. The oxidized starch,
a chemical modification of native starch, produces low viscosity at high solid concentrations and excellent film-forming properties (Kuakpetoon & Wang, 2001).Table 1shows that any combination of extruder inlet temperatures lower than 100 C and processing
temperatures below 120 C, did not produce proper laminates,
probably because no real thermoplasticization of starch was ach-ieved. Additionally, an outlet temperature higher than 90 C on
both NBS and OBS, produced laminates with folds and bubbles, owing toflash expansion of water vapor and glycerol inside the molten material, although in others instances, theflash expansion is used to produce extruded foam of polymers (Zhang, Zhu, Li & Lee, 2012). The best combination of the three temperatures zones (I, II and III) for the proper formation of extruded laminates (TPS for-mation), were 100, 120 and 90C, respectively. All laminates
ob-tained were standardized to a thickness of about 1.5 mm by applying a roll on the samples at the end zone of the extruder during production. Visual examination showed that laminates ob-tained from OBS wereflexible and easy to handle while those from NBS were rigid, brittle and a bit sticky, probably because of some phase separation with diffusion of glycerol to the laminate surface. Overall, laminates from OBS were more homogeneous and more transparent than those from NBS, due to oxidation and subsequent leaching out during native starch modification of pigments, lipids; which are usually forming complex with amylose inside the starch granule, and proteins (Wang & Wang, 2003).
The laminate homogeneity of OBS might be related to the lower viscosity of OBS than NBS (about 1.5 times lower, data not shown), probably the higher viscosity of NBS did not allow a properflux of the molten material through the nuzzle, thus, some wrinkles and bubbles were formed on the laminate surfaces. This behavior of the extruded laminate samples during the laminate formation process, was similar to that observed on previous works using a similar procedure with potato starch. These researches have also found differences on extruder processing conditions, in cases where the viscosity of native starch was three to four times as much as that of oxidized starch (Zhang, Zhang, Wang, Chen & Wang, 2009). Also, it has been found that the higher the content of amylose, the more difficult the starches were to be processed by extrusion, spending twice as much mechanical work than that for waxy starch (Soest & Essers, 1997; Thuwall, Boldizar & Rigdahl, 2006), being somehow consistent with this work, where the amylose concentration pre-sent in NBS (32 g/100 g) was more than twice as much as that of OBS (14 g/100 g). This tendency has been previously mentioned by Wang and Wang (2003), who found that amylose was more prone to depolymerization than was amylopectin at the same hypoclorite level, related to the linear chemical structure or the random arrangement of amylose, that makes it more susceptible to oxida-tive degradation.
3.2. Color
Respect to the laminate color (Table 2), the luminosityL* was bigger for OBS, than that for NBS, but it was smaller than the one
% Solubility ¼
Initial weight dry basis final weight dry basis
Initial weight dry basis
reported previously (L*¼93 0.03) for oxidized starch (0.5e1.5 g/ 100 g) extruded at 95C (Zamudio-Flores, 2005). It seems that the
effect of Maillard reactions caused the decrease in luminosity in both starches (native and oxidized) (Bekedam, Schols, van Boekel & Smith, 2006). The color of the laminates obtained were among the red-yellow color (between the CIELAB a* and b* parameters) (Table 2). However, OBS laminates had a yellower color than NBS laminates, because thea* and h* parameters were higher, amid bigger saturation (C*). This difference infilms color could even be noted visually, being consistent with previous works done previ-ously (Alanis-López, Pérez-González, Rendón-Villalobos, Jiménez-Pérez & Solorza-Feria, 2011).
3.3. Micrographs
Concerning the SEM images as seen inFig. 1, the laminates made with oxidized starch, showed similar number of cracks and pores formation on the surface (magnification 100) to laminates made of NBS. However, on views of lower magnification (40), sample appearance in laminates from oxidized starch was“smoother”than in those of native starch. This was probably caused by the presence of native starch granules which were not fully plasticized during the extrusion process (Fishman, Coffin, Onwulata & Willett, 2006), although some continuity was also observed in all laminates made with both treated and untreated starch, suggesting that these sys-tems might have the potential to remain accessible to some biodegradation factors (e.g. enzymes, chemicals, light).
