BAHAGIAN PENGURUSAN SEKOLAH BERASRAMA PENUH
BAHAGIAN PENGURUSAN SEKOLAH BERASRAMA PENUH
DAN SEKOLAH KECEMERLANGAN
DAN SEKOLAH KECEMERLANGAN
MODUL
MODUL PERFECT SCORE
PERFECT SCORE
SEKOLAH BERASRAMA PENUH
SEKOLAH BERASRAMA PENUH
TAHUN 2011
TAHUN 2011
CHEMISTRY
CHEMISTRY
PANEL PENYEDIA DAN PEMURNI: PANEL PENYEDIA DAN PEMURNI:
Pn. Wan Noor Afifah Binti Wan Yusoff (Ketua)
Pn. Wan Noor Afifah Binti Wan Yusoff (Ketua)
SBPI GOMBAK
SBPI GOMBAK
Pn. Aishah Peong Binti Abdullah
Pn. Aishah Peong Binti Abdullah
SBPI TEMERLOH
SBPI TEMERLOH
Pn. Norini Binti Jaafar
Pn. Norini Binti Jaafar
SEKOLAH SULTAN ALAM SHAH
SEKOLAH SULTAN ALAM SHAH
Pn. Noraini Binti Zakaria
Pn. Noraini Binti Zakaria
SMS SULTAN MOHAMAD JIWA
SMS SULTAN MOHAMAD JIWA
Pn. Rossita Binti Radzak
Pn. Rossita Binti Radzak
SMS TUANKU MUNAWIR
SMS TUANKU MUNAWIR
En Che Malik Bin Mamat
En Che Malik Bin Mamat
SBPI BATU RAKIT
SBPI BATU RAKIT
En Jong Kak Ying
En Jong Kak Ying
SMS KUCHING
SMS KUCHING
En Ooi Yoong Seang
En Ooi Yoong Seang
SMS MUAR
SMS MUAR
Pn Sa’adah Binti
Pn Sa’adah Binti Mohayudd
Mohayuddin
in
SMS SERI PUTERI
SMS SERI PUTERI
Pn
CHEMISTRY PERFECT SCORE MODULE 2011
CHEMISTRY PERFECT SCORE MODULE 2011
CONTENT
CONTENT
1
1 Guidelines &Guidelines & Anwering Techniques Anwering Techniques
Format of an instrument of chemistryFormat of an instrument of chemistry
Construct requirementConstruct requirement
Guidelines for answering paper 1Guidelines for answering paper 1
Guidelines for answering paper 2Guidelines for answering paper 2
Guidelines for answering paper 3Guidelines for answering paper 3
The common command words in paper 2The common command words in paper 2
2
2 Set Set 11
The structure of AtomThe structure of Atom
Chemical Formulae and equationsChemical Formulae and equations
Periodic Table of ElementsPeriodic Table of Elements
Chemical BondsChemical Bonds
3
3 Set Set 22 ElectrochemistryElectrochemistry
Oxidation and ReductionOxidation and Reduction
4
4 Set Set 33
Acids and BasesAcids and Bases
SaltsSalts
Rate of reactionRate of reaction
ThermochemistryThermochemistry
5
5 Set Set 44
Carbon compoundsCarbon compounds
Manufactured Substance in IndustryManufactured Substance in Industry
Chemicals for ConsumersChemicals for Consumers
6
6 Set Set 55
Paper 3 set 1Paper 3 set 1
Paper 3 set 2Paper 3 set 2
CHEMISTRY
CHEMISTRY
PERFECT SCORE MODULE
PERFECT SCORE MODULE
GUIDELINES
GUIDELINES
ANSWERING TECHNIQUES
ANSWERING TECHNIQUES
CHEMISTRY SPM
CHEMISTRY SPM
GUIDELINES AND ANSWERING TECHNIQUES FOR
GUIDELINES AND ANSWERING TECHNIQUES FOR SPM CHEMISTRY PAPERSPM CHEMISTRY PAPER 1.0
1.0 FORMAT FORMAT OF OF AN AN INSTRUMENT INSTRUMENT OF OF CHEMISTRY CHEMISTRY BEGINNING BEGINNING SPM SPM 20032003 No
No Item Item Paper Paper 11 (4541/1) (4541/1) Paper 2 Paper 2 (4541/2) (4541/2) Paper 3 Paper 3 (4541/3) (4541/3) 1
1 Type Type of of instrument instrument Objective Objective test test Subjective Subjective test test Written Written Practical Practical TestTest
2 2
Type
Type of of item item Objective Objective it it Section Section A A :: Structured Item Structured Item Section B : Section B :
Essay restricted response Item Essay restricted response Item Section C :
Section C :
Essay extended response Item Essay extended response Item
Subjective Item : Subjective Item : Structured Item Structured Item
Extended Response Item: Extended Response Item: (Planning an experiment) (Planning an experiment)
3 3
Number
Number of of question question 50 50 (answers (answers all) all) Section Section A A : : 6 6 (answer (answer all)all) Section B : 2 (choose one) Section B : 2 (choose one) Section C : 2 (choose one) Section C : 2 (choose one)
Structured Item : Structured Item : 1/2 items (answer all) 1/2 items (answer all) Extended Response Item : Extended Response Item : 1 item
1 item 5
5 Duration Duration of of time time 1 1 hour hour 15 15 minutes minutes 2 2 hour hour 30 30 minutes minutes 1 1 hour hour 30 30 minutesminutes 2.0
2.0 CONSTRUCT CONSTRUCT REQUIREMENTREQUIREMENT Construct
Construct Paper Paper 1 1 Paper Paper 2 2 Paper Paper 33 Knowledge Knowledge 20 20 m m ( ( No No 1- 1- 20) 20) 14 14 --Understanding Understanding 15 15 m m ( ( No No 2121 – – 35) 35) 21 21 --Application Application 15 15 m m ( ( No No 3636 – – 50) 50) 29 29 --Analysis Analysis - - 21 21 --Synthesizing Synthesizing - - 15 15 --Science
Science process process - - - - 5050 Total
Total mark mark 50 50 100 100 5050 3.0
3.0 TIPS TO SCORE “ A “ CHEMISTRYTIPS TO SCORE “ A “ CHEMISTRY 3.1
3.1 Master Master the the topics topics that that contains contains the the basic basic concepts concepts of of chemistry chemistry :: 1.
1. The The structure structure of of the the atomatom 2.
2. Chemical Chemical Formulae Formulae And And EquationsEquations 3.
3. Periodic Periodic TableTable 4.
4. Chemical Chemical BondBond 3.2
3.2 Familiarize Familiarize with with different different types types of of questions questions as as listed listed below below and and complete complete the the previous previous SPM SPM papers:papers: 1.
1. Objectives Objectives questions questions (MCQ) (MCQ) (Paper (Paper 1)1) 2.
2. Structured Structured questions questions ( ( Paper Paper 2 2 & & 3)3) 3.
3. Essays Essays (Paper (Paper 2)2) 4.
4. Planning Planning an an experiment experiment ( ( Paper Paper 3)3) 5.
5. Draw Draw and and label label the the diagramdiagram 6.
6. Writing Writing chemical chemical equation( equation( balanced balanced equation, equation, ionic ionic equation, equation, half half equation)equation) 3.3
3.3 Try Try to to get get :-
:-
40 marks above for paper 140 marks above for paper 1
60 marks above for paper 260 marks above for paper 2
40 40 marks marks above above for for paper paper 3 3 (Total (Total = = 180/2 180/2 =80 =80 , , A+ A+ in in SPM)SPM) 4.0
4.0 GUIDELINE GUIDELINE FOR FOR ANSWERING ANSWERING PAPER PAPER 11 4.1
4.1 Paper Paper 1 1 questions questions test test students students onon 1.
1. Knowledge Knowledge ( ( Number Number 11 – – 20)20) 2.
2. Understanding ( Understanding ( Number Number 2121 – – 35)35) 3.
3. Application ( Application ( Number Number 3636 – – 50 )50 ) 4.2 Score
4.2 Score in paper 1 Indicates student’s level of in paper 1 Indicates student’s level of understandingunderstandingin chemistry:in chemistry: Less than 20
Less than 20 – – very weakvery weak 20 20 – – 25 25 - - weakweak 26 26 – – 30 30 - - averageaverage 31 31 – – 39 39 - - goodgood 40
40 – – 45 45 - - very very goodgood 46
46 – – 50 50 - - excellent.excellent. 4.3
4.3 Answer Answer all all SPM SPM objective objective questions questions (2003(2003 – – 2010). Objective questions for each year contain all topics. If2010). Objective questions for each year contain all topics. If your score in paper 1 is 4
5.0
5.0 GUIDELINE GUIDELINE FOR FOR ANSWERING ANSWERING PAPER PAPER 2 2 (STRUCTURE (STRUCTURE AND AND ESSAY)ESSAY) 5.1
5.1 Paper Paper 2 2 questions questions test test student student onon 1. Knowledge 1. Knowledge 2. understanding 2. understanding 3. analyzing 3. analyzing 4. synthesizing 4. synthesizing 5.2
5.2 Steps Steps taken taken are:are: 1.
1. Underline Underline thethe command wordcommand word andand marksmarks allocated for each question.allocated for each question. 2.
