Post-Nesting Migration and Mitochondrial DNA Structures of Olive Ridley Turtles (Lepidochelys olivacea) Nested on Beaches in the Birdhead of Papua and the Lesser Sunda Regions,
Indonesia: Conservation Implications
Windia Adnyana
The Faculty of Veterinary Medicine, Udayana University, Bali–Indonesia, Email: [email protected]
ABSTRACT
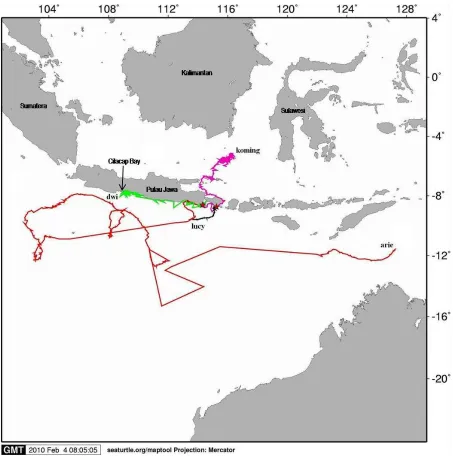
The management area and management unit of olive ridley turtles nested on two geographically separated rookeries; the Papua Birdhead of Peninsula and the western part of the Lesser Sunda regions, were extrapolated using satellite telemetry tracking technique and mtDNA analysis. Nine turtles were outfitted with satellite-linked transmitters after nesting in 2008 and 2009. At the same time, a total of 92 skin samples from 92 different olive ridley turtles from both locations (42 from Papua and 50 from Lesser Sunda) had been collected for mtDNA analysis. Results showed that each population used different migration pathways and feeding grounds. While all Papuan olives migrated southeastward towards relatively similar foraging ground in the waters of Arafura Seas, the Lesser Sunda turtles moved to two different directions, i.e. to the Indian Ocean and the Java Sea. A turtle tag in Alas Purwo showed a remarkable movement. She swam freely, probably searching for food, for almost 3000 km from the water perpendicular to southern side of West Java to the water of Baldwin Bank near the Gulf of Carpentaria in Northern Australia. During inter-nesting periods, both populations used the entire water column in the vicinity of their nesting beaches, suggested that protection of the nesting beach only would not be adequate to secure these turtles. Expansion to the nearby water is needed to give a better protection. In view of the distance to the nearest shores, the Lesser Sunda olives used a much smaller marine area for their interesting movement, which could be practically and easily managed by only the local district governments. On the other hand, a large inter-nesting area used by Papuan olives suggested the need to undertake a combined and coordinative effort between the district, provincial and national government to improve their survival. As a consequence for their migration pathways, the two populations would face different threats. The Papuan olives are most likely to be susceptible to the hunting practice done by a traditional turtle hunters reside in e.g. Key Island, and the Lesser Sunda turtles would prone to being captured by the turtle hunters-linked to the turtle black market in Cilacap Bay of Central Java. Additionally, the Papuan olives might be killed as a bycatch by the tuna longline and shrimp trawls fisheries operate in the Birdhead water and Arafura Seas, respectively. On the other hand, the Lesser sunda olives should be susceptible to the operation of the tuna longline fleets fishing in the Indian ocean. Apart from their different movement areas which brought them to different threats/ consequences, the two olives populations, which are separated by more than 1000 kilometers and interrupted by many seas, are known to be genetically distinct, representing two separate management units; each represents the logical focus for recovery actions. Accordingly, management to restore this population will require local effort to increase survivorship and reduce mortality at the nesting beach and its respective migratory pathways and feeding areas.
13 Introduction
Indonesia is home to six of the seven species of marine turtles, and hosting the last remaining populations of leatherback turtles (Dermochelys coriacea) in the Western Central Pacific. While the green (Chelonia mydas), hawksbill (Eretmochelys imbricata), olive ridley (Lepidochelys olivacea) and the leatherback turtles are found in both nesting and foraging habitats, two other species, the loggerhead (Caretta caretta) and the flatback (Natator depressus) turtles are only reported from a few foraging grounds within Indonesia (Adnyana and Hitipeuw, 2008).