The white arrow on the micrograph of the lateral view of a typical oxidized starch laminate, showed cracks that are not un-common during the SEM study and some of them, might be also produced by cutting effects while conditioning samples. However, the mentioned cracks could not be particularly signals of the me-chanical resistance of the oxidized starch laminates. Nonetheless, some authors (Mali, Grossmann, García, Martino & Zaritzky, 2006) have associated the smooth zone formations with best mechanical properties (e.g. larger shear resistance). Besides, it may well be that glycerol moieties insertion in the oxidized starch laminates, caused an increase in compactness degree, but the material homogeneity during its mixing and passing through the extruder might not be complete, suggesting that a larger residence time in the extruder, to reach a homogeneous laminate because or real TPS production, might still be needed.
3.4. Water vapor permeability (WVP)
It is well known that the water vapor permeability (WVP), is the result of a driving force of water transfer through afilm, given by water chemical potential difference. Its data are suitable to un-derstand possible mass-transfer mechanisms and solutee macro-molecule interactions in degradable films. The WVP of both the oxidized and native banana starch laminates (Fig. 2), decreased substantially at all sampling times during the whole storage period, without reaching an apparent stable minimum value through this time, a reduction of more than four times as much was observed
Fig. 1.Scanning electron micrographies of laminates made from native banana starch (NBS) and oxidized banana starch (OBS) made by extrusion. The white circles show the roughness of the laminates surface. The white arrows point at some fractures and pores. (S)¼superficial view (100), (L)¼Lateral View (40).
Table 2
Color parameters of laminates manufactured with NBS and OBS.
Sample L* a* b* h* C*
NBS 30.240.92a 1.59
0.46a 2.73
0.27a 1.04
0.13a 3.16
0.08a OBS 76.690.98b 3.1
0.23b 13.42
0.3b 1.34
0.04b 13.77 0.21b
from the beginning till the end of storage. This parameter decreased faster than the rate of decrease of similar starch laminates reported formerly, when using lower concentrations of chlorine (0.5e1.5 g/ 100 g) as an oxidant agent (Zamudio-Flores, 2005). Possibly factors as the laminate thickness, the percentage of glycerol added, the effect of the extrusion on the density of the laminate matrix, and even the microcracks observed on the laminates, affected the WVP of the specimens. Some researchers have reported previously similar WVP values for edible starch basedfilms with hydrophilic nature, to those found in this work (Bertuzzi, Castro Vidaurre, Armada & Gottifredi, 2007). However, as shown inFig. 2, a signif-icant difference was observed solely at the beginning of the storage time, being the WVP of the NBS laminates higher than that of OBS samples, this may well be because the oxidation introduced carboxyl and carbonyl functional groups in the starch molecule, with some starch depolymerization. These hydrophilic groups possibly retained more water molecules lowering as a consequence the WVP (Lawal, 2004; Wang & Wang, 2003). Overall, NBS lami-nates showed higher decrease in WVP than OBS lamilami-nates throughout the storage time, suggesting that the oxidation process, did not affect at the same extent as NBS, the diffusion rate of water during the water vapor permeation phenomenon.
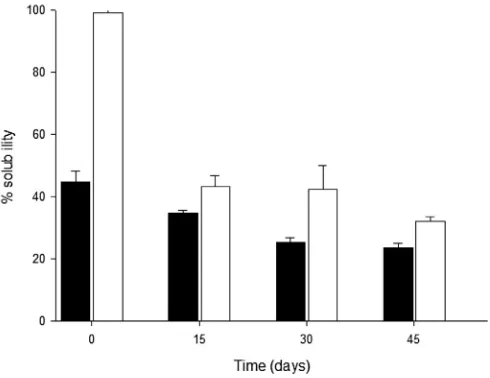
3.5. Solubility
The percent of solubility (measured as the gain of water) at 25C (Fig. 3) were lower than those at 80C (Fig. 4), probably because at the higher temperature, the higher thermal energy involved made the laminates more soluble by structural weakening of its compo-nents, while at 25C, it was solely the water osmotic pressure that contributed to laminates solubilization (Lawal, 2004). The solubility values of the laminates manufactured with native and oxidized starch decreased with storage time at the two temperatures tested, as shown at 25C and 80C. This suggests that a kind of“aging” process might have occurred in the laminate matrix or, in other words, a molecular re-arrangement that reduced the free volume, giving a closer structure and thus, a lower water penetration has taken place. This event might well diminish the polar character of the glycerol-starch composite or TPS. Also, it has been shown that as the temperature and pressure of extrusion is increased, the solubility decreases (Charutigon, Jitpupakdree, Namsree &
Runsardthong, 2008; Hashimoto & Grosmann, 2003). Besides, the pores generated during the extrusion, which might well befilled with water, could be closed partially due to shrinking of the lami-nates with storage time, creating a physical barrier for water intake. For both tests at 25 and 80C, overall, the OBS laminates gained more water than those of NBS laminates. During the oxidation process, undertaken under alkaline conditions, starch gelatinized, resulting thus in higher granules swelling and solubility, which may well account for the higher solubility of the oxidized sample (Lawal, 2004). These results suggest an effect attributed to the oxidation process, that has been shown to take place mainly on the starch amorphous region, i.e., on the amylose domain; producing both carbonyl and carboxyl groups in starch structure, increasing depolymerization and thus, enhancing also its interaction with water by hydrogen bonding (Wang & Wang, 2003). Besides, during the extrusion process there might be a better glycerol (plasticizer) distribution inside the OBS polymeric chains, favoring a higher solubility than in NBS. Also, an increase in amylose content Fig. 3.Solubility of laminates manufactured with native (NBS, ) and oxidized (OBS, ) banana starch, as function of storage time at 25C. Error bars are standard error of the media.