2. MatchMatch thethe command word to the markcommand word to the mark allocated for each question. 1 point is awarded 1 markallocated for each question. 1 point is awarded 1 mark.. 3.
3. Follow Follow the the needs needs of of the the question question (Refer(Refer to the command words, page …….to the command words, page …….)) 4.
4. Unnecessary Unnecessary repetition repetition of of the the statement statement in in the the question question is is not not required.required. 5.3
5.3 Three Three types types of of questions questions which which involve involve experiments experiments in in paper paper 2:2: I.
I. Type Type 11
Describe an experiment on………Include a labeled diagram in
Describe an experiment on………Include a labeled diagram in your answer your answer 1. Diagram
1. Diagram 2. Procedure 2. Procedure 3.
3. Observation/example/data/calculation/equation/sketch Observation/example/data/calculation/equation/sketch of grof graph/conclusionaph/conclusion II.
II. Type Type 22
Describe an experiment………( The diagram will support your
Describe an experiment………( The diagram will support your answer.)answer.) 1.
1. No mark No mark is is allocated for allocated for a a diagramdiagram 2. Procedures
2. Procedures 3.
3. Observation/example/calculation/equation/sketch Observation/example/calculation/equation/sketch of of graph/conclusiongraph/conclusion III.
III. Type Type 33
Describe a chemical/confirmatory
Describe a chemical/confirmatory test for …….test for ……. 1. Procedure 1. Procedure 2. Observation 2. Observation 3. Conclusion 3. Conclusion 6.0
6.0 GUIDELINE GUIDELINE FOR FOR ANSWERING ANSWERING PAPER PAPER 33 6.1
6.1 Structure Structure Question Question 1/2 1/2 test test the the mastery mastery of of 11 11 Scientific Scientific SkillsSkills 1. Observing 1. Observing 2. Classifying 2. Classifying 3. Inferring 3. Inferring 4.
4. Measuring Measuring (burette (burette , , stopwatch, stopwatch, thermometer, thermometer, voltmeter)voltmeter) 5. Predicting
5. Predicting 6.
6. Communicating( Communicating( e.g construct e.g construct table table and and draw draw graph)graph) 7.
7. Space-Time Space-Time RelationshipRelationship 8.
8. Interpreting Interpreting DataData 9.
9. Defining Defining OperationallyOperationally 10.
10. Controlling Controlling VariablesVariables 11. Hypothesizing 11. Hypothesizing
Each answer is allocated mark as follows: 3 marks/2 marks/1 mark/0
Each answer is allocated mark as follows: 3 marks/2 marks/1 mark/0 Score : 11 X 3 = 33Score : 11 X 3 = 33 Example of operational definition:
Example of operational definition: 1. what you do
1. what you do
2. what you observe correctly 2. what you observe correctly Example:
Example:
1. When acid is added into latex,
1. When acid is added into latex, white solidwhite solid is formed.is formed. When acid is added into latex,
When acid is added into latex, latex coagulatedlatex coagulated.- wrong.- wrong
2. When the higher the concentration sodium thiosulphate solution is added
2. When the higher the concentration sodium thiosulphate solution is added into sulphuric acid, time taken for `X~ mark tointo sulphuric acid, time taken for `X~ mark to disappear from sight is shorter.
disappear from sight is shorter.
3. When iron nail is coiled with copper and immersed into jelly mixed with potassium hexacyanoferrate(III) and phenolphthalein 3. When iron nail is coiled with copper and immersed into jelly mixed with potassium hexacyanoferrate(III) and phenolphthalein
solution, blue
solution, blue spot/colouration spot/colouration is is formed.formed.
Operational
Operational definition definition for for What What you you do do What What is is observedobserved 1.
1. Rusting Rusting of of iron iron When When an an iron iron nail nail coiled coiled with with a a lessless electropositive metal is immersed in hot electropositive metal is immersed in hot agar-agar added with potassium
agar-agar added with potassium hexacyanoferrate (III) solution, hexacyanoferrate (III) solution,
Blue spots are formed Blue spots are formed
2.
2. Coagulation Coagulation of of latex latex When When acid acid is is added added to to latex latex White White solid solid is is formedformed 3.
3. Reactivity Reactivity of of Group Group 11 elements
elements
When a metal which is lower in Group 1 is When a metal which is lower in Group 1 is put in a basin half f
put in a basin half filled with waterilled with water
Brighter flame is formed Brighter flame is formed 4.
4. Precipitation Precipitation of of silversilver chloride
chloride
When silver nitrate solution is added to When silver nitrate solution is added to sodium chloride solution
sodium chloride solution
White solid is formed White solid is formed 5. Voltaic
5. Voltaic cell cell When When two two different different metals metals are are dipped dipped into into anan electrolyte
electrolyte
The needle of the The needle of the voltmeter deflects voltmeter deflects 6.
6. An An acid acid When When a a blue blue litmus litmus paper paper is is dipped dipped into into aa substance
substance which is which is dissolved in dissolved in water,water,
Blue litmus paper turns Blue litmus paper turns red
Hypothesis
Hypothesis: Relate manipulated variable followed by responding variable with direction.: Relate manipulated variable followed by responding variable with direction. Example:
Example: 1.
1. The higher The higher temperature of temperature of the reactant the the reactant the higher the rhigher the rate of reactionate of reaction – – 3 marks3 marks The temperature of the reactant affect the rate of reaction
The temperature of the reactant affect the rate of reaction – – 2 marks2 marks
2. Hexene decolourised brown bromine water but hexane does not decolourised brown bromine water. 2. Hexene decolourised brown bromine water but hexane does not decolourised brown bromine water.
3. When acid is added into latex, latex coagulates, when ammonia is added into latex, latex cannot coagulates 3. When acid is added into latex, latex coagulates, when ammonia is added into latex, latex cannot coagulates 6.2
6.2 Question Question 3 3 (essay) (essay) Test Test The The Mastery Mastery of of Planning Planning Experiment .Experiment . Planning should include the following aspects:
Planning should include the following aspects: 1.
1. Aim Aim of of the the experiment/Statement experiment/Statement of of the the problemproblem 2.
2. All All the the variablesvariables 3.
3. Statement Statement of of the the hypothesishypothesis 4.
4. List List of of substances/material substances/material and and apparatusapparatus – – should be separatedshould be separated 5.
5. Procedure Procedure of of the the experimentexperiment 6.
6. Tabulation of dataTabulation of data Score : (5 X 3) + 2 = 17Score : (5 X 3) + 2 = 17 The question normally starts with certain situation related to daily life.
The question normally starts with certain situation related to daily life. Problem statement/ aim of the experiment / hypoth
Problem statement/ aim of the experiment / hypothesis and variable can be concluded from the esis and variable can be concluded from the situation given.situation given. State all the variables
State all the variables
Manipulated variable : Manipulated variable : Responding variable : Responding variable :
Constant variable: list down all the fixed variables to ensure the outco
Constant variable: list down all the fixed variables to ensure the outcome of the responding me of the responding variable is related only tovariable is related only to the manipulated variables.
the manipulated variables. Separate
Separate the substances the substances and apparatusand apparatus -
- Separate the Separate the substances substances and and apparatusapparatus
-- ApparatusApparatus : list down the apparatus for the experiment.: list down the apparatus for the experiment. Example
Example: Rate of reaction: Rate of reaction – – stop watchstop watch Termochemistry - thermometer Termochemistry - thermometer Procedure :
Procedure :
All the steps taken in the procedure must include the apparatus used, quantity and type of
All the steps taken in the procedure must include the apparatus used, quantity and type of substance (powder, solution, lumpssubstance (powder, solution, lumps … etc).
… etc).
No mark is allocated for the diagram. The complete labeled
No mark is allocated for the diagram. The complete labeled diagram can help students in :diagram can help students in : I.
I. Writing Writing the the steps steps taken taken in in the the procedureprocedure II.
II. Listing Listing the the apparatus apparatus and and materialsmaterials Tabulation of data:
Tabulation of data:
The number of columns and rows in the table is related to the manipulated and respondingThe number of columns and rows in the table is related to the manipulated and responding variables
variables
Units must be writtenUnits must be written for all the titles in each row and column of the tablefor all the titles in each row and column of the table
7.0
7.0 THE THE COMMON COMMON COMMAND COMMAND WORDS WORDS IN IN PAPER PAPER 2 2 & & PAPER PAPER 3 3 CHEMISTRYCHEMISTRY
The question norThe question normally starts wmally starts with a command ith a command word.word.
