Endoplasmic Reticulum Stress Proteins in the Rat
Cerebral Cortex and Hippocampus
Biao Chen, Jun Feng Wang, and L. Trevor Young
Background: Sodium valproate is a highly effective
treat-ment for bipolar disorder, but its mechanism of action
remains poorly understood. We recently found with
differ-ential display polymerase chain reaction that valproate
regulates the expression of the endoplasmic reticulum
stress protein GRP78 in the rat cerebral cortex. In our
study, we investigated the effect of this drug on the other
members of the endoplasmic reticulum stress protein
family, GRP94 and calreticulin, and we studied the brain
regional distribution of GRP78, GRP94, and calreticulin.
Methods: Immunohistochemistry was used to measure
protein levels of GRP78, GRP94, and calreticulin after
treatment with sodium valproate (300 mg/kg,
intraperito-neal) in specific rat brain regions.
Results: We report here that chronic treatment with
valproate also increased expression of other members of
the endoplasmic reticulum stress protein family, such as
GRP94 and calreticulin. The brain regional distribution of
these changes was similar for all three proteins, with
marked increase detected in the frontal cortex, parietal
cortex, and CA1 region of the hippocampus.
Conclusions: Because GRP78, GRP94, and calreticulin
possess molecular chaperone activity and bind Ca
21in
the endoplasmic reticulum, the pharmacologic action of
valproate may involve one or more of these processes.
Biol Psychiatry 2000;48:658 – 664 © 2000 Society of
Biological Psychiatry
Key Words: GRP78, GRP94, calreticulin, mood
stabiliz-ers, valproate, anticonvulsants
Introduction
S
odium valproate (VPA) is a first-line treatment for
bipolar disorder (BD), with a broad spectrum of
efficacy in this disorder (Davis et al 2000; Keck et al 1998;
Tohen and Grundy 1999). Despite extensive investigation,
however, the mechanism of its therapeutic action is still
poorly understood. Earlier studies found that chronic
treatment with VPA regulated the expression of several
genes such as Na
1channel subunit (Yamamoto et al 1997)
and
myristoylated
alanine-rich
C
kinase
substrate
(MARCKS; Lenox et al 1996). Using differential display
polymerase chain reaction, we recently reported that
chronic treatment with VPA increased mRNA and protein
expression of 78-kilodalton glucose-regulated protein
(GRP78) in rat brain (Wang et al 1999). This suggests a
novel and potentially clinically relevant mechanism
through which this drug may work.
GRP78, along with GRP94 and calreticulin, are gene
products induced as part of the stress response in
eukary-otic cells. They play an important role in the cellular
response to stress and are involved in endoplasmic
retic-ulum (ER) glycoprotein trafficking, molecular chaperone
activity, and ER Ca
21binding (Brostrom et al 1998; Kim
et al 1987; Little et al 1994; Wooden et al 1991). Because
these proteins act in concert, it was of interest to study the
effects of VPA on other members of the ER protein
family. Moreover, understanding the brain regional
distri-bution of any changes in expression is critical to clarifying
the functional significance of these drug effects. In the
present study, we used immunohistochemistry to study
GRP78, GRP94, and calreticulin expression in rat brain
after chronic treatment with VPA and report here
brain-specific changes in all three members of the ER stress
protein family.
Methods and Materials
Animals and Drug Treatment
Male Sprague–Dawley rats (150 –170 g) were housed two per cage and maintained on a 12-hour light/dark cycle with food and water freely available. Rats were divided into 2 groups: control animals (n5 6) and VPA-treated animals (1 day [1d], 7 days [7d], and 14 days [14d], n56 for each time point). Rats were injected intraperitoneally (IP) daily with saline or VPA (300 mg/kg).
After treatment, rats were anesthetized with sodium
pentobar-From the Department of Psychiatry and Behavioral Neuroscience, McMaster University, Hamilton, Canada.
Address reprint requests to L. Trevor Young, M.D., Ph.D., McMaster University, 1200 Main Street, West, 4N77A, Hamilton Ontario L8N 3Z5, Canada. Received November 25, 1999; revised February 29, 2000; accepted March 6, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
bital (60 mg/kg) and perfused transcardially with 250 mL of 100 mmol/L phosphate buffer (pH 7.5) at room temperature. Brains were removed, rapidly frozen in280°C and serial 20mm coronal sections (from Bregma 22.30 mm to Bregma 24.80 mm) prepared at218°C. The sections were thaw-mounted on poly-L-lysine-coated glass slides. Each slide contained corresponding sections from one animal in each treatment groups (control, 1d, 7d, and 14d) to allow comparisons across groups and minimize differences in background.
