Plant Science 151 (2000) 59 – 66
Oxidative stress and some antioxidant systems in acid rain-treated
bean plants
Protective role of exogenous polyamines
V. Velikova
a,*, I. Yordanov
a, A. Edreva
baInstitute of Plant Physiology‘M.Popo6’,Bulgarian Academy of Sciences,1113Sofia,Bulgaria bInstitute of Genetics‘D.Kostoff’,Bulgarian Academy of Sciences,1113Sofia,Bulgaria
Received 10 March 1999; accepted 27 September 1999
Abstract
The effect of simulated acid rain (AR) (pH 1.8) on H2O2and malonyldialdehyde (MDA) levels and activities of peroxidase and
catalase in bean plants were investigated. The influence of exogenous polyamines spermidine and spermine on these parameters was also studied. AR treatment induced lipid peroxidation and increased level of H2O2in leaves. Pretreatment with spermidine
and spermine prevented these changes. The protective effect of spermine was higher than that of spermidine. The impact of polyamines could be attributed to their acid neutralizing and antioxidant effects, as well as to their ability to stabilize membranes by associating with negatively charged phospholipids. It was also found that AR significantly increased peroxidase and decreased catalase activities at the first hours after treatment. Later, both enzyme activities were enhanced that could contribute to the scavenging and detoxification of active oxygen species. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Phaseolus6ulgaris; Acid rain; Hydrogen peroxide; Lipid peroxidation; Catalase; Peroxidase; Polyamines
www.elsevier.com/locate/plantsci
1. Introduction
Acid rain (AR) induces changes in the cellular biochemistry and physiology of the whole plant. Biological effects of acid deposition on plants are numerous and complex, and include visible
symp-toms of injury (chlorosis and/or necrosis), and
invisible effects such as reduced photosynthesis, nutrient loss from leaves, altered water balance, variation of several enzyme activities [1,2].
Previ-ous data indicated that photosynthetic CO2
fixa-tion and photochemical activity of bean plants were decreased following pH 2.2 – 1.8 AR [3].
The ability of plant to overcome the effect of
the AR stress and to sustain its productivity may be related to the scavenging of stress-induced toxic
oxygen species, such as H2O2(hydrogen peroxide),
OH (hydroxyl radical) and O
2
− (superoxide
radi-cal). Catalases and peroxidases are two major
systems for the enzymatic removal of H2O2 in
plants [4]. Polyamines (PAs) are now regarded as a new class of growth substances [5] and are also well known for their senescence and anti-stress effects due to their acid neutralizing and antioxidant properties, as well as to their mem-brane and cell wall stabilizing abilities [6 – 8]. Scarce data, however, are available on the produc-tion of active oxygen species and antioxidant sys-tems in plants subjected to AR, as well as on the involvement of PAs as protectants against this type of stress.
The aim of the present paper was to investigate the effect of simulated AR (pH 1.8) on lipid
peroxidation, H2O2 content, peroxidase and
cata-Abbre6iations: AR, acid rain; MDA, malonyldialdehyde; PAs,
polyamines; SA, salicylic acid; Spd, spermidine; Spm, spermine; TBA, thiobarbituric acid; TCA, thrichloracetic acid.
* Corresponding author. Tel.: +359-2-979-2682; fax: + 359-273-9952.
E-mail address:[email protected] (V. Velikova)
V.Veliko6a et al./Plant Science151 (2000) 59 – 66
60
lase activities, as well as the possibility of exoge-nous polyamines (spermidine and spermine) to inhibit the accumulation of the above oxidative species in AR-treated bean plants.
2. Material and methods
2.1. Plant material and treatments
Experiments were carried out with 10-day-old
bean plants (Phaseolus 6ulgaris L. cv. Cheren
Starozagorsky) after the emergence of the first composite bean leaf. Plants were grown in a cli-matic chamber at photon flux density about 120
mmol m−1 s−1, temperature 2592°C and 12 h
photoperiod.