Sea turtles usually undertake long migration between their nesting and feeding grounds (Russell, 2005). The home-range between nesting and feeding grounds often encompasses a huge marine areas which are frequently belongs to jurisdiction of more than one country. Therefore, collaboration among multi-layers of authorities (e.g. districts, provinces, and/or countries) is needed for their effective conservation measures (Liew et al, 2000). In this context, knowledge on turtle migration pattern is substantial. However, apart from telemetry works done in green sea turtles nested in Piai Island of Raja Ampat – Papua (Gearhart et al, 2006) and Berau of East Kalimantan (Adnyana et al, 2008), the migration information of the other sea turtle species in Indonesian archipelago is rarely known.
Olive ridley is one of turtle species which are found nesting in the Eastern part of Indonesia. Nesting is year-round with predicted annual nesting density between 400 and 500 nests at each site (Adnyana
and Hitipeuw, 2008). Two known major nesting sites for this species are the Birdhead Peninsula of West Papua and the western side of the Lesser Sunda Region (Alas Purwo of East Java and
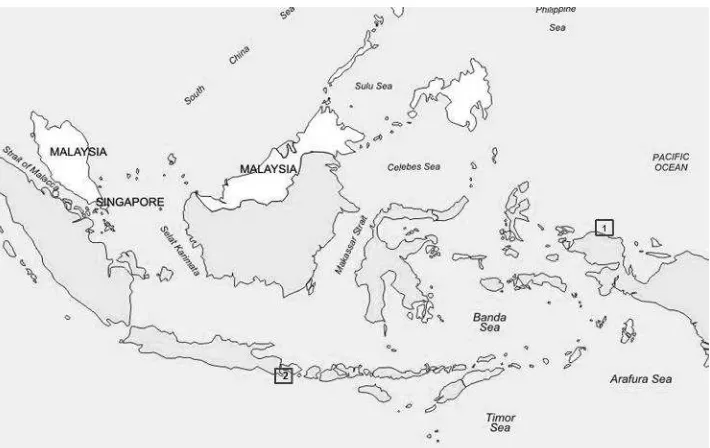
Figure-1: Sampling sites. The square number 1 shows the sampling location in the Birdhead Peninsula of Papua, and number 2 indicates the location of the sampling site in the western part of the Lesser Sunda region
.
During the field work, skin samples were also collected for Mitocondrial DNA analysis, as it has been proven to be particularly effective for detecting population structure in marine turtles (Bowen and Avise 1996; Dethmers et al, 2006). In this particular work, the expected result is a genetic marker which, in combination with satelite tracking data, in the future, can be used to estimate the migratory range of olive ridley from these two broad areas, as well as it applications to major harvests, by-catch and feeding grounds in the region, allowing estimation of which stocks may be adversely affected by human activities.
Materials and Methods
Determining post nesting migration routes
15 (Telonic Inc. USA), Kiwisat 101 and Fastloc (Sirtrack Co, Ltd., New Zealand) were programmed to transmit geographic location to the Argos system on a duty cycle of 12 hrs on and 12 hrs off. The data were processed using Satellite Tracking Analysis Tool-STAT (Coyne and Godley, 2005), generated by Maptool and published in www.seaturtle.org. Minimum distances traveled by tracked turtles were estimated based on great circle distances between subsequent positions along track. The location classes (LC), which is the estimate of accuracy, included to plot the migration routes were LC 3 (±150 m), LC 2 (±350 m), LC 1 (±1000 m), LC 0 (>1000 m), and the location Classes A and B which have
unknown accuracy. LC A and B were visually inspected over spatial projection before used to create
migration movements. The suspected errors were tested and rule out using criteria that shifting speed
of the two consecutive locations was not higher than criterion sea turtle swimming speed at 5 km/hr (Luschi et al 1998; Papi et al 2000). The calculations for speeds and distances only used LC 3, LC 2 and LC 1.
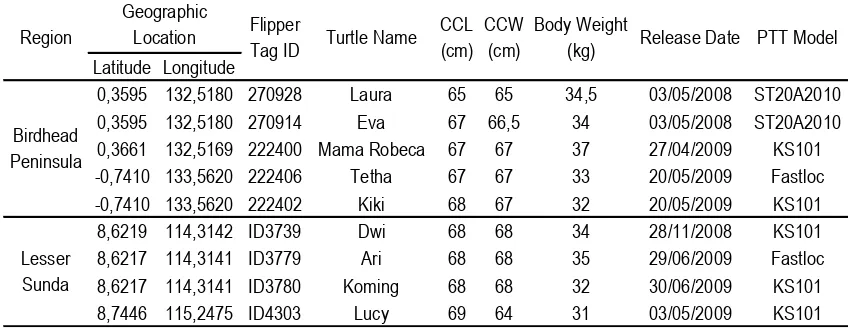
Table 1: Geographic locations (regions), flipper (inconnel) tag, and morphometrics of the post-nesting olive ridley turtles tagged with platform transmitter terminal (PTT) in the Birdhead Peninsula of Papua and the Lesser Sunda turtle rookeries during the 2008-2009 nesting seasons. CCL = the Curved Carapace Length; CCW = the Curved Carapace Width.
