www.elsevier.com / locate / bres
Research report
Activation of purinergic receptors by ATP inhibits secretion in bovine
adrenal chromaffin cells
*
Amy B. Harkins , Aaron P. Fox
Department of Neurobiology, Pharmacology and Physiology, The University of Chicago, 947 E. 58th Street, Chicago, IL 60637, USA Accepted 12 September 2000
Abstract
Autoinhibition is a common mechanism observed in neurons to regulate neurotransmission. Released neurotransmitter interacts with presynaptic autoreceptors to inhibit subsequent release. The requisite elements for autoinhibition are present in chromaffin cells: secretory granules contain millimolar levels of ATP which is coreleased with catecholamines upon stimulation and the cells express purinergic receptors. We were interested to determine whether autoinhibition produced by ATP binding to purinergic receptors plays an important role in catecholamine release from chromaffin cells. In these studies, short depolarizations were used to elicit transmitter release measured by membrane capacitance. We find that stimulation of chromaffin cells results in the release of endogenous ATP which may suppress
21
Ca channel currents and secretion. In the presence of a maximal concentration of ATP, both the amount of secretion and the maximal rate of release are about half that observed in the absence of ATP. ATP-mediated inhibition of secretion was blocked by Reactive Blue-2
21
suggesting the involvement of P2Ypurinergic receptors. Prepulses to positive potentials that relieve the Ca channel block largely relieve
21
the inhibition of secretion. Furthermore, when secretion is plotted as a function of Ca influx there is no apparent change in the relationship between control cells and those stimulated in the presence of ATP and prepulses. These results suggest that ATP diminishes
21 21
secretion by inhibiting Ca influx into the cells. Our results indicate that feedback inhibition by ATP, mediated primarily by Ca channels, may be an important regulator of catecholamine release in chromaffin cells. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Mechanisms of neurotransmitter release
21
Keywords: ATP; Purinergic receptor; Exocytosis; Ca channels; Autoinhibition
21
1. Introduction Ca channel current (I ) by neurotransmitters whichCa
activate a wide variety of G-protein-coupled receptors At most synapses, neuronal communication occurs by [16,17,33]. Autoinhibition appears to involve activation of
21
Ca -dependent vesicular release of chemical neurotrans- presynaptic receptors [34]. Inhibition is most likely me-mitters. Modulation of release can occur by a phenomenon diated via a direct interaction of G-protein bg subunits
21
termed autoinhibition whereby a released neurotransmitter with N- and P/ Q-type Ca channels [5,13,24,26], al-inhibits subsequent release of transmitter through an though other inhibitory pathways are known to exist [25]. autoinhibitory feedback mechanism [19,41]. Neurotrans- This inhibition of ICa is reversible. Inhibition is character-mitters such as noradrenaline, g-aminobutyric acid ized by slowing of activation kinetics, is voltage depen-(GABA), serotonin, enkephalin and somatostatin reduced dent, and is overcome by prepulses to strongly positive
21
Ca influx into cells and diminished release [15,35]. potentials [5,18,38].
21
Inhibition of Ca influx is due to a direct inhibition of the Adrenal chromaffin cells provide an excellent model cell to study mechanisms of transmitter release. In chromaffin cells, many exogenously applied neurotransmitters inhibit *Corresponding author. Tel.: 11-773-702-0020; fax: 1
1-773-702-I through a number of receptors that include D
dopa-1216. Ca 2
E-mail address: [email protected] (A.B. Harkins). mine receptors [6], GABAB receptors [14], a-adrenergic 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
receptors [29], and opioid receptors [1,10,29,42]. Addition- mm in diameter) positioned directly adjacent to the cell. ally, exogenously applied ATP activates P2Y purinergic The outlet pipe was fed by six separate reservoirs equally receptors which inhibit ICa via a G-protein-coupled path- pressurized for similar flow rates (|70 ml / min) and way [1,10,12,21]. Chromaffin cell secretory granules con- controlled by valves operated and timed with the data tain ATP at millimolar concentrations [45] leading to the acquisition software. The exchange time for switching possibility that autoactivation of endogenous purinergic between two solutions was,150 ms. The input rate of the receptors regulates release. perfusion system was matched with the outflow rate of a In this study, we sought to determine whether ATP peristaltic pump to maintain a constant bath volume in could modulate secretion in chromaffin cells. Capacitance order to reduce artifactual capacitance changes.