The following variants were experimented, ap-plying single spraying treatments:
Plants sprayed with cocktail pH 5.6-control;
Plants sprayed with simulated AR pH 1.8 (AR);
Plants sprayed with 1 mM spermidine·3 HCl
(Spd+pH 5.6);
Plants sprayed with 1 mM spermidine·3 HCl+
AR pH 1.8 (Spd+AR);
Plants sprayed with 1 mM spermine·4 HCl
(Spm+pH 5.6);
Plants sprayed with 1 mM spermine·4 HCl+
AR pH 1.8 (Spm+AR).
The AR was prepared according to Seufert et al. [9] and contained the following components:
NH4NO3 (1.3 g l−1), MgSO4·7H2O (3.1 g l−1),
Na2SO4 (2.5 g l−1), KHCO3 (1.3 g l−1),
CaCl2·2H2O (3.1 g l−1). After dilution of initial
solution 1:100, pH value was adjusted to 1.8 with
1 N H3PO4and 1 N H2SO4. The cocktail used to
spray the control plants has the same composition as the AR but the pH value is 5.6. Tween 80
(0.5%, v/v) was used as surfactant. Each plant was
sprayed with 2 ml simulated AR or cocktail pH 5.6. The vessels were covered with lids, which did not permit the AR to contaminate the nutrient solution.
Polyamines were used as foliar sprays applied to plants 24 h before spraying with pH 5.6 cocktail and before the AR treatment. Each plant was sprayed with 2 ml polyamine solution.
The measurements were carried out at different intervals after AR treatment: at the 3rd, 5th, 24th and 168th h.
2.2. Determination of H2O2 content
Hydrogen peroxide levels were determined ac-cording to Sergiev et al. [10]. Leaf tissues (500 mg) were homogenized in ice bath with 5 ml 0.1%
(w/v) TCA. The homogenate was centrifuged at
12 000×g for 15 min and 0.5 ml of the
superna-tant was added to 0.5 ml 10 mM potassium phos-phate buffer (pH 7.0) and 1 ml 1 M KI. The absorbancy of supernatant was read at 390 nm.
The content of H2O2 was given on a standard
curve.
2.3. Determination of the malonyldialdehyde (MDA) content
For the measurement of lipid peroxidation in leaves, the thiobarbituric acid (TBA) test, which determines MDA as an end product of lipid per-oxidation [11], was used. Leaf material (500 mg)
was homogenized in 5 ml 0.1% (w/v) TCA
solu-tion. The homogenate was centrifuged at 10 000×
g for 20 min and 0.5 ml of the supernatant was
added to 1 ml 0.5% (w/v) TBA in 20% TCA. The
mixture was incubated in boiling water for 30 min, and the reaction stopped by placing the reaction tubes in an ice bath. Then the samples were
cen-trifuged at 10 000×g for 5 min, and the
ab-sorbancy of supernatant was read at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of MDA – TBA complex (red pigment) was calculated from the extinction
coefficient 155 mM−1 cm−1.
2.4. Enzyme analysis
For the measurement of enzyme activities, leaf tissue (500 mg) was homogenized in 5 ml 10 mM potassium phosphate buffer (pH 7.0) containing
4% (w/v) polyvinylpyrrolidon (Mr 25 000). The
homogenate was centrifuged at 12 000×g for 30
min and supernatant obtained was used as enzyme extract. All steps in the preparation of the enzyme extract were carried out at 0 – 4°C. An aliquot of the extract was used to determine its protein con-tent by the method of Bradford [12] utilizing bovine serum albumin as standard.
potas-V.Veliko6a et al./Plant Science151 (2000) 59 – 66 61 sium phosphate buffer, pH 7.0, 0.02 – 0.06 ml
en-zyme and 0.6 ml guaiacol 1% (w/v) aqueous
solu-tion. The reaction was started by adding 0.15 ml
of 100 mM H2O2 and the optical density at 470
nm was recorded in a spectrophotometer Shi-madzu against an identical mixture to which no
H2O2 was added. The linear initial reaction rate
was used to estimate the activity, which was
ex-pressed in mmol of guaiacol dehydrogenation
product (GDHP) formed per milligram protein per minute, using the extinction coefficient of 26.6
mM−1 cm−1.