Mitochondrial (mt) DNA Analysis
Total of 92 skin samples of olive ridley turtle had been collected during the nesting seasons of 2008-2009 (Table 2). Application of flipper (inconnel) metal tag (National Band Tag®, USA) to each nesting
turtle during sampling period was done to ensure that each turtle was only sampled once. Samples were also collected from hatchlings at a local turtle hatchery in Bali. In this case, clutches were
Latitude Longitude
0,3595 132,5180 270928 Laura 65 65 34,5 03/05/2008 ST20A2010
0,3595 132,5180 270914 Eva 67 66,5 34 03/05/2008 ST20A2010
0,3661 132,5169 222400 Mama Robeca 67 67 37 27/04/2009 KS101
-0,7410 133,5620 222406 Tetha 67 67 33 20/05/2009 Fastloc
-0,7410 133,5620 222402 Kiki 68 67 32 20/05/2009 KS101
8,6219 114,3142 ID3739 Dwi 68 68 34 28/11/2008 KS101
sampled within 2 weeks time to avoid re-sampling nesting turtles, and only one hatchling per nest was
used in the analysis. Skin samples were collected either from the distal portion of the flippers or the
shoulder/neck area of adults and from dead hatchlings, stored in a NaCl saturated solution of 20% DMSO, and transported to Udayana University for subsequent processes.
Genomic DNA was isolated from 0.1 g of skin tissue using Qiamp™ DNA Mini Kit from Qiagen®, and stored at -20oC for subsequent polymerase chain reaction (PCR). Successful DNA isolation was confirmed by running 2 μL of genomic DNA in an ethidium bromide added 1% Agarose gel. 740 bp segments of the mtDNA control region for all samples were amplified using LTEi9 (GGGAATAATCAAAAGAGAAGG-3’) and H950 (GTCTCGGATTTAGGGGTTT-3’) primers (Abreu-Grobois et al, 2006). The target mitochondrial sequences were amplified by PCR using approximately 50 ng of template genomic DNA in 25 µl reaction volume containing 14.3 µl H2O, 2.5 µl DNA genome,
1.5 units ampli Taq gold polimerase (applied biosystem), 1.5 µl PCR buffer (applied biosystem), 2.5 µl MgCl2 25 Mm, 2 µl dNTP 1 Mm, 1 µl of each primer 10 Mm. The PCR profile comprised an initial denaturation of 5 min at 94°C (to activate the Ampli Taq gold polymerase), followed by 40 cycles of:
94o C for 45 sec (denaturation), 55o C for 45 sec (annealing), 72o C for 45 sec (extension), ended in a final extension in 72o C for 4 min. Electrophoresis was performed to confirm the result of amplification and to determine the length of PCR product. One µl loading dye (bromophenol-blue and cyline cyanol) was added in 2 µl PCR product. Electrophoresis was run for negative and positive control (marker) for 30 minutes in 50 Volt of 1% agarose gel media with etidum bromide dye. PCR product was sent to Macrogen Inc. (Korea) for forward and reverse sequencing.
Sequenced mt-DNA was analyzed by Geneious Pro Trial 4.7.5 and aligned using Clustal X (Thompson
et al 1997). Population parameters, i.e. the percentages of each haplotype as well as haplotype and
nucleotide diversity were analyzed using DNAsp 4.10 (Rozas et al., 2003). DNAsp 4.10 was also used to calculate the variation from each sequence (transition, transversion, and polymorphic sites). Phylogenetic tree was generated using MEGA 4.0 (Kumar et al., 2004). This program was also employed to compare the mtDNA structures of Papuan and Lesser Sunda olive ridleys.