measurements were employed to measure secretion in
response to stimulation. We show that, as previously 2.2. Preconditioned media described [1,10,12,21], ATP significantly reduces ICa. Most
importantly, ATP reduces release by.50%. Stimulation of To determine whether chromaffin cells released suffi-chromaffin cells which results in the release of endogenous cient ATP to activate autoinhibition, cells were plated at ATP suppresses secretion in these cells. The inhibition high density (|10-fold that of experimental dishes). Just mediated by ATP was blocked by Reactive Blue-2 sug- prior to use, a dish of cells was incubated for 5 min in 1 ml gesting the involvement of P2Y receptors. Large prepulses, of the TEA solution (described above) which induced which relieve the inhibition of I , largely reverse theCa depolarization and thus large amounts of secretion. The inhibition of secretion. Plots of secretion as a function of preconditioned solution was removed and immediately
21
Ca influx are not significantly different in the absence of applied to voltage-clamped chromaffin cells. ATP or presence of ATP and prepulses. Our results
indicate that the primary mechanism by which ATP acts to 2.3. Capacitance recordings inhibit secretion from chromaffin cells is by inhibiting I .Ca
ATP autoinhibition may provide an important regulatory Capacitance measurements were made with the phase mechanism for catecholamine release in chromaffin cells. tracking technique [27] in which a 60 mV peak to peak sine wave was superimposed on a holding potential of280 mV [36]. The combination of the sine wave and the holding
2. Materials and methods potential was chosen to provide a good signal to noise ratio
1 21
without activating voltage-dependent Na or Ca chan-2.1. Cell culture and recording configuration nels. Details of the technique can be found in Harkins and Fox [23]. The data were collected at a 500 ms sampling Bovine chromaffin cells were prepared from adrenal rate and filtered at 2 kHz. All current records were glands of animals approximately 18-weeks-old obtained compensated for series resistance and whole-cell capaci-from a local abattoir. The cell isolation procedure has been tance. Single pulse current data were leak subtracted by an described previously [2,10]. Experiments were performed average of 10 hyperpolarizing sweeps. Junction potentials 24–96 h after preparation of the chromaffin cells. of|29 mV were not subtracted from the data. Experiments Chromaffin cells were washed with an external solution were carried out at room temperature (22–248C).
that contained (in mM): 135 NaCl, 2 KCl, 1 MgCl , 52
CaCl , 12 HEPES, and 10 glucose (pH2 57.3, osmolality 2.4. Stimulation protocol |295 mOsm). In some experiments, recordings were made
in an external TEA solution which contained (in mM): 140 Five minutes after the whole-cell configuration was TEA, 10 glucose, 10 HEPES, 0.0001 TTX, and 5 CaCl2 obtained, each cell was stimulated with a train of five step (pH57.3, osmolality |300 mOsm). No differences in depolarizations to110 mV lasting 50 ms with a 100 ms
21
diluted to 100 mM in the external recording solution. For all of the prepulse experiments and many of the non-prepulse experiments, nisoldipine or nitrendipine (1 mM) was added to the external solutions to block any
facilita-21
tion (L-type) Ca current. Because there were no differ-ences in any of the capacitance or current recordings for experiments conducted in the absence or presence of dihydropyridine, all of the non-prepulse data were com-bined. Nisoldipine and nitrendipine (Calbiochem, San Diego, CA) were stored as a 10 mM stock solution in ethanol at 08C and were diluted to 1 mM in the external solution immediately prior to an experiment.
2.5. Analysis
Because multiple stimulations result in variable amounts of run down in the secretory response, the data reported here are largely from the initial train of depolarizations applied to each cell. In many of the experiments (Figs. 1, 2A and B), the only channel blocker employed was cesium
1
in the pipette to block K channels. As a result, these
21 1
current traces contained both Ca and Na channel
1
currents. The Na channel component was completely inactivated 8 ms after activation. Therefore, to measure the
21
Ca influx into cells, the current recordings were leak subtracted and integrated after excluding the first 8 ms of each current trace. For the experiments that were per-formed in channel blocking solutions, i.e. TEA, TTX and
21
cesium (Figs. 2C and 3), the entire Ca current was integrated after leak subtraction. For each cell, the sum of the integrals for five depolarizing steps provided the total
21
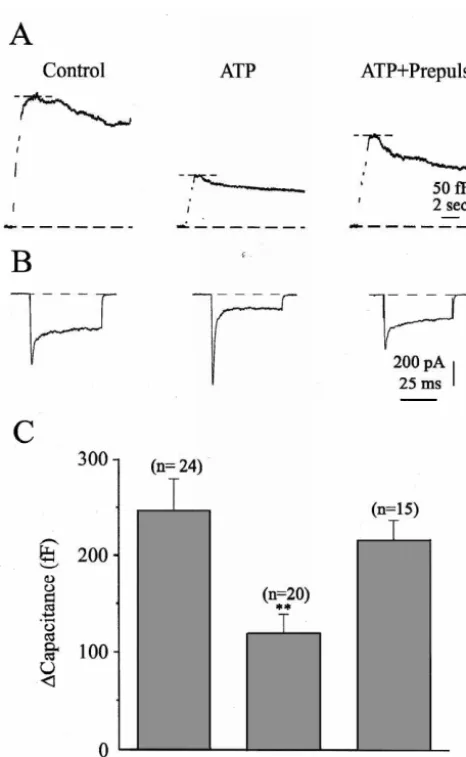
number of Ca ions that entered the cell. Statistical analysis of the data are expressed as mean (X)6standard error of the mean (S.E.M.), and an independent Student’s t-test was performed to test statistical significance. Fig. 1. ATP inhibits granule release from adrenal chromaffin cells. (A) A
representative capacitance trace is shown from three different groups of cells under different stimulation conditions. Left panel shows the
capaci-tance record from a cell stimulated in the absence of ATP (Control) while 3. Results the middle pane shows the capacitance record from a cell stimulated in
the presence of 100mM ATP (ATP). Each cell was stimulated with a
3.1. ATP inhibits secretion from adrenal chromaffin train of five step depolarizations to110 mV (50 ms pulse duration, 100