The activity of catalase (EC 1.11.1.6) was as-sayed by measuring the initial rate of
disappear-ance of H2O2by the method of Kato and Shimizu
[14]. Three ml of catalase assay reaction mixture contained 10 mM potassium phosphate buffer, pH
7.0, 0.1 ml enzyme extract and 0.035 ml H2O2, 3%.
The decrease in H2O2 was followed as decline in
optical density at 240 nm, and the activity was calculated using the extinction coefficient (40
mM−1cm−1) for H
2O2 [14].
2.5. Reagents
Chemicals were purchased from Serva and were of analytical grade.
2.6. Statistics
All results were represented as means9S.E.
from at least five independent series of experi-ments (3 – 5 measureexperi-ments each). The significant
differences were determined by the Student’s t
-test.
3. Results
3.1. H2O2 content
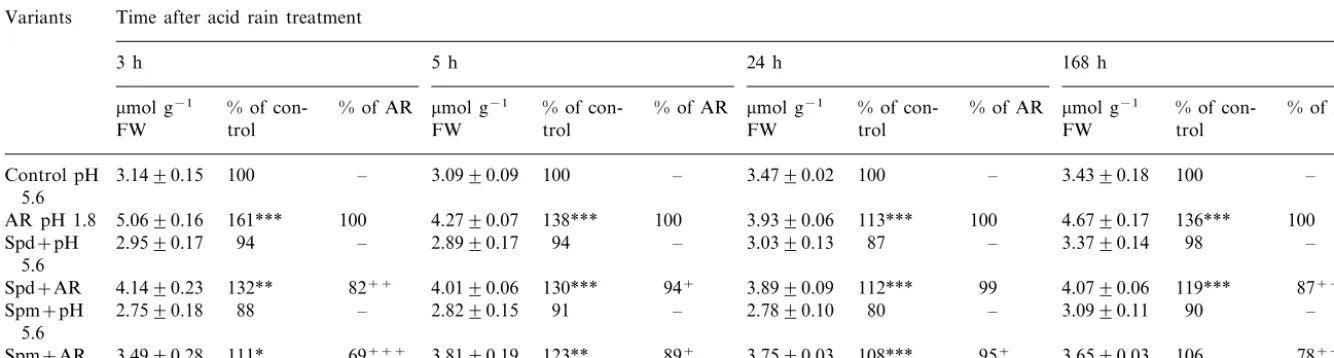
Changes in H2O2content are shown in Table 1.
An increase was observed after AR spraying. The response was more pronounced early after treat-ment, reaching 161% of the control (pH 5.6) at the
3rd h. Later, the level of H2O2 was relatively
decreased but remained higher than in the control. To evaluate the effect of PAs in AR-treated
plants, data for the H2O2 content in the variants
Spd+AR and Spm+AR were compared with
data for AR. It appeared that pretreatment with
PAs interfered with H2O2 accumulation,
particu-larly early after acid application (at the 3rd h):
H2O2 was reduced to 82 (Spd+AR) and 69%
(Spm+AR) of its level in the variant AR. In the
later intervals the effect of PAs was less
pro-nounced (except for Spd+AR and Spm+AR,
168th h). The supply of PAs to plants at pH 5.6
(control) had no significant impact on H2O2
content.