Immunohistochemical Staining
Immunohistochemistry was carried out using the streptavidin-peroxidase method (Elias et al 1989; Ozaki et al 1997). Briefly, sections were treated with 0.3% Triton X-100/phosphate-buff-ered saline (PBS) and incubated in 3% H2O2/methanol to block
endogenous peroxidase. The sections were preincubated with normal serum and then incubated at 4°C for 48 hours with specific primary antisera that recognize GRP78 (Stressgen Bio-technologies, Victoria, Canada), GRP94 (Stressgen Biotecholo-gies), and calreticulin (Upstate Biotechnology, Lake Placid, NY). These primary antisera were used at dilutions of 1:800, 1:800, and 1:1000 for GRP78, GRP94, and calreticulin, respectively. The sections were then incubated for 30 min with biotinylated secondary antibody (Zymed, South San Francisco, CA), and finally with streptavidin-horseradish peroxidase (HRP) conjugate for 30 min at room temperature. The peroxidase reaction was carried out with a DAB Kit (Zymed) according to the manufac-turer’s protocol. The specificity of immunoreactivity was tested by incubating rat brain sections with no primary antibody or after the addition of nonimmune rabbit serum in which no immuno-staining was observed.
Quantitative Image Analysis
Immunohistochemically stained sections were examined at 503 magnification by creating a digitized image with a microcom-puter imaging device (MCID) image analysis system (Brock University, St. Catherines, Canada) attached to a light micro-scope (Zeiss Axioskop, Oberkochen, Germany) with a high-resolution charge-coupled device (CCD) camera (MTI CCD 72). The density of immunoreactive positive neurons in frontal cortex, parietal cortex, dentate gyrus, and CA1 and CA3 of hippocampus were measured using standard landmarks. Back-ground values were obtained at a ten small boxes cursor in the molecular layer of cortex. The density of immunostaining in different regions was expressed as relative optical density (ROD), which was the density of each region divided by the background value of the same section. Six sections from each animal per each antibody through each region of interest were counted, and the same regions were compared as closely as possible between animals.
Statistical Analysis
Data were presented as the mean6SEM. from six independent experiments. Statistical analysis of data was performed with one way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test.
Results
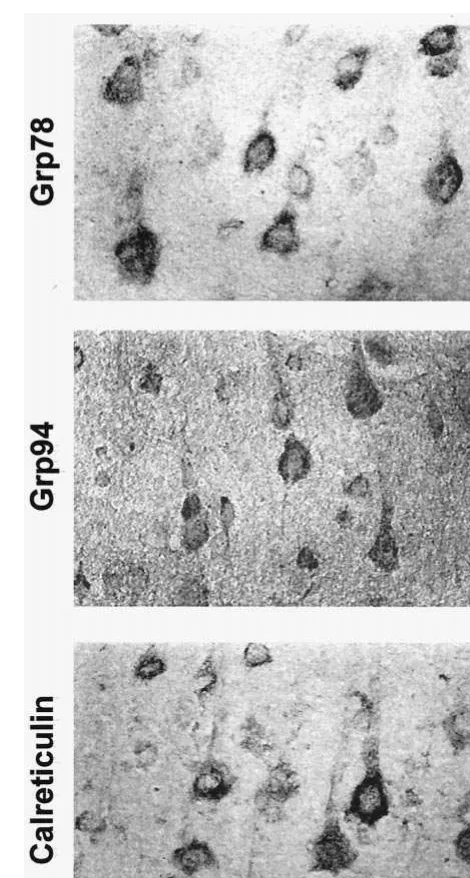
GRP78, GRP94, and calreticulin immunoreactivity were
restricted to the cytoplasm of neurons with substantial
overlap across the expression pattern for each protein
(Figure 1). In saline-treated rats, immunoreactive positive
neurons of GRP78, GRP94, and calreticulin were
distrib-uted throughout the entire brain. The pyramidal cells in the
parietal cortex showed particularly strong
immunoreactiv-ity to GRP78, GRP94, and calreticulin when compared
with other cells (Figures 2, 3, and 4). The
immunoreac-tivity of GRP94 and calreticulin was relatively weaker in
Figure 1. Immunohistochemical staining of GRP78, GRP94, and calreticulin of pyramidal cell laminae of the rat parietal cortex treated with saline. Magnification 3363.Valproate Increases ER Stress Proteins BIOL PSYCHIATRY 659
dentate gyrus compared with CA1 and CA3 regions of
hippocampus (Figures 3 and 4).