ms interpulse duration). The right panel shows the capacitance record of a cells cell stimulated in the presence of 100 mM ATP but preceded by
depolarizing prepulses to1100 mV (lasting 100 ms) 10 ms prior to the In order to determine whether ATP can serve as an test depolarization (ATP1Prepulse). For each cell, the peak exocytotic
autoinhibitory neurotransmitter, ATP (100mM) was direct-response was measured at the maximal change in the capacitance record
ly applied to adrenal chromaffin cells while monitoring following the depolarizing stimulus (dashed line at the top of each
capacitance trace). The maximum rate of exocytosis was determined by secretion with membrane capacitance measurements. ATP finding the largest change in capacitance that occurred during any of the reduced both the peak and the maximal rate of exocytosis 50 ms depolarizations. (B) plots currents elicited by the first depolariza- from chromaffin cells when compared with control cells. tion of a train, for the same three conditions plotted in panel A. Note that
1 Fig. 1A shows representative capacitance traces from each
the early current in each trace is Na current. The prepulse protocol
1 group of cells. Because there is run down in secretion
largely inhibited the Na current. (C) plots the average peak change in
membrane capacitance observed in the absence of ATP (Control, n524), when two sets of stimulations are applied to single cells in the presence of ATP (ATP, n520), and in the presence of ATP but (|30%, see Fig. 2A), the control data, ATP data, and with prepulse stimulation (ATP1Prepulses, n515). ATP significantly ATP1prepulse data presented in Fig. 1 are from the initial reduced the average change in capacitance from 248 fF (632 fF, n524)
stimulations of different cells. Fig. 1A shows the capaci-under control conditions to 119 fF (620 fF, n520, **P,0.01). Prepulses
tance trace obtained from three typical cells stimulated in the presence of ATP restored the peak of the capacitance change to 216
1
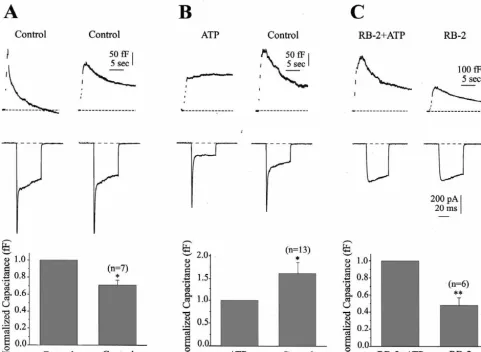
Fig. 2. ATP inhibits catecholamine secretion when elicited with two stimulations in the same cell. Each cell was stimulated twice with identical trains of depolarizations applied 5 min apart. (A) top, plots the capacitance response from a representative cell that was stimulated twice, while (A) middle, plots the current elicited by the first depolarization of each train. (A) bottom, plots the capacitance response of seven cells stimulated the first and second time under control conditions. The peak exocytotic response from the second stimulation was normalized to the first stimulation. On average, the second control
1
response was 71% (66%, n57, P,0.005) of the first control response. These cells were recorded with the Na -based solution. (B) top, plots capacitance, while the middle panel plots current from cells stimulated first in the presence of ATP and then in the absence of ATP. (B) bottom, shows the average capacitance response of 13 cells stimulated first in the presence of ATP followed by a second stimulation after ATP had been washed away for 5 min. On average, the second control response was 58% (623%, n513, P,0.05) larger than the first response observed in the presence of ATP. These cells were
1
recorded with the Na -based solution. (C) The P2Yantagonist Reactive Blue-2 (RB-2, 100mM), blocked the inhibition of secretion produced by ATP. (C) top, plots capacitance, while the middle panel plots current from cells stimulated first in the presence of ATP and RB-2 and then in the presence of RB-2 alone. (C) bottom, shows the average capacitance response from six cells. The first stimulation in the presence of RB-2 and ATP elicited a larger peak secretory response than the second stimulation in the presence of RB-2 alone. On average, the second stimulation was 49% (69.7%, n56, P,0.001) of the first stimulation. The TEA based solution was used for the experiments shown in (C).
capacitance trace was measured as indicated by the upper ATP-treated cell, the peak of the capacitance trace was 112 dashed line. Although it is possible that release is not fF and the maximal rate of exocytosis was 534 fF / s. These constant during each 50 ms depolarization, we calculate data suggest that ATP can act on purinergic autoreceptors the maximal rate of exocytosis by dividing the largest to inhibit evoked release.
capacitance change observed during a single depolarization The inhibition produced by ATP was similar but not by the 50 ms pulse duration of the depolarization. For the identical throughout each depolarization in the train. In the control cell (in the absence of ATP, left panel), the peak of first depolarization, ATP inhibited secretion from 5568.3 the capacitance trace was 281 fF and the maximal rate of fF (n524) to 29.365.7 fF (n517) while for the fifth exocytosis was 1124 fF / s. The middle panel (Fig. 1A) stimulation, secretion was reduced from 13.663.0 fF (n5
shows the capacitance trace from a different cell that was 24) to 9.662.2 fF (n517). In the first depolarization, ATP
21 6 6
exposed to ATP for 5 s prior to stimulation using a inhibited Ca influx from 25.1310 ions (62.6310
6 6
of ATP is mediated entirely by block of ICa or whether there is a secondary effect of ATP on the secretory machinery, we used 100 ms prepulses to 1100 mV to relieve the inhibition of I . These prepulses are notCa
expected to directly affect the secretory machinery. Nor
21
should the prepulses alter Ca -influx as the
depolariza-21
tions reach or exceed the equilibrium potential for Ca .