Fig. 4.Solubility of laminates manufactured with native (NBS, ) and oxidized (OBS, ) banana starch, as function of storage time at 80C. Error bars are standard error of the media.
decreased the index of solubility, as mentioned above; the native starch used to obtain laminates had higher amylose content (32 g/ 100 g) than its oxidized counterpart (14 g/100 g).
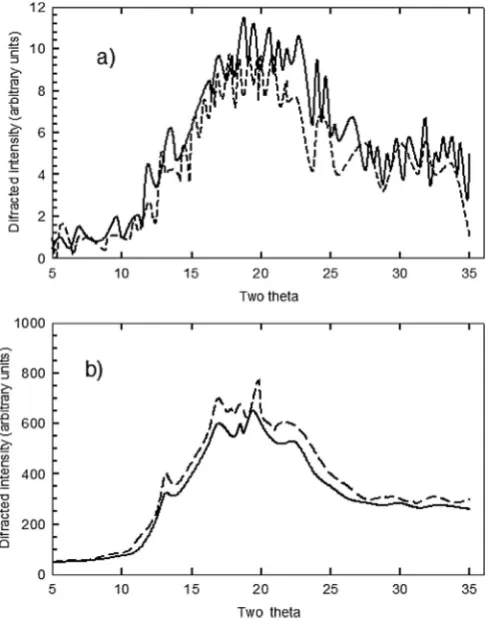
3.6. Laminates crystallinity
The X-ray diffraction of laminates manufactured with native and oxidized starches showed similar patterns at 15 and 45 days, as seen inFig. 5. At thefirst testing time (fifteen days), both NBS and OBS laminates were mainly amorphous, but with the storage time, crystallinity peaks were formed. At the second testing time (forty five days), the percent of crystallinity for the oxidized starch lam-inates (19.77 g/100 g) was overall, similar to that of the native starch laminates (20.07 g/100 g). This might be because the overall starch crystallinity is attributed mainly to amylopectin, which in the oxidation process is less depolymerised than amylose (Wang & Wang, 2003). However, these results need further investigation, since different trends have been reported previously, for example, the manufacture of banana starch oxidation by double screw extrusion, produced sheets with higher crystallinity than those of sheets from native starch (Alanis-López et al., 2011). However, Wang and Wang (2003)indicated that there were no significant changes in starch crystallinity even after oxidation with 2 g/100 g hypochlorite, due to oxidation taking place mainly in the amor-phous lamellae, rather than in the crystalline lamellae. Thus, further research is needed tofind out if this effect might pass over films during production.