17
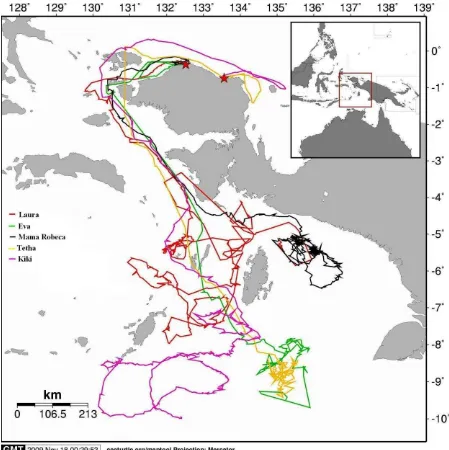
After completing her nesting period, all turtles swam for about 34 – 64 days to reach the Arafura Sea. When leaving the Birdhead Papua water, two olives from Kaironi beach crossing the waters at the northern side of Waigeo Island of Raja Ampat, but the three olives from Jamursba Medi took the southern side of Waigeo Island and Dampier Strait for their pathways down to the Arafura Sea. After that, the routes taken were almost the same which mostly near coastal areas with the average distance to the nearest shores were between 13 – 33 miles. Except Mama Robeca and Laura (two olives released from Jamursba Medi which end up in the Seram Sea; the northern part of Arafura Sea), the other three turtles seemed to cross the Kei Islands waters before reaching their feeding grounds further south in the Arafura Sea. Kiki seemed to be the most active turtle with the widest coverage feeding
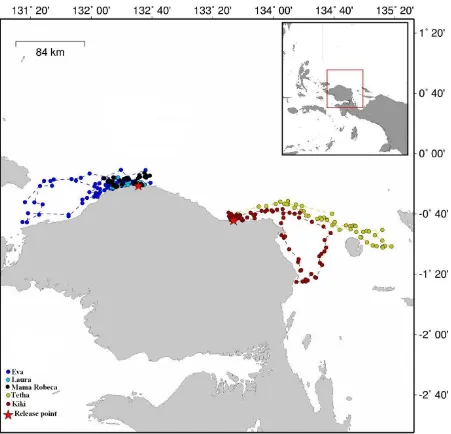
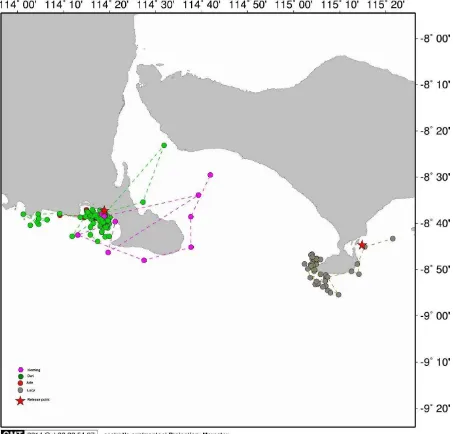
Figure 3. Inter-nesting areas used by Papuan olives. The released points were Jamursba Medi (JM) and Kaironi beaches (golden triangles at the left and right sides). Notes that JM turtles used water areas around ±100 miles in the left side of the released point, and their Kaironi counterparts occupied water areas around ±130 miles in the right side of the released point. No turtles used the water between JM and Kaironi for their inter-nesting area.
21 kph and swam within 0 – 4 miles from the nearby shores. The water depths in this inter-nesting area varied between -0.49 to -79.41 m. Re-nesting was not necessarily to occur at the same beach (Alas Purwo). While a turtle named Arie, most likely, re-nested on a beach which was ± 70 miles at the western side of the point of release, a turtle named Dwi was apparently re-nested on the beach of Perancak – Bali at the south-west area of Bali. On the other hand, Lucy which was released from Serangan Island was predicted to re-nest on a beach around 11.5 miles to the west from the point of release (Figure 4).
After completing their inter-nesting period, the turtles moved to three different directions. Two turtles
23 Figure 5. Inter-nesting areas used by the Lesser Sunda olives. The released points were Alas Purwo Beach (AP, left red star) and Serangan – Bali (right red star). Notes: the inter-nesting areas for Alas Purwo olives situated up to 4 miles from nearby shores and included the beaches ± 20 miles to the west of the released point and Perancak Beach (Bali) in the north-east. An olive released from Serangan Island of Bali seemed to use the southern tip of Bali as her inter-nesting site.