Students must know the meaning of the command word to make sure that theStudents must know the meaning of the command word to make sure that the answer given is according to theanswer given is according to the question’s requirement.
question’s requirement.
Match the command word to the mark allocated for each question.Match the command word to the mark allocated for each question. Command
Command word word Explanation/exampleExplanation/example Name/State the Name/State the name name (paper 2 & 3) (paper 2 & 3)
Give the name , not the formula. Give the name , not the formula.
Example: Name the main element added to copper to form bronze. Example: Name the main element added to copper to form bronze. Wrong
Wrong answer answer : : Sn.Sn. Correct answer : Tin Correct answer : Tin State
State (paper 2 & 3) (paper 2 & 3)
Give brief answer only. Explanation is not required. Give brief answer only. Explanation is not required. Example :
Example : State one substance State one substance which can conduct electricity which can conduct electricity in solid state.in solid state. Answer
Answer : : CopperCopper
State the State the observation observation (Paper 2 & 3) (Paper 2 & 3)
Write what is observed physically. Write what is observed physically. Example 1
Example 1 : : State one observation wState one observation when magnesium powder is ahen magnesium powder is added to hydrochloricdded to hydrochloric acid.
acid. [ [ 1 1 mark]mark]
Wrong answer
Wrong answer : : Hydrogen Hydrogen gas gas is is released.released. Correct answer
Correct answer : : Gas bubbles Gas bubbles are releasedare released
Indicate the change of colour , give the initial and final colour of the substance/chemical. Indicate the change of colour , give the initial and final colour of the substance/chemical. Example 2
Example 2 : : What What is is the the colour colour change change of of copper(II) copper(II) suphate suphate solution. solution. [2 [2 marks]marks] Wrong answer
Wrong answer : : The solution The solution becomes colourlessbecomes colourless Correct answer
Correct answer : : The blue colour of The blue colour of the solution becomes cthe solution becomes colourlessolourless
Explain Explain (Paper 2 & 3) (Paper 2 & 3)
Give the answer with reasons to explain certain statement /
Give the answer with reasons to explain certain statement / fact / observation/ principal.fact / observation/ principal. Example 1
Example 1 : : Explain Explain why why bronze bronze is is harder harder than than pure pure copper copper [4 [4 marks]marks] Correct answer
Correct answer ::
-- Copper Copper atoms atoms in in pure pure copper copper are are all all of of the the same same size size and and ...1...1
-- they they are are arranged arranged in in layers layers that that can can slide slide easily easily when when force force is is applied applied ...1...1
-- The presence of tin atoms in bronze that are different in size disturb theThe presence of tin atoms in bronze that are different in size disturb the orderly
orderly arrangement arrangement of of atoms atoms in in bronze. bronze. ...1...1
-- This This reduces reduces the the layer layer of of atoms atoms from from sliding. sliding. ...1...1 What is meant by..
What is meant by.. (Definition)
(Definition) (Paper 2 & 3) (Paper 2 & 3)
Give the exact meaning Give the exact meaning Example:
Example: What is meant by hydrocarbon.What is meant by hydrocarbon. Wrong answer:
Wrong answer: A compound that contains carbon and hydrogenA compound that contains carbon and hydrogen Correct answer
Correct answer : : A compound A compound that contains carbon that contains carbon hydrogen onlyhydrogen only
Describe chemical Describe chemical test test (Paper 2 & 3) (Paper 2 & 3) State the
State the methodmethod to conduct the test ,to conduct the test , observationobservation andand conclusion.conclusion. Example
Example : : Describe Describe how how to to identify identify the the ion ion present present in in the the solution solution . . [3 [3 marks]marks] Answer
Answer : : - Pour - Pour in 2 in 2 cmcm33of the solution in a test tube. of the solution in a test tube. Add a few drops of sodiumAdd a few drops of sodium hydroxide
hydroxide solution solution and and the the test test tube tube is is shake shake the the test test tube tube ...1...1 -
- A A reddish reddish brown brown precipitate precipitate formed. formed. ...1...1 - Fe
- Fe3+3+ions presentions present ………1………1
Describe gas test. Describe gas test. (Paper 2 & 3) (Paper 2 & 3)
State
State the method the method to conduct to conduct the test the test observation and observation and conclusionconclusion.. Example:
Example: Describe Describe the the confirmatory confirmatory test test for for gas gas released released at at the the anode anode (oxygen). (oxygen). [ [ 3 3 marks]marks] Wrong answer
Wrong answer : : Test with a Test with a glowing wooden glowing wooden splinter.splinter. Correct answer:
Correct answer: - Place - Place a a glowing glowing wooden wooden splinter splinter to to the the mouth mouth of of the the test test tube tube ....…….1…….1 -
- The The glowing glowing wooden wooden splinter splinter is is lighted lighted up up ...……1……1 -
- Oxygen Oxygen gas gas is is released released ....…….1…….1
Describe an Describe an experiment experiment ( ( 8 - 8 - 10 mar10 marks)ks) (Paper 2) (Paper 2)
-- No mark is awarded for the No mark is awarded for the diagram. diagram. The diagram can help students wThe diagram can help students write the steps taken in therite the steps taken in the procedure.
procedure.
-- List List of of materials materials 1m1m
-- List List of of apparatus apparatus 1m1m
-- Procedure Procedure - - ( ( 55 – – 8 m)8 m)
-- Observation/tabulation of data/ calculation/sketch of the graph/ chemical equation /ionic equationObservation/tabulation of data/ calculation/sketch of the graph/ chemical equation /ionic equation /conclusion …… etc.
/conclusion …… etc.
-- Any additional details relevant derived from the question.Any additional details relevant derived from the question.
Plan an Plan an experiment experiment ( 17 marks) ( 17 marks) ( Paper 3) ( Paper 3)
Answer the question according the requirement : Answer the question according the requirement :
Problem statement/Aim of experimentProblem statement/Aim of experiment
HyphotesisHyphotesis
VariablesVariables
List of substances and apparatusList of substances and apparatus
ProcedureProcedure
Tabulation of dataTabulation of data Note: For question
Note: For question 3, unlike PEKA 3, unlike PEKA report report students only need students only need to answer accordito answer according to what is ng to what is stated instated in the question.
the question. - No mark for
- No mark for the diagram. Diagram can the diagram. Diagram can help student help student writing the steps writing the steps taken in the procedure.taken in the procedure. Can be obtained from the diagram
Describe the Describe the process process …… Describe Describe thethe structure …. structure …. Describe
Describe and writeand write equation… equation… Describe how Describe how …… (Paper 2 & 3) (Paper 2 & 3)
Give relevant details derived from the question. Give relevant details derived from the question.
Predict Predict (Paper 2 & 3) (Paper 2 & 3)
Make a prediction for
Make a prediction for something that might something that might happen based on factshappen based on facts Example
Example : : Experiment 1 is repeated using a larger beaExperiment 1 is repeated using a larger beaker. Predict the increase in temperatureker. Predict the increase in temperature Answer : The increase in temperature is lower than experiment 1
Answer : The increase in temperature is lower than experiment 1.. Compare
Compare (Paper 2) (Paper 2)
Give the
Give the similarities and differencessimilarities and differences between two items/ situationsbetween two items/ situations
Differentiate Differentiate (Paper 2) (Paper 2)
Give differences
Give differences between two items/situationsbetween two items/situations
Example : State three differences between ionic and covalent compound. Example : State three differences between ionic and covalent compound.
Answer : State three properties of ionic compound and three properties covalent compound Answer : State three properties of ionic compound and three properties covalent compound Draw a labeled Draw a labeled diagram of the diagram of the apparatus apparatus (Paper 2) (Paper 2) Draw
Draw a complete set a complete set up of apparup of apparatusatus (i)
(i) Functional Functional set set up up of of apparatusapparatus (ii)
(ii) Complete Complete labellabel (iii)
(iii) Shade Shade solid, solid, liquid liquid and and gas gas correctly.correctly. (iv)
(iv) Draw Draw an an arrow arrow and and labellabel ’ heat’ if ’ heat’ if the experimthe experiment involves ent involves heatingheating
Draw
Draw a a diagramdiagram toto show
show the the bondingbonding formed in the formed in the compound compound (Paper 2) (Paper 2) (i)Ionic compound
(i)Ionic compound – – The number of electrons in each shell is correct, 2 electrons in the first shellThe number of electrons in each shell is correct, 2 electrons in the first shell and 8 electrons in the second and third shell.
and 8 electrons in the second and third shell. –
– Show the charge of each particle.Show the charge of each particle. –
– Write the symbol of each element at Write the symbol of each element at the centre of the ion.the centre of the ion. (ii) Covalent compound
(ii) Covalent compound
The number of electrons in each shell is correct, 2 electrons in the first shell andThe number of electrons in each shell is correct, 2 electrons in the first shell and 8 electrons in the second and third shell.
8 electrons in the second and third shell.