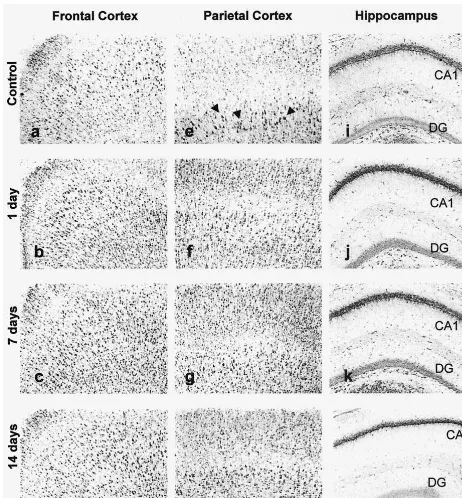
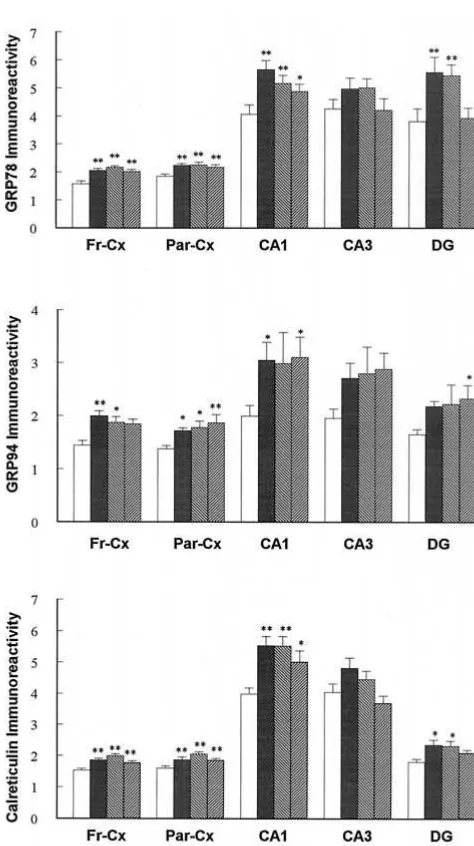
GRP78 immunoreactivity was significantly increased
(Figure 5A) by intraperitoneal injection of VPA for 1, 7,
and 14 days in both frontal and parietal cortex (p
,
.01).
Sodium valproate increased GRP78 expression by 30%,
38%, and 28% in the frontal cortex and 21%, 21%, and
17% in the parietal cortex. Although VPA treatment for 1,
7, and 14 days had no effect on GRP78 expression in CA3
region of hippocampus, VPA significantly increased
GRP78 expression in CA1 and dentate gyrus (p
,
.05).
VPA increased GRP78 expression by 38%, 27%, and 20%
at 1, 7, and 14 days in CA1; by 46% and 43% at 1 and 7
days in dentate gyrus (p
,
.01).
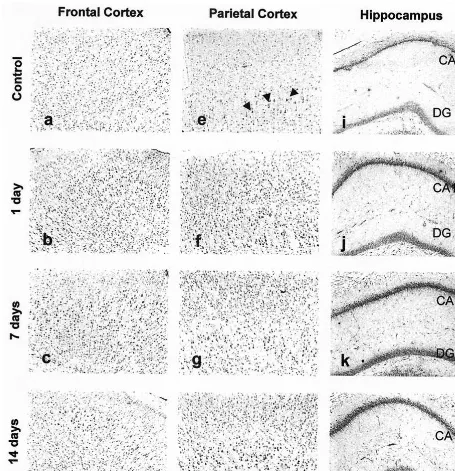
Similarly, calreticulin immunoreactivity was
signifi-cantly increased (Figure 5B) after intraperitoneal injection
of VPA for 1, 7, and 14 days in frontal cortex, parietal
cortex, CA1, and dentate gyrus, but not in the CA3 region.
VPA increased calreticulin expression by 22%, 38%, and
5% at 1, 7, and 14 days in frontal cortex (p
,
.01); by
18%, 29%, and 16% at 1, 7, and 14 days in parietal cortex
(p
,
.01); by 39%, 38%, and 25% at 1, 7, and 14 days in
CA1 (p
,
.05); and by 30% and 28% at 1 and 7 days in
dentate gyrus (p
,
.05).