Mitochondrial-DNA Structures
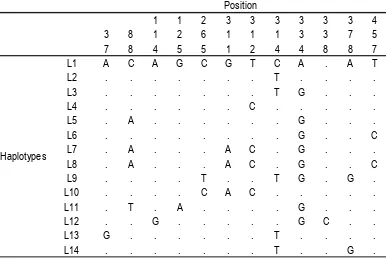
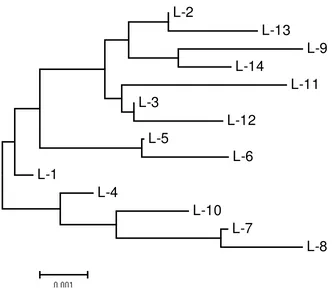
from two different rookeries revealed 12 polymorphic sites and 14 distinct haplotypes (Table 3 and 4). The haplotypes identified in this work can be grouped into three categories. Those that are (i) found only in the Birdhead Peninsula of Papua rookery (L5, L6, L7, L8, L9, L10, L11, L12), (ii) identified only the Lesser Sunda rookery (L13, L14, L1, L2, L3, L4), and (iii) a low frequency of a single haplotype (L3) which was observed to occur in both populations. The most frequent haplotype in the olive populations of the Birdhead of Papua and the Lesser Sunda regions was L5 (28%) and L1 (51.9%), respectively.
Table 3. Polymorphic sites from 14 mtDNA olive ridley sequenced haplotypes found in the Birdhead of Papua and the Lesser Sunda region. Numbering was based on the different position within the 545-bp length. genetic distance, which shows the genetic difference among two haplotypes from all haplotypes
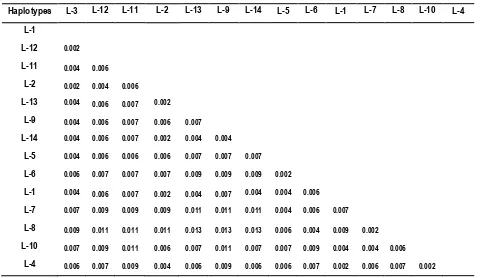
25 sequences, varied between 0.002 to 0.011 (Table 5). Comparison between the genetic distances for both locations is presented as a phylogenetic tree in Figure 4.
Figure 4. Phylogenetic tree showing the genetic distances of haplotypes olive ridley populations in the Birdhead of Papua and the Lesser Sunda regions. Scale bar shows the genetic distance of 0.001.
Three-rounds pairwise tests, i.e. computing conventional F-Statistics from haplotype frequencies, comparing the pairs of population samples by means of Distance method of Tamura and Nei, and differentiating sample by Exact Test based on the haplotype frequencies (Excoffier et al, 2006) were performed and all showed that the haplotypes composition of Papuan olive ridley turtles were significantly different to the Lesser Sunda turtles.
DISCUSSION
Many olive ridley rookeries in Indonesia have undergone serious declines over the last few decades
27 paramount issue for their conservation management. This is the first report of olive ridley migration study from the Birdhead of Papua and the Lesser Sunda region. These geographically distant rookeries, which are separated by more than 1000 kilometers and interrupted by many seas, such as the Java Sea, Sulawesi, and Maluku Sea, as expected, hosted olive ridley populations which are genetically distinct, representing two separate management units. The two populations have also different management areas which are indicated by their post-nesting migration movements. Therefore, each population represents the logical focus for recovery actions. When this population undergoes depletion, restoration via natural colonization is unlikely except over the very long-term (100 or 1000’s generations) (Dethmers et al, 2006). Accordingly, management to restore this population will require
local effort to increase survivorship and reduce mortality at the nesting beach and its respective migratory pathways and feeding areas.
During inter-nesting periods, both Papuan and Lesser Sunda olive ridley turtles using the entire water column in the vicinity of their nesting beaches. This finding suggests that protection of the nesting beach only, as it is today, is inadequate to secure these turtles. Expansion to the nearby water is needed to give a better protection. Indonesian law stipulated that marine areas within 4 and 12 miles from the beach line are under the jurisdiction of regency/municipality and provincial governments, respectively. Marine area beyond 12 miles from the beach line is belongs to the national Government jurisdiction. In this context, based on the observed inter-nesting movements, protection made and issued by the local district government of Banyuwangi (East Java) should be adequate to improve the protection of the Lesser Sunda (Alas Purwo) olives. This action should also beneficial to protect part of the inter-nesting areas for Sukamade green turtle rookery; an important green turtle nesting beach situated about 100 kilometers at the western side of Alas Purwo nesting beach (Adnyana, 2007). On the other hand, considering a huge inter-nesting area used by Papuan olives, a coordinative effort should be in place between the district, provincial and national government to improve the survival of the Papuan olives.