The number of atoms sharing pair of electrons is correct.The number of atoms sharing pair of electrons is correct.
Write the symbol of each element at the center of each atom in the molecule.Write the symbol of each element at the center of each atom in the molecule.
Draw graph Draw graph (Paper 3) (Paper 3)
Draw graph as follows : Draw graph as follows :
Label the two axis with the correct unitLabel the two axis with the correct unit
Choose suitable scale, the size of the graph should be at least Choose suitable scale, the size of the graph should be at least ¾ of the size of the grap¾ of the size of the graphh paper.
paper.
Plot all the points correctlyPlot all the points correctly
Smooth graph ( curve or straight line )Smooth graph ( curve or straight line )
For For the determination the determination of the rate of the rate of reactionof reaction (i)
(i) Draw Draw a a tangent tangent at at the the curve.curve. (ii)
(ii) Draw Draw a a triangle triangle at at the the tangenttangent Calculate the gradient of the tangent Calculate the gradient of the tangent Draw the energy
Draw the energy level diagram level diagram ( Paper 2) ( Paper 2)
Draw an arrow for the vertical axis only and label with energy.Draw an arrow for the vertical axis only and label with energy.
Draw two horizontal lines for the reactants and productsDraw two horizontal lines for the reactants and products Draw the Draw the arrangement of arrangement of particles in solid, particles in solid, liquid and gas. liquid and gas. (Paper 2) (Paper 2)
Solid: Draw at least three layers of particles closely packed in orderly manner and they are notSolid: Draw at least three layers of particles closely packed in orderly manner and they are not overlap.
overlap.
Liquid : The particles packed closely but not in orderly mannerLiquid : The particles packed closely but not in orderly manner
Gas Gas : T: The partihe particles are cles are very very far aparfar apart from t from each each otherother Draw the direction of
Draw the direction of electron flow
electron flow (Paper 2 /3) (Paper 2 /3)
Draw the direction for the flow of electrons on the c
Draw the direction for the flow of electrons on the c ircuit, not through the solution.ircuit, not through the solution. Write chemical Write chemical equation equation (Paper 2 & 3) (Paper 2 & 3)
Write the balanced chemical equationWrite the balanced chemical equation
Differentiate :Differentiate : (i)
(i) Balanced chemical Balanced chemical equationequation (ii)
(ii) Ionic Ionic equationequation (iii)
(iii) Half Half equation equation for for oxidationoxidation (iv)
(iv) Half Half equation equation for for reductionreduction Calculate
Calculate (Paper 2 & 3) (Paper 2 & 3)
Show all the steps takenShow all the steps taken
Give final answer with unit.Give final answer with unit. Classify
Classify (Paper 3) (Paper 3)
CHEMISTRY PERFECT SCORE MODULE
CHEMISTRY PERFECT SCORE MODULE
SET
SET
1.
1. The Str
The Structure o
ucture of Atom
f Atom
2.
2. Chemical Formulae
Chemical Formulae and
and Equations
Equations
3.
3. Periodic Table
Periodic Table of
of Elements
Elements
4. C
PAPER 2 : SECTION A [STRUCTURE] PAPER 2 : SECTION A [STRUCTURE]
1
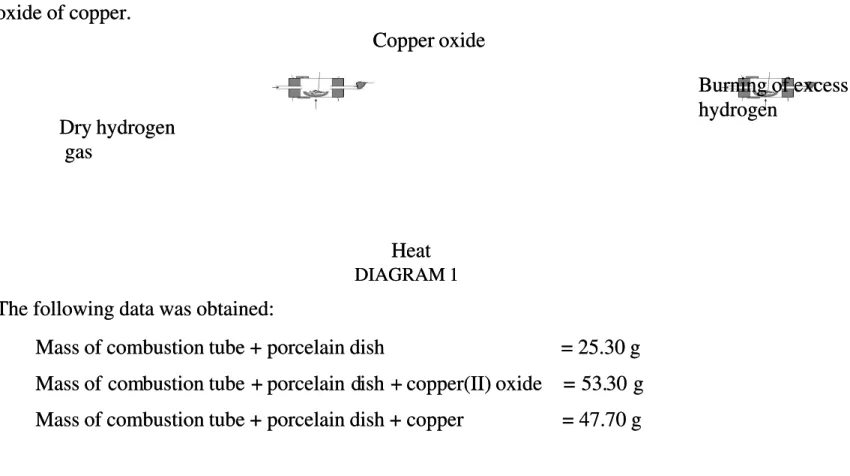
1 Diagram Diagram 1 shows 1 shows the apparatus the apparatus set-up used set-up used in an experimin an experiment to determent to determine the emine the empirical formula of pirical formula of anan oxide of copper.
oxide of copper.
DIAGRAM 1 DIAGRAM 1
The following data was obtained: The following data was obtained:
Mass
Mass of of combustion combustion tube tube + + porcelain porcelain dish dish = = 25.30 25.30 gg Mass
Mass of comof combustion bustion tube + tube + porcelain dporcelain dish + ish + copper(II) copper(II) oxide oxide = 53.= 53.30 g30 g Mass
Mass of of combustion combustion tube tube + + porcelain porcelain dish dish + + copper copper = = 47.70 47.70 gg
(a)
(a) What What is is meant meant by by empirical empirical formula?formula? ... ... ……….. ……….. [1 mark] [1 mark] (b)
(b) Write a chemical equation Write a chemical equation for the reaction used to produce for the reaction used to produce hydrogen gas.hydrogen gas.
……...
……...
[2 marks] [2 marks] (c)
(c) How How to to ensure ensure that that all all the the copper copper oxide oxide is is completely completely reacted?reacted?
……….. ……….. ... ………...………... ………...………... [1 mark] [1 mark] (d)
(d) Based Based on on the the data data given, given, determine determine the the empirical empirical formula formula of of the the copper copper oxide.oxide.
[4 marks] [4 marks] (e)
(e) Write the chemical equation for Write the chemical equation for the reaction between hydrogen and the the reaction between hydrogen and the oxide of copper.oxide of copper.
…………
………...…...
[2 marks] [2 marks] (f)
(f) After the reaction After the reaction is completed, is completed, hydrogen gas hydrogen gas is allowed is allowed to to flow continuously flow continuously until the copuntil the copperper is cooled to room temperature. Explain why .
is cooled to room temperature. Explain why .
………….……… ………….………...……... Dry hydrogen Dry hydrogen gas gas Heat Heat Copper oxide Copper oxide Burning of excess Burning of excess hydrogen hydrogen
(g)
(g) The empirical formula for magnesium oxide can The empirical formula for magnesium oxide can be determined by direct heating of be determined by direct heating of magnesium.
magnesium. Draw
Draw a labeled a labeled diagram diagram to show to show apparatus sapparatus set-up et-up to carry to carry out this out this experiment.experiment.
[ 2 marks] [ 2 marks] 2
2 (a) (a) 70.2 70.2 g g of of aluminium aluminium carbonate carbonate decomposed decomposed easily easily when when heated heated to to produce produce aluminium aluminium oxide oxide basedbased on
on the the following following equation.equation.
[Relative atomic mass: Al: 27 ; C: 12; O: 16; 1 mole of gas occupied 24 dm
[Relative atomic mass: Al: 27 ; C: 12; O: 16; 1 mole of gas occupied 24 dm33at room condition]at room condition] (i)
(i) Write Write the the formula formula of of Aluminium Aluminium carbonate.carbonate.
…………..………... …………..………...
[1 mark] [1 mark] (ii)
(ii) Write a balanced chemical Write a balanced chemical equation equation for for the the reaction reaction above.above.
………... ………...
[2 marks] [2 marks] (iii)
(iii) Calculate the Calculate the mass mass of of aluminium aluminium oxide oxide that that is is produced.produced.
[3 marks] [3 marks] (iv)
(iv) Calculate Calculate the vthe volume olume of carbon of carbon dioxide dioxide gas pgas produced roduced at at room room conditions conditions ..
[3 marks] [3 marks]
...(s)
(b)
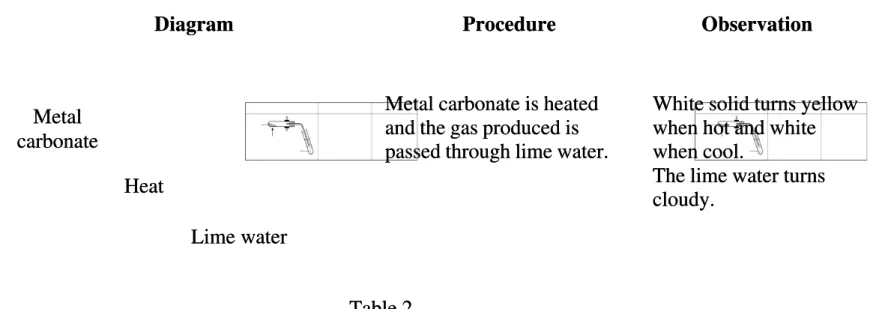
(b) Table Table 2 2 shows shows an an experiment experiment to to investigate investigate the the effect effect of of heat heat on on metal metal carbonate.carbonate. Diagram
Diagram ProcedureProcedure ObservationObservation
Metal carbonate is heated Metal carbonate is heated and the gas produced is and the gas produced is passed through lime water. passed through lime water.