When comparing the development of crystallinity versus time of storage, three peaks of wide shape and low intensity are noto-rious after 45 days of storage in both native and oxidized starch laminates: at 2q¼12.7, 17.12 and 22.22 respectively. Besides, a
well-defined peak (2
q
¼ 19.68) is observed. The signal observed at 2q
¼17.2 is attributed to the type B crystallinity due to external and short chains of amylopectin in glycerol (Soest & Essers, 1997). The diffraction pattern intensities VH type 2q
¼12.7, 19.7, and 22.22 might well be the result of recrystallization during storage, of the remaining amylose in OBS laminates, and that already present in NBS laminates (Kuakpetoon & Wang, 2006). Mali, Grossmann, García, Martino & Zaritzky (2002) also found the B-type pattern in yam starchfilms, which remained almost the same even after ninety days storage, with no significant effect in crystallinity from glycerol addition, suggesting that changes in this property might be related to starch source.3.7. Thermal properties
Table 3shows the transition temperatures (Tf), and enthalpies (
D
H) at its endothermic peaks, related mainly to starch crystallites melting, for laminate samples evaluated as function of the time of storage. These peak temperatures ranged from 166 to about 217C, as an indication of thermal decomposition of the starch bio-polymers (amylose and amylopectin). Authors like Mali et al. (2002)mentioned that amylose recrystallization requires temper-atures higher than 140C to be detected by DSC, being consistent with these results and those of XRD previously shown. The fact that no signals were detected before the above mentioned temperature range, suggests that starch gelatinization duringfilm obtainment was complete.Chang, Cheah & Seow (2000)have suggested that recrystallization of amylose and perhaps to a lesser extent of amylopectin, may take place during thefilm-forming process, an event that is known to be time-dependent and is likely to occur during storage. As time passed out, an increase in the temperature an enthalpy of melting was observed for both NBS and OBS lami-nates, being the melting temperature of the OBS lamilami-nates, higher than those of the NBS solely within thefirstfifteen days offilms manufacture, when both NBS and OBS laminates were mainly amorphous; but changing this trend from thirty days storage on-wards.Betuzzi et al. (2007), found that thefinal degree of crystal-linity in starchfilms depends on the ability of amylose chains to form crystals, as well as the mobility of the chains during the crystallization process. This suggests that the higher amylose con-tent in NBS might be responsible of the higher melting temperature as the storage time increased. However, the enthalpy (D
H) values associated with melting of the crystalline phase, were lower for OBS laminates than those of NBS laminates, possibly because of the degradation of the crystalline lamellae expected in OBS laminates (Betuzzi et al., 2007). Thus, it is notorious that the oxidation process modified the molecular arrangement in a form that it weakened the starch structure, but rendered it more stable, because despite of the transition (melting) temperature being higher for NBS laminatesTable 3
Thermal properties of the NBS and OBS laminates during storage*.
Samples Tf(C) DH(J/g)
NBS0 188.550.72a 42.78
0.35a
NBS 15 194.510.59b 87.55
1.97b
NBS 30 215.480.54c 96.00
1.21c
NBS 45 216.920.50c 115.08
1.98d
OSF0 200.500.21d 39.79
0.62a
OSF15 203.230.46d 62.30
2.5e
OSF30 211.880.85e 75.712
0.91f
OSF45 216.660.33c 83.86
0.92b
*mean values of three measurementsstandard error. 0, 15, 30 and 45 are the storage days. Same suffixes in the same column indicate no significant differences (a¼0.05).Tf¼transition temperature,DH¼transition or melting enthalpy, NBS: native banana starch; OBS: oxidized banana starch.
than those from OBS, the amount of energy (enthalpy) needed for this transition was lower, a trend that has also been observed in previous works (Alanis-López et al., 2011; Jouppila & Ross, 1997).
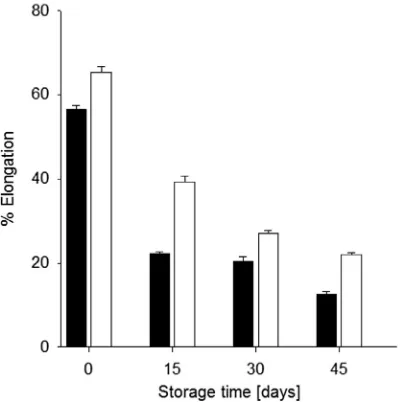
3.8. Laminates texture
Respect to the mechanical properties; tensile strength (TS, MPa) (Fig. 6), percent of elongation (E, %) (Fig. 7), and elasticity modulus (EM, slope of forceedeformation plot, MPa) (Fig. 8); of laminates made with oxidized starch, was bigger in all tests than those of the native starch: being consistent with the results of Alanis-López et al. (2011), where it was postulated that the processing temper-ature essayed, probably affected the oxidized starch laminates mechanical resistance. The relationship among TS vs. E and EM varied conversely with time of storage; i.e., as the %E decreased, both the TS and the EM increased. Laminates from OBS increased its mechanical properties (TS and EM), respect to those of NBS, possibly because of the oxidation process itself (e.g., change from hydroxyl to carbonyl groups, increasing hydrogen bonds), that
enhanced crosslinks formation in starch network, increasing the strength of those samples. This behavior has been reported previ-ously for potato starch laminates and it was attributed also to the increment of crystallinity (Soest & Essers, 1997), besides, the lower %E shown by NBS laminates with storage time, is consistent with its brittle texture mentioned above. Although further research is needed to understand the changes in laminates from oxidized starch during longer storage times, this study have shown that, from its overall functional properties, oxidized banana starch might have the potential of application in laminates production.