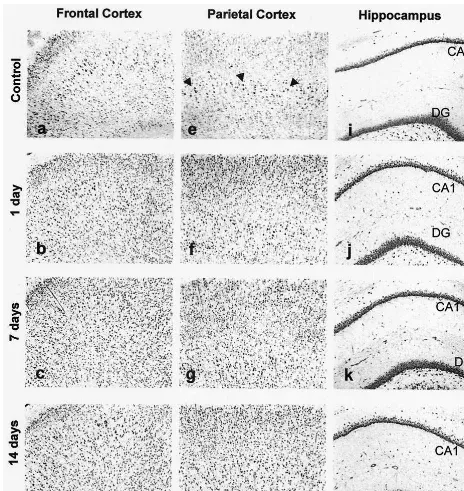
Finally, intraperitoneal injection of VPA for 1, 7, and 14
days significantly increased GRP94 immunoreactivity in
all five measured regions including frontal cortex, parietal
cortex, CA1, CA3, and dentate gyrus. VPA increased
GRP94 expression by 38%, 29%, and 27% at 1, 7, and 14
days in frontal cortex (p
,
.05); by 24%, 28%, and 35%
Figure 3. GRP94 immunoreactivity in different brain regions of the rat treated with sodium valproate (VPA). Rats were treated with saline (a, e, and i) and VPA (300 mg/kg, intraperitoneal) for 1 day (b, f, and j), 7 days (c, g, and k), and 14 days (d, h, and l). Arrowheads point to pyramidal cells. Magnification 1003. DG, dentate gyrus.Valproate Increases ER Stress Proteins BIOL PSYCHIATRY 661
at 1, 7, and 14 days in parietal cortex (p
,
.05); by 53%,
49%, and 55% at 1, 7, and 14 days in CA1 (p
,
.05); and
32%, 35%, and 41% at 1, 7, and 14 days in dentate gyrus.
Discussion
We previously demonstrated that VPA increased GRP78,
one of the ER stress proteins in rat cerebral cortex (Wang et
regulate the cellular response to stress to bring about its
therapeutic effects.
ER stress proteins function in ER glycoprotein
traffick-ing, have molecular chaperone activity, and protect cells
from the deleterious effects of damaged proteins by
binding and disposing of malfolded protein (Kim et al
1987; Little et al 1994; Wooden et al 1991). ER stress
proteins are also Ca
21binding proteins and play an
important role in maintaining ER Ca
21homeostasis
(Lie`vremont et al 1997). Because VPA appears to increase
expression of ER stress proteins that perform these
partic-ular processes, our findings suggest that VPA treatment
may target one or more of these processes. Although the
mechanism of the regulation of ER stress proteins by VPA
is not yet known, it is possible that this regulation is
important in the pharmacologic action of VPA.
The effect of VPA on ER stress protein expression may
also be relevant to the pathophysiology of BD. It has been
known that intracellular free Ca
21in peripheral blood
cells was increased in BD subjects (Dubovsky et al 1992).
Increasing evidence suggests that altered
polyphosphoino-sitide (PI)– generated second messenger signaling occurs
in blood cells and brain tissue obtained from BD patients,
which may relate to these increased intracellular Ca
21levels (Jope et al 1996; Wang et al 1997). Under these
conditions, an increase in ER stress proteins might be
expected to bind ER Ca
21and prevent depletion of ER
stores due to increased PI signaling and in turn lower
intracellular Ca
21levels. Some recent evidence suggests
that VPA inhibits 5-hydroxytryptamine-induced Ca
21in-flux in C6 glioma cells (Yamaji et al 1996), consistent
with this hypothesis.
VPA-induced increases in ER stress proteins may have
a neuroprotective role in specific brain regions in which
cellular damage may occur in BD. Although there is no
direct evidence to support this hypothesis, there is
increas-ing interest in the role of ER stress proteins in central
nervous system (CNS) disease and injury. GRP78
expres-sion is induced after CNS injuries (Lowenstein et al 1994),
such as seizures, global ischemia, and acute trauma, and in
neurodegenerative diseases like Alzheimer’s (Hamos et al
1991). ER stress proteins induction by chronic VPA
treatment may play a neuroprotective role by clearing
malfolded proteins.