21
Prepulses relieved ATP mediated inhibition of the Ca channel current, and largely reversed ATP’s inhibitory effect on exocytosis. Fig. 1A (right panel) shows a capacitance trace from a cell exposed to ATP for 5 s prior to stimulation. In this experiment, prepulses were applied to reverse the ATP-mediated inhibition of I . The peak ofCa
the capacitance trace was 207 fF and the maximal rate of exocytosis was 1112 fF / s.
Fig. 1B plots currents elicited by the first depolarization of a train, from three representative cells under ‘Control’, ‘ATP’ and ‘ATP1Prepulse’ conditions shown in Fig. 1A.
1
Please note that Na currents were unblocked, variable
1
between cells, and that prepulses largely inhibited the Na
21
current. On average, ATP reduced the total Ca influx
6 6
under control conditions from 100.3310 ions (69.2310
6 6
ions, n524) to 69.4310 ions (65.6310 ions, n520, P,0.01), a 31% reduction. Depolarizing prepulses
re-21 6 6
stored the total Ca influx to 105.2310 ions (65.7310 ions, n514) in the continued presence of ATP. Therefore, prepulse stimulation in the presence of ATP relieved the
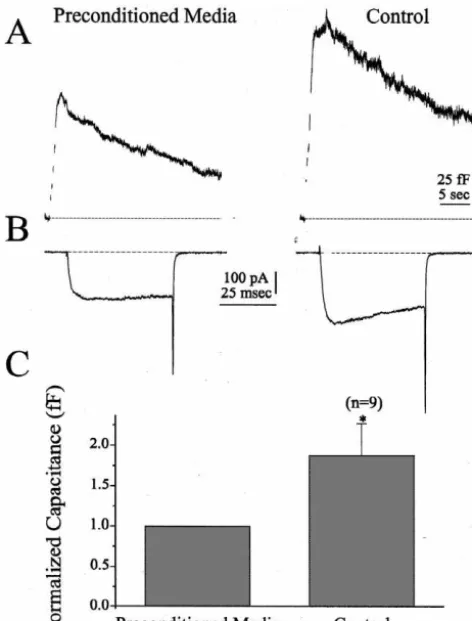
21
Fig. 3. Preconditioned media inhibited secretion. Densely plated inhibition of the Ca channel current. Fig. 1C summarizes chromaffin cells were induced to secrete by a 5 min incubation in the the average peak exocytotic response for each group of depolarizing TEA-based solution, after which the ‘Preconditioned Media’
cells. On average, ATP reduced the peak exocytotic was collected and applied to voltage-clamped cells. (A) shows
representa-response by 52% compared to control conditions (Fig. 1C), tive capacitance traces from a cell stimulated twice, first in the presence
a statistically significant response (P,0.01). Prepulses in of Preconditioned Media (left panel) and then in the Control solution
(right panel). (B) plots the ICarecorded during the first depolarizations of the presence of ATP restored the peak exocytotic response each train. (C) plots average capacitance data from nine cells exposed to to 87% of control cells (Fig. 1C), a value not statistically Preconditioned Media. The peak exocytotic response from the second
different than control. ATP also reduced the maximal rate stimulation was normalized to the first stimulation. The Control response
of exocytosis from 1308 fF / s (6162, n524) under control was 188% (639%, n59, P,0.05) of that obtained in the presence of
conditions to 666 fF / s (694, n520, P,0.01) in the Preconditioned Media. Data for all nine cells were obtained in TEA-based
solution. presence of ATP, a 49% reduction. Prepulses in the
presence of ATP restored the maximal rate of secretion to 1115 fF / s (6120, n515), 85% of control cells. Thus, prepulse stimulation completely relieved the inhibition of
21
while for the fifth stimulation, Ca influx was reduced I , and largely reversed the peak secretory response andCa
6 6 6
from 15.9310 ions (61.3310 ions, n524) to 12.9310 maximal rate of exocytosis. These data suggest that ATP
6
ions (61.1310 ions, n520). acts on purinergic autoreceptors to primarily inhibit
secre-21
tion through a Ca channel-dependent mechanism. 3.2. The inhibition of secretion by ATP is relieved by
prepulses 3.3. ATP inhibits secretion when both control and ATP responses are elicited in the same cell
21
A wide variety of neurotransmitters can inhibit Ca
channel current, and in many cases the inhibition is Because secretion can run down as a function of time thought to be mediated bybgsubunits of G-proteins [13]. within a single cell, the data shown in Fig. 1 was obtained This inhibitory interaction, which is voltage-dependent, is by stimulating individual cells once, in either the absence relieved by prepulses to strongly depolarizing potentials or presence of ATP, and then analyzing each cell
in-21
shows capacitance data from a representative cell stimu- collected and immediately applied to a chromaffin cell. lated with two trains of depolarizations, separated by 5 Each cell was stimulated twice, first in the presence of min, both in the absence of ATP (control). Fig. 2A Preconditioned Media, then in its absence (Control). Fig. (middle) shows the currents elicited by the first depolariza- 3A illustrates that the Preconditioned Media inhibited tion of each train. The bottom panel (Fig. 1A), summarizes secretion. Representative capacitance traces were recorded capacitance data from seven cells. The peak change in from a single cell in the presence (left panel) and absence capacitance of the second stimulus was reduced to 71% of (right panel) of the Preconditioned Media. Fig. 3B shows the first stimulus. On average, total ICa was reduced 8% that ICa was inhibited by the Preconditioned Media. Fig.