4. Conclusion
Laminates from OBS showed clearer color (higher luminosity) than those from NBS. Superficial views of laminates (SEM) from OBS were“smoother”than those of NBS. However, in lateral views, both laminates made from OBS and NBS showed cracks and pores formation on the surface. No significant differences were observed in WVP values between NBS and OBS laminates. The percent of solubility of the laminates from both NBS and OBS decreased with storage time, irrespective of the measuring temperature. Similar type B patterns and percent of crystallinity were observed in lam-inates from both NBS and OBS. As time passed out, an increase in the temperature an enthalpy of melting was observed for both NBS and OBS laminates, but the
D
Hvalues for OBS laminates were lower than those of NBS specimens. The strength of laminates made with OBS, was higher than those of NBS in all tests. The relationship among TS vs. %E and EM varied conversely with storage time.Acknowledgments
The authors are grateful to Instituto Politécnico Nacional (Pro-jects 20121051 and 20131083), CONACYT (Project 60565-Z), SNI, COFAA, EDI and SIP- in Mexico.
References
Alanis-López, P., Pérez-González, J., Rendón-Villalobos, R., Jiménez-Pérez, A., & Solorza-Feria, J. (2011). Extrusion and characterization of thermoplastic starch sheets from“Macho”banana.Journal of Food Science, 76(6), E465eE471. Fig. 6.Tensile strength of starch laminates made from native (NBS, ) and oxidized
(OBS, ) banana starches. Error bars are standard error of the media.
Fig. 7.Percent of elongation of starch laminates made from native (NBS, ) and oxidized (OBS, ) banana starches. Error bars are standard error of the media.
ASTM. (1989).Standard test methods for water vapor transmission of materials in sheet form. E96e80. Philadelphia: ASTM International.
ASTM. (1995).Standard test methods for tensile properties of thin plastic sheeting, methods D-6388 M 93 and D-888-95a. Philadelphia: American Society for Testing and Materials.
Bekedam, E. K., Schols, H. A., van Boekel, M. A. J. S., & Smith, G. J. (2006). High molecular weight melanoidins from coffee brew.Journal of Agriculture and Food Chemistry, 54(20), 7658e7666.
Bertuzzi, M. A., Castro Vidaurre, E. F., Armada, M., & Gottifredi, J. G. (2007). Water vapor permeability of edible starch basedfilms.Journal of Food Engineering, 80, 972e978.
Chang, Y. P., Cheah, P. B., & Seow, C. C. (2000). Plasticizingeantiplasticizing effects of water on physical properties of tapioca starchfilms in the glassy state.Journal of Food Science, 65(3), 445e451.
Charutigon, C., Jitpupakdree, J., Namsree, P., & Runsardthong, V. (2008). Effects of processing conditions and the use of modified starch and monoglyceride on some properties of extruded rice vermicelli.LWT-Food Science and Technology, 41(4), 642e651.
Du, X., MacNaughtan, B., & Mitchell, J. R. (2011). Quantification of amorphous content in starch granules.Food Chemistry, 127(1), 188e191.
Fishman, M. L., Coffin, D. R., Onwulata, C. I., & Willett, J. L. (2006). Two stage extrusion of plasticized pectin/poly(vinyl alcohol) blends.Carbohydrate Poly-mers, 65(4), 421e429.
Garcia, M. A., Ponotti, A., & Zaritzky, N. E. (2006). Physicochemical, water vapor barrier and mechanical properties of corn starch and chitosan compositefilms. Starch/Stärke, 58, 453e463.
Gilbert, G. A., & Spragg, S. P. (1964). Iodometric determination of amylose. In R. I. Whistler (Ed.),Methods in carbohydrate chemistry(pp. 168e169). New York: Academic Press.