White solid turns yellow White solid turns yellow when hot and white when hot and white when cool.
when cool.
The lime water turns The lime water turns cloudy.
cloudy.
Table 2 Table 2 Based on the experiment:
Based on the experiment: (i)
(i) Name the metal carbonate used.Name the metal carbonate used.
………...
………... [1 mark] [1 mark] (ii)
(ii) State the name of the State the name of the products formed.products formed.
……….………... ……….………...
[1 mark] [1 mark] (iii)
(iii) Write Write a a chemical chemical equation equation for for the the reaction.reaction.
…….………... …….………...
[2 marks] [2 marks] 3
3 Table 3 shows the Table 3 shows the proton number and the numproton number and the number of neutrons for atoms P, Q, R ber of neutrons for atoms P, Q, R and S.and S.
Table 3 Table 3 (a)
(a) (i) (i) What What is is meant meant by by thethe proton number proton number ??
………... ………...
[1 mark] [1 mark] (ii)
(ii) What What is is the the nucleon nucleon number number of of atom atom P?P?
……….………... ……….………...
[1 mark] [1 mark] (b)
(b) Write the symbol for atom Q in the formWrite the symbol for atom Q in the form
..………….……… ..………….………
[1 mark] [1 mark] (c)
(c) Which atoms have the same Which atoms have the same number of valence electrons?number of valence electrons?
……… ………
Atom
Atom Proton numberProton number Number of neutronsNumber of neutrons P P 33 44 Q Q 1616 1717 R R 1616 1616 S S 1919 2020 Heat Heat Metal Metal carbonate carbonate Lime water Lime water
X
X
A A Z Z(d)
(d) (i) (i) Which Which atoms atoms are are isotopes?isotopes?
………... ………...
[1 mark] [1 mark] (ii)
(ii) State a reason State a reason for your answer for your answer in (d) (i).in (d) (i).
………..
………...
[1 mark] [1 mark] (e)
(e) Diagram 3 shows Diagram 3 shows a graph of tempa graph of temperature against time oerature against time of substance f substance X X when it is when it is heated until itheated until it boils.
boils.
(i)
(i) What What is is the the melting melting point point of of substance substance X?X?
……….……… ……….………
[1 mark] [1 mark] (ii)
(ii) Complete the Complete the table table below below by by stating stating the the physical physical state state of of substance substance X X at at the the section section ABAB and DE.
and DE.
Section
Section Physical statePhysical state AB AB DE DE [1 mark] [1 mark] (iii)
(iii) Explain why Explain why the the temperature temperature remains remains constant constant fromfrom
tt
11totott
22..……… ……… ……… ……… [2 marks] [2 marks] Diagram 3 Diagram 3
C
C
E
E
Temperature / Temperature / ooC CB
B
D
D
F
F
A
A
Time / Time / ss 777 777 63 63tt
11tt
22tt
33tt
444
4 Table 4 shows the proton number of a few elements in Period 3.Table 4 shows the proton number of a few elements in Period 3.
Element
Element SodiumSodium MagnesiumMagnesium AluminiumAluminium ChlorineChlorine ArgonArgon Proton number
Proton number 1111 1212 1313 1717 1818
Table 4 Table 4 Based on Table 4,
Based on Table 4, answer the following questions:answer the following questions: (a)
(a) State two State two elements which elements which are are metals.metals.
……… ………
[1 mark] [1 mark] (b)
(b) Chlorine is in Group 17 in the Periodic Table of Elements. What is another name for group 17?Chlorine is in Group 17 in the Periodic Table of Elements. What is another name for group 17?
……….……… ……….………
[1 mark] [1 mark] (c)
(c) Write the electron arrangement of alWrite the electron arrangement of aluminium atom.uminium atom.
……….. ………..
[1 mark] [1 mark] (d)
(d) (i) (i) Arrange Arrange the the element element in in Table Table 4 4 according according to to descending descending order of order of atomic atomic sizessizes .. ………. ………. [1 mark] [1 mark] (ii)
(ii) Explain your Explain your answer answer in in (d) (d) (i).(i).
……… ……… ……… ……… [2 marks] [2 marks] (e)
(e) When sodium is burnt in chlorine When sodium is burnt in chlorine gas, sodium chloride is formed.gas, sodium chloride is formed. (i)
(i) StateState oneone observation for the reaction.observation for the reaction.
……… ………
[1 mark] [1 mark] (ii)
(ii) Write the chemical equation for the reaction.Write the chemical equation for the reaction.
……… ………
[2 marks] [2 marks] (iii)
(iii) StateState oneone physical property of sodium chloride.physical property of sodium chloride.
……… ………
[1 mark] [1 mark]
5
5 Diagram 5 shows the symDiagram 5 shows the symbols of atom of bols of atom of elements U, V, W and X.elements U, V, W and X.
U
U
7
7
3
3
V
V
1
12
2
6
6
W
W
1
19
9
9
9
X
X
20
20
10
10
Diagram 5 Diagram 5 (a)(a) (i) (i) Which Which element element is an is an inert inert gas?gas? ...
... [1 mark] [1 mark] (ii)
(ii) Give a reason for your answer in (a) (i).Give a reason for your answer in (a) (i). ...
... [1 mark] [1 mark] (b)
(b) Element W exists as diatomic molecule.Element W exists as diatomic molecule.
State the type of chemical bond in molecule W. State the type of chemical bond in molecule W. ...
... [1 mark] [1 mark] (c)
(c) Element V can react with element W to form a compound.Element V can react with element W to form a compound. (i)
(i) Write Write the the chemical chemical formula formula for for the the compound compound formed.formed. ...
...
[1 mark] [1 mark] (ii)
(ii) Draw the electron arrangement for Draw the electron arrangement for the compound formed.the compound formed.
[2 marks] [2 marks] (iii)
(iii) StateState oneone physical property for the compound formed.physical property for the compound formed. ...
... [1 mark] [1 mark] (d)
(d) Element U reacts with elemElement U reacts with element W to form ent W to form a compound .a compound . (i)
(i) State the type of State the type of the compound produced.the compound produced. ...
...
[1 mark] [1 mark] (ii)
(ii) Explain briefly Explain briefly how the comhow the compound pound is formed.is formed. ... ... ... ... ... ... [3 marks] [3 marks] (iii)
(iii) Draw the electron arrangement for Draw the electron arrangement for the compound formed.the compound formed.
[2 marks] [2 marks]
X
X
Y
Y
Y
Y
PAPER
PAPER 2: 2: SECTION SECTION B B [ESSAY][ESSAY] 6
6 (a) (a) Diagram Diagram 6.1 6.1 shows shows the the electron electron arrangement arrangement for for atom atom of of an an element element from from Group Group 17 17 in in thethe Periodic
Periodic Table Table of of Element.Element.
Diagram 6.1 Diagram 6.1 Based on Diagram 6.1,
Based on Diagram 6.1, (i)
(i) Write the electron arrangement for the atom and state the name of the element.Write the electron arrangement for the atom and state the name of the element.
[2 marks] [2 marks] (ii)
(ii) Write a chemical equation for the reaction between the element and iron.Write a chemical equation for the reaction between the element and iron.
[2 marks] [2 marks] (b)
(b) Table Table 7.2 7.2 shows shows the the observation observation of of the the reaction reaction between between Group Group 1 1 elements elements X X , , Y Y and and Z Z withwith water.
water. Group
Group I I elementelement ObservationObservation X
X
[Proton number =3] [Proton number =3]
X moves slowly o
X moves slowly on the water surface with a soft ‘hiss’ sound.n the water surface with a soft ‘hiss’ sound.
A colourless solution that turns red lit
A colourless solution that turns red litmus paper blue is formed.mus paper blue is formed. Y
Y
[Proton number =11] [Proton number =11]
Y moves rapidly and randomly on the water surface with a ‘hiss’ Y moves rapidly and randomly on the water surface with a ‘hiss’
sound. A colourless solution that turns red lit
sound. A colourless solution that turns red litmus paper blue ismus paper blue is formed.. formed.. Z Z [Proton number =19] [Proton number =19] Z burns with a
Z burns with a reddish-purple flame, moves very rapidly andreddish-purple flame, moves very rapidly and
randomly on the water surface with ‘hiss’ and ‘pop’ sound. A randomly on the water surface with ‘hiss’ and ‘pop’ sound. A
colourless solution that turns red litmus paper blue
colourless solution that turns red litmus paper blue is formed..is formed.. Table 6.2
Table 6.2 Based on Table 6.2
Based on Table 6.2 (i)
(i) Arrange X, Y and Z in descending order of reactivity of Group I elements towards water.Arrange X, Y and Z in descending order of reactivity of Group I elements towards water. Compare and explain the reactivity X and Z with water.