Sea has long been known as one of the important fishing areas for demersal fisheries, especially shrimp in Indonesia. The coastal water which is situated between the Province of Maluku and Papua are known to be relatively far from local community areas, allows only industrial scale fisheries to operate. Based on the President Decree number 85/1982, shrimp trawl fishing areas are permitted to operate between the 130o E to the North until the 10 m isobath, including the waters around Kei Islands, Tanimbar, Aru and West Papua. Annually, 250 - 526 trawl fishing vessels were recorded to operate in this area (Purbayanto et al, 2004), targeting the panaeids and tiger shrimps. Although the use of Turtle Excluder Device (TED) is compulsory for any trawls fishing in this area, but most, if not all of the crews use the device while fishing as it is considered reducing their prawn capture (Wiadnyana,
2005). As the selectivity of the shrimp trawl is low, different fish species and other animals such as sea turtles are unavoidably caught. Prasetyo (2006) reported that 116 sea turtle of various species were incidentally captured during the fishing activity of 55 prawn vessels during 2006. They were composed of 112 green sea turtles, 2 olive ridleys, and 2 unidentified species but presumably the loggerheads. Another report specified that in 2005 and 2006, a total of 133 and 26 sea turtles were incidentally caught by trawlers fishing in this area (Zaenudin et al, 2007). While in 2005 the species of the captured turtles was not defined, in 2006 they were consisted of 14 olives, 8 greens, and 4 loggerhead turtles.
29
Nufit. The Nufit is the right to capture the Tabob (sea turtles) for subsistence needs. The target species
is usually the leatherback turtle (Suarez and Starbird, 1995), but other species, including olive ridley will not be spared (Manuputty and Ingratubun, 2012).
Most of the Lesser Sunda olive ridley turtles swim within the Indian Ocean water. The telemetry result showed that they used both coastal and open ocean regions. During their coastal movement, the turtle might be captured incidentally by artisanal fishers. A report published by Profauna (2005) revealed sea turtles and their products were massively traded in at least 6 locations; 2 in Central Java (Cilacap Bay and Samas Jogjakarta), 1 in East Java (Puger Banyuwangi), and 3 in West Java (Pangandaran,
Pelabuhan Ratu, and Pangumbahan). One of the tracked turtle (named Dwi) was observed end up in Cilacap Bay, the turtle trade center in Central Java which is located a few hundred kilometers at the western side of the nesting beach. Nowadays, although it is prohibited by law, but products of olive ridley turtles (formalin-fixed whole body) can be easily observed traded illegally in this bay. Nevertheless, a genetic work needs to be done to ensure the connection of the traded olive ridley and the Lesser Sunda nesting population.
Additionally to possibly being captured for black market at various places in the southern coast of Java notably the Cilacap Bay, similar to the Papuan olives, the migration pathways of Lesser Sunda Olives were also overlapped with the operation of longline tuna fishery fishing in the Indian Ocean. The interaction between the turtles and the fishery was reported, and included only the olive ridley species. For example, observers following the trips of longline vessels in 2006 found the incidental fishing of 12 olives by Bali-based tuna fleets and 5 by the vessels based in Pelabuhan Ratu of West Java (Zaenudin et al, 2007). The fact that there was no other turtle species but olive incidentally captured by this type of fishery in this particular water, again, perhaps relates to the nature of the olive ridley as a carnivore animal. Again, this finding suggests the necessity of promoting fishing gear modification in the tuna longline industry.
REFERENCES
Proceedings of the 26th Annual Symposium on Sea Turtle Biology and Conservation. International
Sea Turtle Society, Athens, Greece. Available from
http://www.iucn-mtsg.org/genetics/meth/primers/abreu_grobois_etal_new_dloop_primers.pdf
.
2. Adnyana W (2007). Tracking and Genetic Analysis of Sea Turtles in the Eastern Part of Indonesia. A Technical Progress Report (Part 1) to WWF Indonesia. 19 p.
3. Adnyana W and C Hitipeuw (2008). Sea Turtles in Indonesia: Nesting Abundance, Migration Pathways, Major Threats and Management Issues. Paper presented in the workshop on By Cetch Reduction Techniques in Sea Turtles: Where Have We Been and Where Do We Go Next? Lesson from Indonesia, Malaysia, and Philippines. Denpasar, Bali – Indonesia, 18 -19 August 2008.