6 6
from 142310 ions (62.7310 ions) for the first stimula- 3C shows average data from nine cells demonstrating that
6 6
tion to 131310 ions (62.3310 ions) for the second the contents of chromaffin cell secretory granules could stimulation. When the first stimulation was delivered in the function to autoinhibit secretion. When the first stimulation presence of ATP and the second in the absence of ATP, the was delivered in the presence of the Preconditioned Media change in capacitance increased 58% from stimulus 1 to and the second in the control solution, the change in peak stimulus 2 (Fig. 2B). Total ICa increased 54% from 693 capacitance increased 88% from stimulus 1 to stimulus 2
6
6 6
(Fig. 3C). Total I increased 24% from 142310 ions 10 ions (66.0310 ions) for the first stimulation in the Ca
6
6 6
(69.5310 ions) for the first stimulation in the Pre-presence of ATP to 106310 ions (69.5310 ions) for the
6 6
conditioned Media to 176310 ions (610.5310 ions) for second stimulation in the absence of ATP. Thus, the data
the second stimulation in the control solution. shown in Fig. 2A and B strongly suggest that ATP
inhibited the secretory response in chromaffin cells and
21
3.6. ATP inhibits secretion via a Ca channel-that the inhibition was due to a direct inhibition of the
dependent mechanism calcium current.
Although the prepulse data are good evidence that ATP 3.4. ATP acts through a P2 y receptor to inhibit secretion 21
acts via a Ca channel-dependent mechanism, further evidence was available from the change in capacitance and Both the trypanocidal drug suramin and the anthra- 21
total Ca influx data for each cell. Fig. 4 shows a plot of quinone-sulfonic acid derivative Reactive Blue-2 (RB-2)
the maximal secretory response elicited as a function of have been used as P purinergic receptor antagonists. Both2
drugs possess only limited selectivity for the receptor [11,20]. Between the two antagonists, RB-2 exhibits a somewhat higher degree of selectivity for the P2Y receptor than does suramin (although this may vary between species and tissue type) so we tested this putative P2Y receptor antagonist on the ATP-mediated inhibition in chromaffin cells. RB-2 (100mM) prevented the inhibition of secretion associated with ATP application (Fig. 2C). This group of cells was stimulated twice, both times in the presence of RB-2. The initial stimulation with ATP (and RB-2) pro-duced a larger secretory response than the second stimula-tion without ATP which is likely caused by run down. The peak change in capacitance of the second stimulation was reduced to 49% of the first stimulation, and total ICa was
6 6
increased 3% from 275310 ions (628.5310 ions) for
6 6
the first stimulation to 283310 ions (630.0310 ions) for the second stimulation. Please note that this set of experiments was carried out in a TEA-based solution which suppressed the Na1 current and which may have accelerated the run down of secretion.
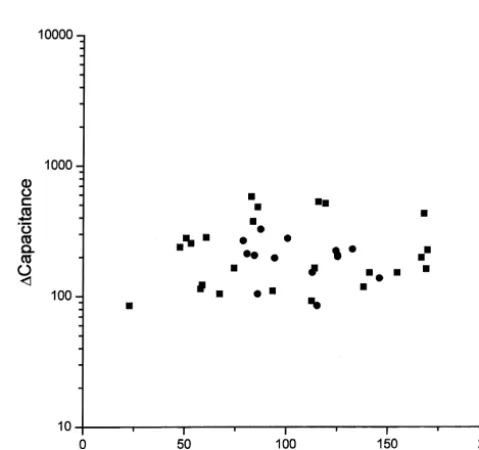
21
Fig. 4. ATP inhibits secretion via inhibition of Ca channel current. For each cell, the sum of the integrated current for the five depolarizations
21
3.5. Release of endogenous ATP from chromaffin cells (number of Ca ions) is plotted as a function of the maximal change in inhibits secretion membrane capacitance. The scatter plot shows all of the cells from the cells stimulated in the absence of ATP (h) and in the presence of ATP
with prepulses (s). The prepulse protocol is identical to that used in Fig.