Compare and explain the reactivity X and Z with water.
[6 marks] [6 marks] (ii)
(ii) Compare the chemical property of X, Y and Z. Give a reason for your answer.Compare the chemical property of X, Y and Z. Give a reason for your answer.
[2 marks] [2 marks] (c)
(c) Diagram Diagram 6.3 6.3 shows shows the the electron electron arrangement arrangement of of a a molecule molecule XYXY22..
Diagram 6.3 Diagram 6.3
Based on Diagram 6.3, write the electron arrangement for atom element X and element Y. Based on Diagram 6.3, write the electron arrangement for atom element X and element Y. Explain briefly how the molecule is formed from atom X and atom Y.
Explain briefly how the molecule is formed from atom X and atom Y. Explain the position of element Y in the Period Table of Element. Explain the position of element Y in the Period Table of Element.
[8 marks] [8 marks]
7.
7. Table 7.1 shows the electron arrangement for atoms W, X and Y. These letters are not the actual symbols of Table 7.1 shows the electron arrangement for atoms W, X and Y. These letters are not the actual symbols of the elements.
the elements.
Table 7.1 Table 7.1 (a)
(a) State the position of element X in the Periodic Table of Elements.State the position of element X in the Periodic Table of Elements. Explain how you determine the group and the period of element X. Explain how you determine the group and the period of element X.
[4 marks] [4 marks] (b)
(b) Atoms Atoms W W and and Y Y can can form form chemical chemical bonds bonds with with atom atom X.X. Explain how
Explain how the bond the bond is formed is formed between :between : (i)
(i) Atoms Atoms Y Y and and XX (ii)
(ii) Atoms W and XAtoms W and X
[10 marks] [10 marks] (c)
(c) Table Table 7.2 7.2 shows shows physical physical property property of of compound compound P P and and compound compound Q.Q.
Table 7.2 Table 7.2 State the type of
State the type of bond in compound P and compound Q.bond in compound P and compound Q. By choosing
By choosing oneone physical property, explain why there is a difference between the propertyphysical property, explain why there is a difference between the property of the compounds.
of the compounds.
[6 marks] [6 marks] PAPER
PAPER 2 2 SECTION SECTION C: C: ESSAYESSAY 8
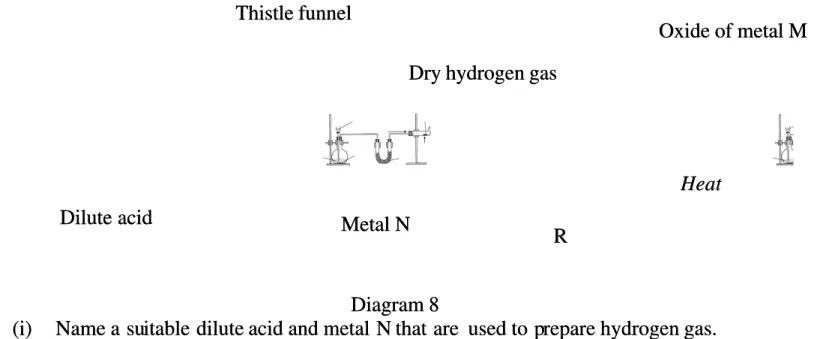
8 (a) (a) Diagram Diagram 8 8 shows shows the the apparatus apparatus set set up up to to determine determine the the empirical empirical formula formula of of oxide oxide of of metal metal M. M. MM is less reactive than hydrogen.
is less reactive than hydrogen.
Diagram 8 Diagram 8 (i)
(i) Name Name a sua suitable dilute itable dilute acid acid and and metal N metal N that are that are used used to pto prepare repare hydrogen hydrogen gas.gas.
[2 marks] [2 marks] (iii)
(iii) Suggest a suitable chemical subsSuggest a suitable chemical substance for R tance for R and state the function of R.and state the function of R.
[2 marks] [2 marks] (iv)
(iv) State the example of oxide of mState the example of oxide of metal M. Describe the redoxs etal M. Describe the redoxs reaction that occurs in the comreaction that occurs in the combustionbustion tube based on the changes in oxidation number.
tube based on the changes in oxidation number.
[6 marks] [6 marks] Element
Element Electron arrangementElectron arrangement W W 2.42.4 X X 2.8.72.8.7 Y Y 2.8.8.12.8.8.1 Physical Property
Physical Property Compound PCompound P Compound QCompound Q Melting point
Melting point HighHigh LowLow
Electrical conductivity
Electrical conductivity Can conduct electricity inCan conduct electricity inmolten state or aqueous solutionmolten state or aqueous solution
Cannot conduct Cannot conduct
electricity in molten and electricity in molten and solid states solid states Dilute acid Dilute acid Thistle funnel Thistle funnel R R Heat Heat Oxide of metal M Oxide of metal M Dry hydrogen gas
Dry hydrogen gas
Metal N Metal N
(b)
(b) The The information information below below is is about about hydrocarbon hydrocarbon YY
Empirical formula of Y is CHEmpirical formula of Y is CH22
Molar Molar mass mass of of Y Y = = 56 56 gmolgmol
-1 -1
(i)
(i) Determine the molecular formula for hydrocarbon Y.Determine the molecular formula for hydrocarbon Y. [Relative atomic mass
[Relative atomic mass of C =12 , of C =12 , H = 1 ]H = 1 ]
[2 marks] [2 marks] (ii)
(ii) Describe an experiment to prepare Describe an experiment to prepare hydrocarbon Y in the laboratory from its correspondinghydrocarbon Y in the laboratory from its corresponding alcohol.
alcohol.
In your answer, include the diagram of
In your answer, include the diagram of the appratus set-up, materials used, andthe appratus set-up, materials used, and procedure.
procedure.
[8 marks] [8 marks] 9
9 (a) (a) (i) (i) What What is is meant meant by by empirical empirical formula?formula?
[1 mark] [1 mark] (ii)
(ii) Diagram Diagram 9.1 9.1 shows shows the the apparatus apparatus set-up set-up used used to to determine determine the the empirical empirical formula formula of of oxideoxide of metal X.
of metal X.
Diagram 9.1 Diagram 9.1 Suggest one suitable oxide of metal X.
Suggest one suitable oxide of metal X.
Write a balanced chemical equation involved. Write a balanced chemical equation involved.
[3 marks] [3 marks] (b)
(b) Diagram 9.2 Diagram 9.2 shows the shows the apparatus set-up apparatus set-up used to used to determine the determine the empirical formula empirical formula of of another another oxide of oxide of metal.metal.
(h)
(h) Suggest one Suggest one suitable oxide of suitable oxide of the the metal.metal.
[1 mark] [1 mark] (ii) Based one diagram 9.2, describe how y
(ii) Based one diagram 9.2, describe how you could determine the empirical formula of the ou could determine the empirical formula of the named metal oxidenamed metal oxide in the
in the laboratory. laboratory. Your description Your description should includeshould include -- procedure of experimentprocedure of experiment
-- tabulation of resultstabulation of results
-- calculation of calculation of the the empirical formempirical formulaula
[10 marks] [10 marks] (c) A carbon compound contains 84.6% of
(c) A carbon compound contains 84.6% of carbon and 15.4% of hydrogen by mass.carbon and 15.4% of hydrogen by mass.
The relative molecular mass of this compound is 70. Calculate the molecular formula of this compound. The relative molecular mass of this compound is 70. Calculate the molecular formula of this compound.
[Relative atomic mass: C, 12; H, 1] [Relative atomic mass: C, 12; H, 1]
[5 marks] [5 marks]
Heat Heat Oxide
Oxide of metal of metal XX Dry hydrogen gas
Dry hydrogen gas
Diagram 9.2 Diagram 9.2 Heat Heat Metal Metal
CHEMISTRY PERFECT SCORE MODULE
CHEMISTRY PERFECT SCORE MODULE
SET
SET
1.
1. Electrochemistry
Electrochemistry
2.
PAPER
PAPER 2: 2: SECTION SECTION A A [STRUCTURE][STRUCTURE] 1
1 Diagram 1 shows the apparatus set- up for the combination of electrolytic cell and chemical cell.Diagram 1 shows the apparatus set- up for the combination of electrolytic cell and chemical cell.
Diagram 1 Diagram 1 (a)
(a) Which cell is the electrolytic cell?Which cell is the electrolytic cell?
………..……….……….. ………..……….………..
[1 mark] [1 mark] (b)
(b) Based on Cell IBased on Cell I (i)
(i) State the negatif terminal of the cell .State the negatif terminal of the cell .
…………..………... …………..………...
[1 mark] [1 mark] (ii)
(ii) Draw the flow of electron in Diagram 1.Draw the flow of electron in Diagram 1.