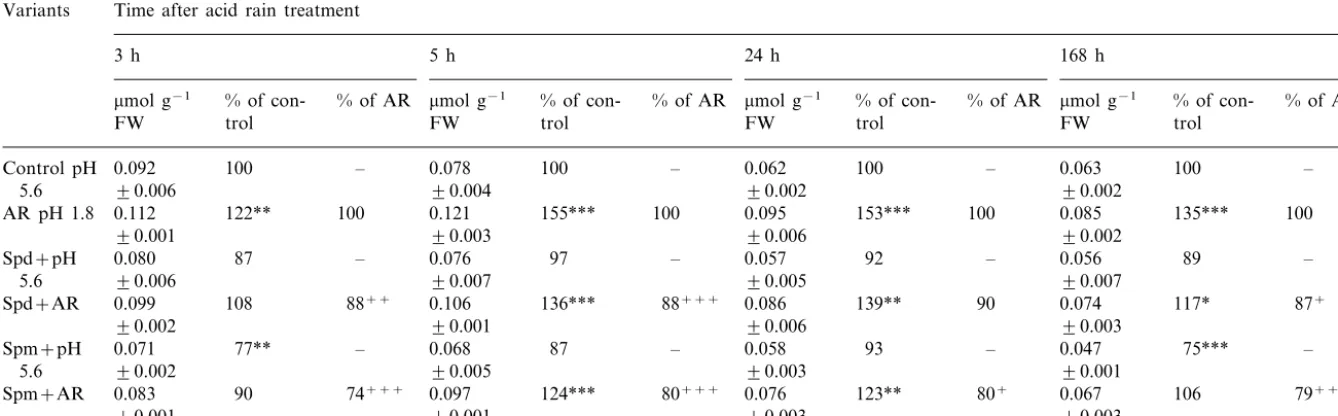
3.2. Lipid peroxidation
Variations in the content of MDA are presented in Table 2. An increase of MDA following acid application was scored having a maximum at the 5th h. The response amounted to 122, 155, 153 and 135% of the control (pH 5.6) at the 3rd, 5th, 24th and 168th h, respectively. Preliminary supply of Spm attenuated the effect of AR during the whole period studied: the MDA content in variant
Spm+AR was reduced to 74 (3rd h), 80 (5th and
24th h) and 79% (168th h) of its value in acid sprayed plants (AR). The reduction caused by Spd was 88% in the first two intervals but later it was less expressed. The level of MDA was not signifi-cantly influenced by polyamine application at pH 5.6 (except for Spm, 3rd and 168th h).
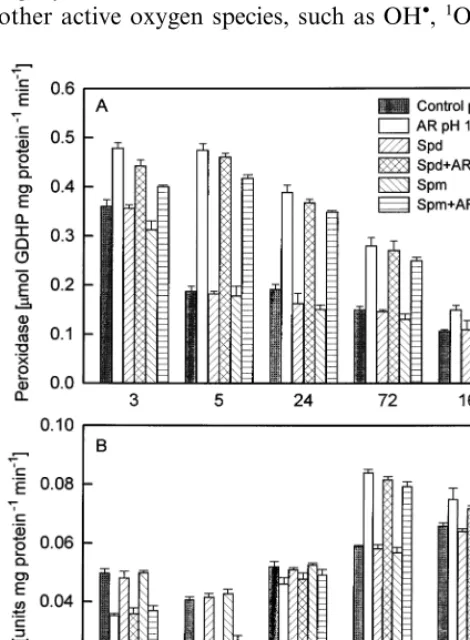
3.3. Peroxidase and catalase acti6ities
Fig. 1 represents the changes of peroxidase and catalase activities. The results obtained showed that peroxidase was higher in acid-treated plants, particularly in the first hours after spraying: at the 3rd h peroxidase increased to 133% and at the 5th h it was more than 2-fold higher (253%) as com-pared with the control plants (pH 5.6). At the 24th, 72nd and 168th h peroxidase activity gradu-ally decreased but remained higher than control (202, 187 and 140%, respectively, of the control pH 5.6).