Plates of chromaffin cells were exposed to depolarizing
21
total Ca influx from the first stimulation of each cell for cells [43], PC12 cells [30], and endothelial cells [44]. the control condition (squares) and the cells treated with These apparently conflicting results are explained by the ATP and prepulses (circles). No segregation between the fact that cells express different classes of purinergic two groups of cells is apparent and they all appear to receptors. For instance, chromaffin cells express both
21
follow the same Ca influx versus secretion relationship. ionotropic (P ) and metabotropic (P ) purinergic re-2X 2Y
This analysis suggests that if there is an additional effect of ceptors [8,40]. P2X receptors are ligand activated ion
21 21
ATP on secretion, other than of Ca channel inhibition, channels that are highly permeable to Ca whereas P2Y
the effect is relatively small. receptors are G-protein-coupled receptors [4]. Thus, the actions of ATP vary depending on the purinergic re-ceptor(s) activated. For example, ATP, acting through
21
4. Discussion Ca -permeable P2X receptors, can evoke secretion
[3,8,28,37,40], while activation of metabotropic P2Y
re-21
These studies were carried out to determine whether ceptors has been shown to release Ca from intracellular ATP released from bovine adrenal chromaffin cells could stores [8,28]. Preparations enriched for adrenaline-con-bind to purinergic autoreceptors and inhibit subsequent taining chromaffin cells, like ours, appear to express release of secretory granules. The secretory granules predominately P2Y receptors [4,8,32]. We have not ob-contain high concentrations of both catecholamines and served any inward current in response to rapid (,150 ms) ATP [45], which when released will increase the local application of ATP (Drs K.P.M. Currie and A.P. Fox, concentration of ATP. In a previous study, dispersed unpublished observation), nor have we observed any chromaffin cells in culture released ATP in the range of increase in secretion measured with membrane capacitance 10–100 mM with the K for Id Ca inhibition of |0.5 mM in response to rapid (,150 ms) application of ATP [10]. A similar ‘Preconditioned Media’ suppressed both ICa (authors’ unpublished observations), as would be expected and secretion in our experiments. Direct external applica- if P2X receptors were present. As described in this study, tion of 100mM ATP to single cells inhibited secretion by activation of chromaffin cell P2Y receptors inhibited ICa
21 21
suppressing Ca influx through Ca channels. The and effectively inhibited exocytosis.
inhibitory action of ATP was largely reversed by strongly Others have studied the inhibitory effect of ATP on
21
depolarizing prepulses that relieved the Ca current secretion. A study by Powell et al. [39] reported that
21
inhibition. Plots of secretion versus Ca influx, in the 2-methylThioATP (2-MeSATP), an ATP analog, inhibited
21
absence of ATP or in the presence of ATP1prepulses, both Ca influx and secretion in bovine chromaffin cells. would be expected to reveal differences between groups of Previously, Chern et al. [9] observed an inhibition of cells if ATP had significant effects on the secretory secretion when ATP activated putative P2Y receptors to
21 21
machinery. No obvious differences were observed. Taken inhibit Ca influx via Ca channels. Our data are in together, these data suggest that ATP inhibits secretion partial agreement with an earlier study by Lim et al. [31] in
21
mainly through a Ca channel-dependent mechanism. rat chromaffin cells which reported that 100 mM ATP
21
However, since prepulses relieved Ca current inhibition, inhibited secretion by 59% compared with control values. but were not quite as effective in restoring the secretory In the Lim et al. study [31], secretion was evoked with a response, we cannot rule out a small inhibitory effect that single depolarization lasting one second. There are several may act directly on the exocytotic machinery but which is important differences between the Lim et al. study and the
21
not mediated by Ca channels. present manuscript that are worth noting. First, Lim et al.
21
Previous studies have shown that neurotransmitters reported that ATP inhibited secretion through both Ca
-21 21
partially and reversibly block Ca channel currents by dependent and Ca -independent mechanisms and con-acting through G-protein-coupled receptors. For example, cluded that the large majority of inhibition was mediated in bovine adrenal chromaffin cells, dopamine [6], norad- by a calcium channel independent pathway. Although we renaline [29], opioids [10,29], and ATP [1,10,12,21,37] could not completely rule out a calcium channel indepen-have all been shown to act through G-protein-coupled dent pathway, our data suggest that it is small and receptors to inhibit I . We have found that ATP reversiblyCa relatively unimportant. Second, in the Lim et al. study, inhibits, on average, 31% of I . This inhibition is in goodCa Reactive Blue-2 by itself produced large increases or agreement with other experimental results in bovine decreases in membrane capacitance. Thus, they were not chromaffin cells under similar recording conditions in able to link the changes in capacitance to P2Y receptors. In
21
which ATP inhibited, on average, 30–60% of Ca current our study we saw no changes in capacitance that were due [10,12,21]. Some earlier studies found that ATP first to Reactive Blue-2 application. This antagonist blocked
21
caused a rapid increase in Ca influx followed by a both the inhibition of calcium current and the inhibition of
21
slower and sustained inhibition of ICa [12,37]. Ca secretion suggesting that both were a result of the same P2Y
imaging studies have shown that ATP caused a rapid receptor activation. Third, Lim et al. did not study release
21 21
21
entry and Ca release from internal stores in adrenal chromaffin exogenous ATP. In our study we link the two and suggest
cells. Differential sensitivity to UTP and suramin, J. Biol. Chem. that this process occurs during physiological conditions
270 (1995) 5098–5106.
[10]. [9] Y.J. Chern, M. Herrera, L.S. Kao, E.W. Westhead, Inhibition of
Our data suggest that, among its many functions, ATP catecholamine secretion from bovine chromaffin cells by adenine nucleotides and adenosine, J. Neurochem. 48 (1987) 1573–1576. may act as an autoinhibitor of granule release from
[10] K.P. Currie, A.P. Fox, ATP serves as a negative feedback inhibitor chromaffin cells. In the adrenal gland, splanchnic nerve 21
of voltage-gated Ca channel currents in cultured bovine adrenal activity releases acetylcholine, which depolarizes chromaf- chromaffin cells, Neuron 16 (1996) 1027–1036.