[1 mark] [1 mark] (iii)
(iii) State the observation at copper electrode.State the observation at copper electrode.
……… ………
[1 mark] [1 mark] (c)
(c) Write half Write half equation equation for for the the reaction reaction at at copper copper electrodeelectrode..
…………..………
…………..………...….………...….
[2 marks] [2 marks] (d)
(d) Based on the Cell IIBased on the Cell II (i)
(i) State the energy change in the cell.State the energy change in the cell.
……….……… ……….………
[1 mark] [1 mark] (ii)
(ii) What can be observe at What can be observe at the copper(II) sulphate solution?the copper(II) sulphate solution?
….……… ….………
[1 mark] [1 mark] (iii)
(iii) Explain yExplain your answer our answer in in d(ii)d(ii)
………..………….……….. ………..………….……….. [2 marks] [2 marks] Copper(II) sulphate Copper(II) sulphate solution solution Cell II Cell II Cell I Cell I V V Magnesium Magnesium electrode
2
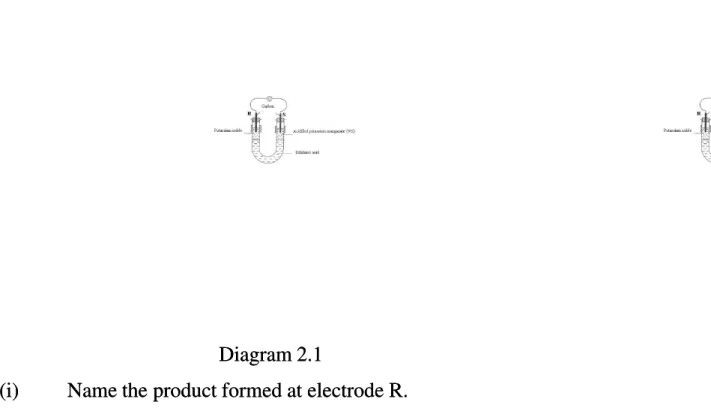
2 (a)(a) Diagram 2.1 shows the apparatus set-up to investigate the transfer of electrons at a distanceDiagram 2.1 shows the apparatus set-up to investigate the transfer of electrons at a distance between potassium iodide solution and acidified potassium manganate(VII) solution.
between potassium iodide solution and acidified potassium manganate(VII) solution.
Diagram 2.1 Diagram 2.1 (i)
(i) Name Name the the product product formed formed at at electrode electrode R.R.
..……….
..……….
…………...…………...[1 mark] [1 mark] (ii)
(ii) Complete the Complete the half half equation equation for for the the reaction reaction at at electrode electrode S.S. MnO MnO44 --+ + ... ... HH++ + + ... ... e e → Mn→ Mn2+2+ + ... H+ ... H22OO [1 mark] [1 mark] (iii)
(iii) State State the the change change in oin oxidation xidation number number of of manganese manganese and and name name the the process process that that occurs occurs at at S.S.
Change in oxidation number : ………...………...………... Change in oxidation number : ………...………...………...
Name of process :
Name of process : ... [2 marks] [2 marks] (iv)
(iv) Suggest Suggest a a substance substance that that can can replace replace potassium potassium iodide iodide solution solution in in order order to to obtain obtain the the samesame reaction. reaction.
…………
…………
... [1 mark] [1 mark] (b)(b) Diagram Diagram 2.2 2.2 shows shows the the set set up up of of the the apparatus apparatus to to investigate investigate the the reactivity reactivity of of metals metals J, J, K K and and L.L. The different metals are heated
The different metals are heated consecutively.consecutively.
Diagram 2.2 Diagram 2.2
Table 2.2 shows the observation of t
Table 2.2 shows the observation of the experiment.he experiment.
Metal
Metal ObservationsObservations Colour of residueColour of residue
Hot Cold
Hot Cold
J
J Burns Burns brightlybrightly YellowYellow WhiteWhite K
K Glows dimlyGlows dimly Black Black Black Black
L
L Burns Burns with with a a very very bright bright flameflame WhiteWhite WhiteWhite
Table 2.2 Table 2.2 (i)
(i) State the name of metal J.State the name of metal J.
………..
……….. ………...………...
[1 mark] [1 mark] (ii)
(ii) Write a Write a chemical chemical equation equation for for the the reaction reaction between between metal metal J J and and oxygen.oxygen.
……….………...
……….………... [2 marks] [2 marks] (iii)
(iii) Based Based on on the the observation observation in in Table 2.1Table 2.1, , arrange arrange metals metals J, J, K anK and L d L in in ascending ascending order order of of reactivity towards oxygen.
reactivity towards oxygen.
………..
……….. .……..……... [1 mark] [1 mark] (iv)
(iv) A mixture of metal J and oxide of metal L is heated strongly.A mixture of metal J and oxide of metal L is heated strongly. Predict an observation. Give a reason.
Predict an observation. Give a reason.
.. ………...
………..
………... [2 marks] [2 marks]
PAPER
PAPER 2: 2: SECTION SECTION B B [ESSAY][ESSAY] 3
3 (a) (a) Table Table 3.1 3.1 shows shows the the electrical electrical conductivity conductivity of of two two different different compoundscompounds Compound
Compound Electrical Electrical conductivityconductivity Propanone (C
Propanone (C33HH66O) O) Cannot Cannot conduct conduct electricityelectricity
Sodium
Sodium chloride chloride solution solution (NaCl) (NaCl) Conduct Conduct electricityelectricity Table 3.1
Table 3.1
Referring to Table 3.1, explain why there is a difference in the electrical conductivity. Referring to Table 3.1, explain why there is a difference in the electrical conductivity.
[4 marks] [4 marks] (b)
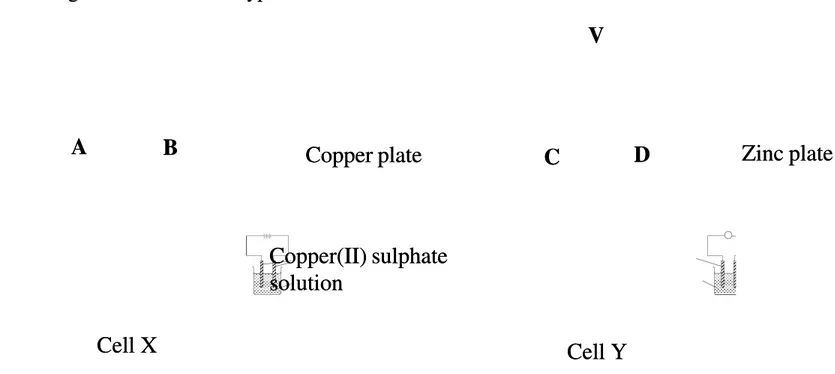
(b) Diagram Diagram 3.1 3.1 shows shows two two types types of of cells.cells.
Diagram 3.1 Diagram 3.1
Compare and contrast cell X and cell Y. Include in your answer the observations and half Compare and contrast cell X and cell Y. Include in your answer the observations and half equations for the reactions at the electrodes in both cells.
equations for the reactions at the electrodes in both cells.
[8 marks] [8 marks] (c)
(c) An An experiment experiment is is carried carried out out to to determine determine the the position position of of metals metals silver, silver, L L and and M M in in thethe electrochemical series. Diagram 3.2 shows the results of
electrochemical series. Diagram 3.2 shows the results of the experiment.the experiment.
Experimen Experimen
I
I II II IIIIII
Observation
Observation Grey solid depositedGrey solid deposited
Colourless solutionColourless solution
Grey solid depositedGrey solid deposited
Light blue solutionLight blue solution No changeNo change
Diagram 3.2 Diagram 3.2
Copper plate
Copper plate
Copper(II) sulphate
Copper(II) sulphate
solution
solution
Zinc plate
Zinc plate
A
A
B
B
V
V
C
C
D
D
Cell X
Cell X
Cell Y
Cell Y
L
L
Silver nitrate
Silver nitrate
solution
solution
M
M
Silver nitrate
Silver nitrate
solution
solution
M
M
L nitrate
L nitrate
solution
solution
(i)
(i) Based on the Based on the results in Diagresults in Diagram 3.2, arrange the ram 3.2, arrange the electroposivity of melectroposivity of metals silver, L and etals silver, L and M in ascendingM in ascending order.
order.
Explain your answer. Explain your answer.
[7 marks] [7 marks] (ii)
(ii) Based on observation in exBased on observation in experimen II, suggest one poperimen II, suggest one possible metal for Mssible metal for M
[1 mark] [1 mark] 4
4 (a) (a) The following The following is the is the chemical equation chemical equation of a of a redoxs reredoxs reactionaction Zn + Pb(NO
Zn + Pb(NO33))22 → → Zn(NOZn(NO33))22 + + PbPb
Referring to the above chemical equation, Referring to the above chemical equation, (i)
(i) Write Write half equation half equation for the for the oxidation oxidation and the and the reduction reactionsreduction reactions..