In conclusion, chronic treatment with the mood
stabi-lizer VPA upregulates expression of ER stress proteins
GRP78, GRP94, and calreticulin in rat cerebral cortex and
hippocampus, which may have particular relevance for the
prophylactic effect of VPA in the long term management
of patients with BD. The molecular chaperone and Ca
21binding activities of ER stress proteins may be involved in
the pharmacologic action of VPA.
This work is supported by grants from the Ontario Mental Health Foundation and the Stanley Foundation. B.C. is supported by a Wyeth Ayerst Fellowship. LTY is a Career Scientist of the Ontario Ministry of Health.
References
Brostrom CO, Brostrom MA (1998): Regulation of translational initiation during cellular responses to stress. Prog Nucleic
Acid Res Mol Biol 58:79 –125.
Figure 5. Expression of GRP78, GRP94, and calreticulin in different regions of the rat treated with sodium valproate (300 mg/kg, intraperitoneal). Immunoreactive granule density in the frontal cortex (Fr-Cx), parietal cortex (Par-Cx), hippocampus CA1, hippocampus CA3, and dentate gyrus (DG) regions is expressed as relative optical density. The data are means 6 SEMs (n56). *p,.05 and **p,.01, when compared with control animals.
Valproate Increases ER Stress Proteins BIOL PSYCHIATRY 663
Davis LL, Ryan W, Adinoff B, Petty F (2000): Comprehensive review of the psychiatric uses of vaproate. J Clin
Psycho-pharmacol 20(suppl 1):1S–17S.
Dubovsky SL, Murphy J, Thomas M, Rademacher J (1992): Abnormal intracellular calcium ion concentrations in platelets and lymphocytes of bipolar patients. Am J Psychiatry 149: 118 –120.
Elias ME, Margiotta M, Gaborc D (1989): Sensitive and detection efficiency of the peroxidase antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC), and peroxidase-labeled avidin-biotin (LAB) methods. Am J Clin Pathol 92:62– 67.
Hamos JE, Olas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA (1991): Expression of heat shock proteins in Alzheimer’s disease. Neurology 41:345–350.
Jope RS, Song L, Li PP, Young LT, Kish SJ, Pacheco MA, et al (1996): The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem 66:2402–2409.
Keck PE Jr, McElroy SL, Strakowski SM (1998): Anticonvul-sants and antipsychotics in the treatment of bipolar disorder.
J Clin Psychopharmacol 59(suppl 6):74 – 81.
Kim YK, Kim KS, Lee AS (1987): Regulation of the glucose-regulated protein genes by beta-mercaptoethanol requires de novo protein synthesis and correlates with inhibition of protein glycosylation. J Cell Physiol 133:533–559.
Lenox RH, McNamara RK, Watterson JM, Watson DG (1996): Myristoylated alanine-rich C kinase substrate (MARCKS): A molecular target for the therapeutic action of mood stabilizers in the brain? J Clin Psychiatry 57(suppl 13):23–31. Lie`vremont JP, Rizzuto R, Hendershot, Meldolesi J (1997): BiP,
a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca21. J Biol Chem 49:30873– 30879.
Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS (1994): The glucose-regulated proteins (GRP78 and GRP94): Functions, gene regulation, and applications. Crit Rev Eukaryot Gene
Expr 4:1–18.
Lowenstein DH, Gwinn RP, Seren MS, Simon RP, McIntosh TK (1994): Increased expression of mRNA encoding calbindin-D28K, the glucose-regulated proteins, or the 72 kDA heat-shock protein in three models of acute CNS injury. Brain Res
Mol Brain Res 22:299 –308.
Ozaki N, Chuang DM (1997): Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem 69:2336 –2344.
Tohen M, Grundy S (1999): Management of acute mania. J Clin
Psychopharmacol 60(suppl 5):31–34.
Wang JF, Bown CD, Young LT (1999): Differential display PCR reveals novel targets for the mood stabilizing drug valproate including the molecular chaperone GRP78. Mol Pharmacol 55:521–527.
Wooden SK, Li-Jing L, Navarro D, Qadri I, Pereira L, Lee AS (1991): Transactivation of the GRP78 promoter by mal-folded proteins, glycosylation block, and calcium iono-phore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-1. Mol Cell
Biol 11:5612–5622.
Yamaji T, Kagaya A, Uchitomi Y, Yokata N, Yamawaki S (1996): Effects of carbamazepine and sodium valproate on 5-HT-induced calcium increase in individual C6 rat glioma cells. Neuropsychobiology 34:22–25.