21
fin cells, activates Ca channels and releases secretory [11] H.H. Dalziel, D.P. Westfall, Receptors for adenine nucleotides and
21
nucleosides: subclassification, distribution, and molecular characteri-granules in a Ca -dependent manner. Release of secretory
zation, Pharmacol. Rev. 46 (1994) 449–466. granules would be expected to increase the concentration
[12] M. Diverse-Pierluissi, K. Dunlap, E.W. Westhead, Multiple actions of ATP in the local environment. In the animal, the cells of extracellular ATP on calcium currents in cultured bovine are tightly clumped in the gland as opposed to our cultured chromaffin cells, Proc. Natl. Acad. Sci. USA 88 (1991) 1261–1265. cells which are sparsely distributed at the bottom of a dish. [13] A.C. Dolphin, Mechanisms of modulation of voltage-dependent calcium channels by G proteins, J. Physiol. (Lond.) 506 (1998) Thus, although secretion is polarized in the gland, there is
3–11. a strong likelihood that diffusion is restricted, thereby
[14] P. Doroshenko, E. Neher, Pertussis-toxin-sensitive inhibition by (2) increasing inhibition [7]. By acting on P21 2Y autoreceptors, baclofen of Ca signals in bovine chromaffin cells, Pflugers Arch.¨ ATP may inhibit Ca channels and thus operate in a 419 (1991) 444–449.
[15] K. Dunlap, G.D. Fischbach, Neurotransmitters decrease the calcium feedback loop to inhibit subsequent granule release. These
component of sensory neurone action potentials, Nature 276 (1978) data indicate that feedback autoinhibition by ATP may be
837–839.
an important regulator of catecholamine release in [16] K. Dunlap, G.D. Fischbach, Neurotransmitters decrease the calcium chromaffin cells. conductance activated by depolarization of embryonic chick sensory
neurones, J. Physiol. (Lond.) 317 (1981) 519–535.
21
[17] K. Dunlap, J.I. Luebke, T.J. Turner, Exocytotic Ca channels in mammalian central neurons, Trends Neurosci. 18 (1995) 89–98.
Acknowledgements [18] K.S. Elmslie, W. Zhou, S.W. Jones, LHRH and GTP-gamma-S
modify calcium current activation in bullfrog sympathetic neurons, Neuron 5 (1990) 75–80.
This work was supported by NIH awards to A.P.F. We
[19] M.A. Enero, S.Z. Langer, R.P. Rothlin, F.J. Stefano, Role of the thank Drs Kevin P.M. Currie and Chien-Yuan Pan for
a-adrenoceptor in regulating noradrenaline overflow by nerve preparation of the adrenal chromaffin cells, Paolo Nucifora stimulation, Br. J. Pharmacol. 44 (1972) 672–688.
for help with software, and Dr Kevin P.M. Currie for [20] B.B. Fredholm, M.P. Abbracchio, G. Burnstock, J.W. Daly, T.K. helpful discussions and critical review of the manuscript. Harden, K.A. Jacobson, P. Leff, M. Williams, Nomenclature and classification of purinoceptors, Pharmacol. Rev. 46 (1994) 143–156. [21] L. Gandia, A.G. Garcia, M. Morad, ATP modulation of calcium channels in chromaffin cells, J. Physiol. (Lond.) 470 (1993) 55–72.
References [22] O.P. Hamill, A. Marty, E. Neher, B. Sakmann, F.J. Sigworth,
Improved patch-clamp techniques for high-resolution current record-¨
[1] A. Albillos, L. Gandia, P. Michelena, J.A. Gilabert, M. del Valle, E. ing from cells and cell-free membrane patches, Pflugers Arch. Eur. Carbone, A.G. Garcia, The mechanism of calcium channel facilita- J. Physiol. 391 (1981) 85–100.
tion in bovine chromaffin cells, J. Physiol. (Lond.) 494 (1996) [23] A.B. Harkins, A.P. Fox, Activation of nicotinic acetylcholine
687–695. receptors augments calcium channel-mediated exocytosis in rat
[2] C.R. Artalejo, S. Rossie, R.L. Perlman, A.P. Fox, Voltage-dependent pheochromocytoma (PC12) cells, J. Gen. Physiol. 111 (1998) 257–
21
phosphorylation may recruit Ca current facilitation in chromaffin 269.
cells, Nature 358 (1992) 63–66. [24] S. Herlitze, D.E. Garcia, K. Mackie, B. Hille, T. Scheuer, W.A.
21
[3] T. Asano, K. Otsuguro, T. Ohta, T. Sugawara, S. Ito, Y. Nakazato, Catterall, Modulation of Ca channels by G-protein beta gamma Characteristics of ATP-induced catecholamine secretion from adren- subunits, Nature 380 (1996) 258–262.
al chromaffin cells of the guinea-pig, Comp. Biochem. Physiol. C. [25] B. Hille, Modulation of ion-channel function by G-protein-coupled Pharmacol. Toxicol. Endocrinol. 112 (1995) 101–108. receptors, Trends Neurosci. 17 (1994) 531–536.
[4] E.A. Barnard, J. Simon, T.E. Webb, Nucleotide receptors in the [26] S.R. Ikeda, Voltage-dependent modulation of N-type calcium chan-nervous system. An abundant component using diverse transduction nels by G-protein beta gamma subunits, Nature 380 (1996) 255– mechanisms, Mol. Neurobiol. 15 (1997) 103–129. 258.