[4 marks] [4 marks] (ii)
(ii) Identify Identify substance substance that that is is oxidized oxidized and and reduced. reduced. Explain Explain your your answer answer in in term term of of transfer transfer of of electrons.
electrons.
[4 marks] [4 marks]
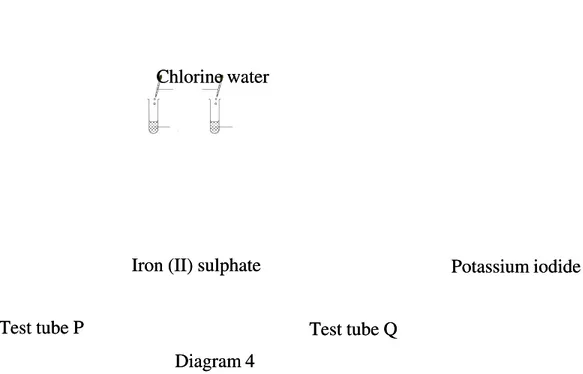
(b)
(b) Diagram 4 shows two Diagram 4 shows two redox reactions redox reactions that take place in that take place in test tubes P and test tubes P and Q.Q.
(i)
(i) State the observations and write the ionic equation for the reaction in test tubes P and QState the observations and write the ionic equation for the reaction in test tubes P and Q respectively.
respectively.
[6 marks] [6 marks] (ii)
(ii) State the name of the product formed in each test tube P and QState the name of the product formed in each test tube P and Q
[4 marks] [4 marks] (iii)
(iii) Describe a confirmation test to verify the product in test tube QDescribe a confirmation test to verify the product in test tube Q
[2 marks] [2 marks]
Diagram 4 Diagram 4 Iron (II) sulphate Iron (II) sulphate Chlorine water Chlorine water Test tube P Test tube P Potassium iodide Potassium iodide Test tube Q Test tube Q
PAPER
PAPER 2: 2: SECTION SECTION C C [ESSAY][ESSAY] 5
5 (a) (a) Diagram Diagram 5 5 shows shows the the apparatus apparatus set-up set-up and and observations observations for for experiments experiments 1 1 using using 1.0 1.0 moldmmoldm-3-3 aqueous solution of compound XSO
aqueous solution of compound XSO44and experiment II using 0.0001 moldmand experiment II using 0.0001 moldm-3-3aqueous solution of aqueous solution of
compound XY compound XY22..
Experiment
Experiment Apparatus Apparatus set-up set-up ObservationObservation
II
Cathode: Cathode:
Brown solid deposited Brown solid deposited
Anode: Anode:
A colorless gas is produced. A colorless gas is produced.
II II
Cathode: Cathode:
Brown solid deposited Brown solid deposited
Anode: Anode:
A colorless gas is produced. A colorless gas is produced. The
The gas relight gas relight a glowing a glowing splinter.splinter.
Diagram 5 Diagram 5 (i)
(i) In both experiment I and experiment II, the product formed at cathode is the same. UsingIn both experiment I and experiment II, the product formed at cathode is the same. Using your knowledge of factors affecting the selective
your knowledge of factors affecting the selective discharge of ions at the edischarge of ions at the electrodes,lectrodes, -- suggest one possible cation for Xsuggest one possible cation for X2+2+ionion
-- write the half equation for the reaction at the cathodewrite the half equation for the reaction at the cathode -- state the name of the product at cathodestate the name of the product at cathode
[4 marks] [4 marks] (ii)
(ii) Name the product formed at anode in experiment I. Describe a confirmatory test to identify theName the product formed at anode in experiment I. Describe a confirmatory test to identify the gas produced.
gas produced.
[3 marks] [3 marks] (iii)
(iii) SuggestSuggest oneone possible anion for Ypossible anion for Y--ion in experiment II.ion in experiment II.
Name the product at the anode and explain the formation of the product in the experiment. Name the product at the anode and explain the formation of the product in the experiment.
[6 marks] [6 marks] (b)
(b) Pure Pure copper copper metal metal is is used used to to make make copper copper wire.wire. Describe
Describe how to purify the copper mhow to purify the copper metal using an electrolysis process.etal using an electrolysis process. Include a labelled diagram in your answer.
Include a labelled diagram in your answer.
[7 marks] [7 marks]
6
6 (a) (a) Diagram Diagram 6.1 6.1 shows shows the the apparatus apparatus set-up set-up to to study study the the effect effect of of metals metals P P and and Q Q on on the the rusting rusting of of ironiron nail. The results are recorded after
nail. The results are recorded after three days.three days. Experiment
Experiment Observation Observation after after 3 3 daysdays
II
Dark blue precipitateDark blue precipitate
Iron nail rustIron nail rust
II II
Solution turns pink.Solution turns pink.
Iron nail does not rustIron nail does not rust
Diagram 6.1 Diagram 6.1
(i) Name
(i) Name oneone possible metal for metal P and metal Qpossible metal for metal P and metal Q
[2 marks] [2 marks] (ii)
(ii) Explain why there is a difference in obExplain why there is a difference in observation in Experiment I and Iservation in Experiment I and II.I.
[8 marks] [8 marks] (b)
(b) Diagram 6.2 Diagram 6.2 shows a shows a redox reaction bredox reaction between bromine water etween bromine water and iron(IIand iron(II) sulphate solution.) sulphate solution.
(i)
(i) Describe the redox Describe the redox reaction that occur reaction that occur in the test tube. in the test tube. Your answer shouYour answer should include the ionld include the ionicic equation and observations.
equation and observations.
[8 marks] [8 marks] (ii)
(ii) Based on the oxidation reaction in (b) (i), describe a chemical test to identify the product formedBased on the oxidation reaction in (b) (i), describe a chemical test to identify the product formed in the test tube.
in the test tube.
[2 marks] [2 marks] Agar-agar solution with potassium
Agar-agar solution with potassium hexacyanoferrate(III) and
hexacyanoferrate(III) and phenolphthalein solution. phenolphthalein solution.
Iron nail wrapped with metal Iron nail wrapped with metal PP
Agar-agar solution with potassium Agar-agar solution with potassium hexacyanoferrate(III) and
hexacyanoferrate(III) and phenolphthalein solution. phenolphthalein solution.
Iron nail wrapped with metal Iron nail wrapped with metal QQ
Diagram 6.2
Diagram 6.2
CHEMISTRY PERFECT SCORE MODULE
CHEMISTRY PERFECT SCORE MODULE
SET
SET
1. Acids and Bases
1. Acids and Bases
2. Salts
2. Salts
3. Rate of reaction
3. Rate of reaction
4. Thermochemistry
4. Thermochemistry
PAPER 2 SECTION A [STRUCTURE] PAPER 2 SECTION A [STRUCTURE] 1.
1. Diagram 1 shows foDiagram 1 shows four test tubes ur test tubes labeled A,B,C and D labeled A,B,C and D which are used which are used to study the to study the relationship betweenrelationship between pH
pH value of value of acid and acid and alkali with alkali with the the molarity.molarity. pH paper pH paper
A
A B B C C DD
5 cm
5 cm33HCl HCl 5 5 cmcm33HCl HCl 5 cm5 cm33NaOH NaOH 5 5 cmcm33 NaOHNaOH 0.1 mol dm
0.1 mol dm-3-3 0.01 mol dm0.01 mol dm-3-3 0.1 mol dm0.1 mol dm-3-3 0.01 moldm0.01 moldm-3-3
(a)
(a) Determine Determine which which solution solution hashas (i)
(i) highest highest pH pH value?value?
……….……… ……….……… ...
[1 mark] [1 mark] (ii)
(ii) lowest pH lowest pH value?value?
………... ………...
[1 mark] [1 mark] (b)
(b) If If the the hydrochloric hydrochloric acid acid in in test test tube tube B B is is replaced replaced with with 5 5 cmcm33of 0 .1 mol dmof 0 .1 mol dm-3-3ethanoic acid,ethanoic acid, predict the pH value
predict the pH value of the solution. Explain your answerof the solution. Explain your answer
………
………
...………..
………..
…. …. ……… ………. ………. [3 marks] [3 marks] (c)(c) Excess Excess of of magnesium magnesium powder powder is is added added to to 5 5 cmcm33of 0.1moldmof 0.1moldm-3-3 hydrochloric acid in test tube Ahydrochloric acid in test tube A (i)
(i) Name Name the the products products formed.formed. .. ……...……….. ……...……….. [1 mark] [1 mark] (ii)
(ii) Write an Write an ionic ionic equation equation for for the the reaction reaction in in test test tube tube AA
……… ………
[1 mark] [1 mark] (iii)
(iii) Calculate Calculate volume volume of of hydrogen hydrogen gas gas released released at at room room conditions conditions in in test test tube tube AA [1 mol
[1 mol of of gas occupies gas occupies 24 dm24 dm33at room conditions]at room conditions]