4. Adnyana W, LP Soede, G Gearheart, M Halim (2008). Status of green turtle (Chelonia mydas) nesting and foraging populations of Berau, East Kalimantan, Indonesia, including results from tagging and telemetry. Indian Ocean Turtle Newsletter No. 7: 2 – 11.
5. Bowen BW and JC Avise (1996). Conservation genetics of marine turtles. In: Conservation
Genetics. Case Histories from Nature (eds Avise JC, Hamrick JL), Chapman & Hall, New York.
6. Coyne MS and BJ Godley (2005). Satellite Tracking and Analysis Tool (STAT): an integrated system for archiving, analyzing and mapping animal tracking data. Marine Ecology Progress Series
301: 1-7.
7. Dethmers K, D Broderick, C Moritz, NN FitzSimmons, CJ Limpus, S Lavery, S Whiting, M Guinea, RIT Prince, R Kennett (2006). The genetic structure of Australasian green turtles (Chelonia
mydas): exploring the geographical scale of genetic exchange. Molecular Ecology, 15:3931-3946.
8. Excoffier L, G Laval, and S Schneider (2006). Arlequin ver 3.01 An Integrated Software Package for Population Genetics Data Analysis. Computational and Molecular Population Genetics Lab (CMPG), Institute of Zoology, University of Berne, Baltzerstrasse 63012 Bern, Switzerland
9. Gearheart G, F Ballamu, B Samper (2006).Satellite-tracking of green turtles (Chelonia mydas) in Raja Ampat, Papua, Indonesia. 26th Annual Symposium on Sea Turtle Biology and Conservation. Island of Crete, Greece, 3-8 April 2006. P 90.
10. Kumar S, K Tamura, and M Nei (2004). MEGA 3: Integrated Software for Moleculer Evolutionary Genetics Analysis and Sequence Aligment. Briefings in Bioinformatics 5:150-163.
11. Liew HC, EH Chan, F Papi, P Luschi (1995). Long distance migration of green turtles from Redang Island, Malaysia: The need for regional cooperation in sea turtle conservation. Proceedings of the International Congress of Chelonian Conservation. 6-10 July, 1995. Gonfaron, France. : pp 73-75
31 13. Manuputty J dan MA Ingratubun (2012). Upaya Penyelamatan Penyu Belimbing (Dermochelys coriacea) di Perairan Kei Maluku Tenggara, Melalui Pengembangan Kawasan Konservasi Laut.
Paper presented in the Mini-Simposium: Menakar Keberhasilan Program Konservasi Penyu Laut di
Indonesia. Mataram 21 October 2012. 9 p.
14. Papi F, P Luschi, S Akesson, S Capogrossi, GC Hays (2000). Open-Sea Migration of Magnetically Disturbed Sea Turtles. The Journal of Experimental Biology 203, 3435–3443.
15. Profauna (2005). Final Report on the Sea Turtle Trade on the South Coast of Java. Downloaded in 18 December 2012 from http://www.profauna.net/sites/default/files/downloads/publication-2005-sea-turtle-trade-in-java.pdf
16. Purbayanto A, SH Wisudo, RI Wahju, J Santoso, B Pramono, A Marpaung (2004). Upaya Mengurangi dan Memanfaatkan Hasil Tangkapan Samping (Bycatch) Di Laut Arafura. Paper presented in Konferensi Nasional IV Sumberdaya Pesisir dan Laut. Balikpapan, Kalimantan Timur.
14 – 16 September 2004. 16 p.
17. Thompson JD, TJ Gibson, F Plewniak, F Jeanmougin, DG Higgins (1997). The clustal_x Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic
Acids Research, 25, 4876–4882.
18. Rozas, J., JC Sánchez-DelBarrio, X Messeguer, and R Rozas (2003). DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496-2497
19. Russell AP (2005). Migration in amphibians and reptiles: An overview of patterns and orientation mechanisms in relation to life history strategy. In Elewa AM Migration of organisms: climate
22. Zainudin IM, LP Soede, C Hitipeuw, W Adnyana (2007). Interaction of Sea Turtles with Indonesian Fisheries – Preliminary Findings. Indian Ocean Turtle Newsletter No. 6: 1-10.
ACKNOWLEDGEMENT
This work was part of a larger study entitled Tracking and Genetic Analysis of Marine Turtles in Indonesia; a
collaborative effort between WWF Indonesia and Udayana University to define turtle-based MPA networks in