[5] B.P. Bean, Neurotransmitter inhibition of neuronal calcium currents [27] C. Joshi, J.M. Fernandez, Capacitance measurements. An analysis of by changes in channel voltage dependence, Nature 340 (1989) the phase detector technique used to study exocytosis and
endo-153–156. cytosis, Biophys. J. 53 (1988) 885–892.
21
[6] L. Bigornia, C.N. Allen, C.R. Jan, R.A. Lyon, M. Titeler, A.S. [28] K.T. Kim, E.W. Westhead, Cellular responses to Ca from extracel-Schneider, D2 dopamine receptors modulate calcium channel cur- lular and intracellular sources are different as shown by
simulta-21
rents and catecholamine secretion in bovine adrenal chromaffin neous measurements of cytosolic Ca and secretion from bovine cells, J. Pharmacol. Exp. Ther. 252 (1990) 586–592. chromaffin cells, Proc. Natl. Acad. Sci. USA 86 (1989) 9881–9885. [7] G. Callewaert, R.G. Johnson, M. Morad, Regulation of the secretory [29] T. Kleppisch, G. Ahnert-Hilger, M. Gollasch, K. Spicher, J. response in bovine chromaffin cells, Am. J. Physiol. 260 (1991) Hescheler, G. Schultz, W. Rosenthal, Inhibition of voltage-dependent
21
C851–C860. Ca channels via alpha 2-adrenergic and opioid receptors in
¨
[8] E. Castro, J. Mateo, A.R. Tome, R.M. Barbosa, M.T. Miras-Portug- cultured bovine adrenal chromaffin cells, Pflugers Arch. 421 (1992)
21
[30] S. Koizumi, H. Uneyama, M. Ikeda, S. Ueno, K. Inoue, Inhibition [38] N.J. Penington, J.S. Kelly, A.P. Fox, A study of the mechanism of
21
by imipramine of ATP-evoked responses in rat pheochromocytoma Ca current inhibition produced by serotonin in rat dorsal raphe cells, Biochem. Biophys. Res. Commun. 244 (1998) 342–346. neurons, J. Neurosci. 11 (1991) 3594–3609.
21
[31] W. Lim, S.J. Kim, H.D. Yan, J. Kim, Ca -channel-dependent and [39] A.D. Powell, A.G. Teschemacher, E.P. Seward, P2Y purinoceptors -independent inhibition of exocytosis by extracellular ATP in inhibit exocytosis in adrenal chromaffin cells via modulation of
¨
voltage-clamped rat adrenal chromaffin cells, Pflugers Arch. 435 voltage-operated calcium channels, J. Neurosci. 20 (2000) 606–616. (1997) 34–42. [40] F. Reichsman, S. Santos, E.W. Westhead, Two distinct ATP re-[32] L.F. Lin, M.C. Bott, L.S. Kao, E.W. Westhead, ATP stimulated ceptors activate calcium entry and internal calcium release in bovine
catecholamine secretion: response in perfused adrenal glands and a chromaffin cells, J. Neurochem. 65 (1995) 2080–2086.
subpopulation of cultured chromaffin cells, Neurosci. Lett. 183 [41] K. Starke, Influence of extracellular noradrenaline on the (1995) 147–150. stimulation-evoked secretion of noradrenaline from sympathetic [33] D. Lipscombe, S. Kongsamut, R.W. Tsien, Alpha-adrenergic inhibi- nerves: evidence for ana-receptor-mediated feed-back inhibition of tion of sympathetic neurotransmitter release mediated by modulation noradrenaline release, Naunyn Schmiedeberg’s Arch. Pharmacol. of N-type calcium-channel gating, Nature 340 (1989) 639–642. 275 (1972) 11–23.
21
[34] R.J. Miller, Presynaptic receptors, A. Rev. Pharmacol. Toxicol. 38 [42] W.A. Twitchell, S.G. Rane, Opioid peptide modulation of Ca(
)-1 21
(1998) 201–227. dependent K and voltage-activated Ca currents in bovine adrenal [35] A.W. Mudge, S.E. Leeman, G.D. Fischbach, Enkephalin inhibits chromaffin cells, Neuron 10 (1993) 701–709.
release of substance P from sensory neurons in culture and decreases [43] C. Villalobos, S.R. Alonso-Torre, L. Nunez, J. Garcia-Sancho, action potential duration, Proc. Natl. Acad. Sci. USA 76 (1979) Functional ATP receptors in rat anterior pituitary cells, Am. J.
526–530. Physiol. 273 (1997) C1963–C1971.
[36] E. Neher, A. Marty, Discrete changes of cell membrane capacitance [44] U.M. Vischer, C.B. Wollheim, Purine nucleotides induce regulated
21
observed under conditions of enhanced secretion in bovine adrenal secretion of von Willebrand factor: involvement of cytosolic Ca chromaffin cells, Proc. Natl. Acad. Sci. USA 79 (1982) 6712–6716. and cyclic adenosine monophosphate-dependent signaling in endo-[37] K. Otsuguro, T. Ohta, S. Ito, Y. Nakazato, Modulation of calcium thelial exocytosis, Blood 91 (1998) 118–127.
¨