Goñi, I., García, A., & Saura-Calixto, F. (1997). A starch hydrolysis procedure to estimate glycemic index.Nutrition Research, 17(3), 427e437.
González, A. E. (2003).Obtención de almidón a partir de plátano completo (Musa paradisiaca L. BSci. Thesis. México: Universidad Tecamac.
Gurgel, M., Vieira, A., da Silva, M. A., dos Santos, L. O., & Beppu, M. M. (2011). Natural-based plasticizers and biopolymerfilms: a review.European Polymer Journal, 47(3), 254e263.
Hashimoto, J. M., & Grosmann, M. V. E. (2003). Effects of extrusion conditions on quality of cassava bran/cassava starch extrudates.International Journal of Food Science & Technology, 38(5), 511e517.
Hermansson, A. M., & Svegmark, K. (1996). Developments in the understanding of starch functionality.Trends in Food Science & Technology, 7, 345e353. Jouppila, K., & Roos, Y. H. (1997). The physical state of amorphous corn starch and its
impact on crystallization.Carbohydrate Polymers, 32(2), 95e104.
Kuakpetoon, D., & Wang, Y. (2001). Characterization of different starches oxidized by hypochlorite.Starch/Stärke, 53(5), 211e218.
Kuakpetoon, C. A., & Wang, Y. (2006). Structural characteristics and physicochem-ical properties of oxidized corn starches varying in amylose content. Carbohy-drate Research, 341(11), 1896e1915.
Kuakpetoon, C. A., & Wang, Y. (2008). Locations of hypochlorite oxidation in corn starches varying in amylose content.Carbohydrate Research, 343(1), 90e100. Lawal, S. O. (2004). Composition, physicochemical properties and retrogradation
characteristics of native, oxidized, acetylated and acid-thinned new cocoyam (Xanthosoma sagittifolium) starch.Food Chemistry, 87, 205e218.
Mali, S., Grossmann, M. V. E., García, M. A., Martino, M. N., & Zaritzky, N. E. (2002). Microstructural characterization of yam starchfilms.Carbohydrate Polymers, 50(4), 379e386.
Mali, S., Grossmann, M. V. E., García, M. A., Martino, M. N., & Zaritzky, N. E. (2006). Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources.Journal of Food Engineering, 75(4), 453e460.
Montgomery, D. C. (1991).Design and analysis of experiments. New York: John Wiley and Sons.
Sangseethong, K., Termvejsayanon, N., & Sriroth, K. (2010). Characterization of physicochemical properties of hypochlorite and peroxide oxidized cassava starches.Carbohydrate Polymers, 82(4), 446e453.
Soest, J. J. G., & Essers, P. J. J. (1997). Influence of amylose-amylopectin ratio on properties of extruded starch plastic sheets.Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 34(9), 1665e1689.
Su, B., Xie, F., Li, M., Corrigan, A. P., Yu, L., Li, X., et al. (2009). Extrusion processing of starchfilm.International Journal of Food Engineering, 5(1), 1e12.
Thomas, D. J., & Atwell, W. A. (1999).Starches (handbook series). St. Paul, MN: American Association of Cereal Chemists.
Thuwall, M., Boldizar, A., & Rigdahl, M. (2006). Extrusion processing of high amylose potato starch materials.Carbohydrate Polymers, 65(4), 441e446.
Wang, Y. J., & Wang, L. (2003). Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite.Carbohydrate Polymers, 52(3), 207e217.
Wurzburg, O. B. (1986).Modified starches: Properties and uses. Boca Raton, Florida: CRC Press.
Zamudio-Flores, P. B. (2005). Elaboración de películas degradables de almidón de plátano, evaluación de sus propiedades mecánicas y de barrera. MSci. Thesis. Yautepec, Morelos. México: Centro de Desarrollo de Productos Bióticos-IPN.
Zhang, P., Whistler, R. L., BeMiller, J. N., & Hamaker, B. R. (2005). Banana starch: production, physicochemical properties, and digestibilityda review. Carbohy-drate Polymers, 59(4), 443e458.
Zhang, Y.-R., Zhang, S.-D., Wang, X.-L., Chen, R.-Y., & Wang, Y. Z. (2009). Effect of carbonyl content on the properties of thermoplastic oxidized starch. Carbohy-drate Polymers, 78(1), 157e161.