In contrast to peroxidase, activity of catalase tended to decrease at the first hours after AR application: it was 71 and 53% of the control pH 5.6 at the 3rd and 5th h, respectively. At the end of experimental period catalase activity increased to 142 (72nd h) and 114% (168th h).
amount-V
.
Veliko
6
ae
ta
l
.
/
Plant
Science
151
(2000)
59
–
66
62
Table 1
Effects of simulated acid rain (AR, pH 1.8) applied independently or in combination with 1 mM spermidine·3 HCl (Spd) and 1 mM spermine·4 HCl (Spm) on H2O2content
in bean leaves 3, 5, 24 and 168 h after acid treatmenta
Variants Time after acid rain treatment
168 h 24 h
3 h 5 h
mmol g−1
% of
con-mmol g−1 % of AR % of con- % of AR mmol g−1 % of con- % of AR mmol g−1 % of con- % of AR
FW trol
trol trol
trol FW
FW FW
100 –
Control pH 3.1490.15 100 – 3.0990.09 3.4790.02 100 – 3.4390.18 100 –
5.6
100 3.9390.06 113***
AR pH 1.8 5.0690.16 161*** 100 4.2790.07 138*** 100 4.6790.17 136*** 100
94 – 3.0390.13 87 – 3.3790.14
2.8990.17 98
94 – –
Spd+pH 2.9590.17 5.6
4.1490.23 132** 82++ 130*** 94+ 3.8990.09 112*** 99 4.0790.06 119*** 87++
Spd+AR 4.0190.06
91 – 2.7890.10
Spm+pH 2.7590.18 88 – 2.8290.15 80 – 3.0990.11 90 –
5.6
89+ 3.7590.03 108*** 95+ 3.6590.03 106
3.8190.19 78+++
69+++ 111*
3.4990.28 123**
Spm+AR
aData are presented as
mmol g−1FW, as % of the control (pH 5.6) and as % of the AR (pH 1.8) values.
*PB0.05; **PB0.01;
***PB0.001 (statistical differences with control, pH 5.6). +PB0.05;
++PB0.01;
V
.
Veliko
6
ae
ta
l
.
/
Plant
Science
151
(2000)
59
–
66
63
Table 2
Effects of simulated acid rain (pH 1.8) applied independently or in combination with 1 mM spermidine·3 HCl (Spd) and 1 mM spermine·4 HCl (Spm) on malonyldialdehyde (MDA) level in bean leaves 3, 5, 24 and 168 h after acid treatmenta
Variants Time after acid rain treatment
168 h 24 h
3 h 5 h
mmol g−1
mmol g−1 % of con- % of AR % of con- % of AR mmol g−1 % of con- % of AR mmol g−1 % of con- % of AR
FW trol
trol trol
FW FW
trol FW
0.078
0.092 100 –
Control pH 100 – 0.062 100 – 0.063 100 –
90.002 90.002
90.006 90.004
5.6
100 155*** 100 0.095
122** 153*** 100 0.085
0.112 135***
AR pH 1.8 0.121 100
90.006 90.002
90.001 90.003
0.076
– 97 – 0.057
87
0.080 92
Spd+pH – 0.056 89 –
90.007 90.007
90.006
5.6 90.005
0.099 136*** 88+++
Spd+AR 108 88++ 0.106 0.086 139** 90 0.074 117* 87+
90.003
90.001 90.006
90.002
87 – 0.058 93
0.068 –
0.071 77** – 0.047 75*** –
Spm+pH
90.005
90.002 90.003 90.001
5.6
0.067 124*** 80+++ 0.076 123** 80+
0.097 74+++
90 106 79+++
Spm+AR 0.083
90.001 90.003
90.001 90.003
aData are presented as
mmol g−1FW, as % of the control (pH 5.6) and as % of the AR (pH 1.8) values.
*PB0.05; **PB0.01;
***PB0.001 (statistical differences with control, pH 5.6). +PB0.05;
++PB0.01;
V.Veliko6a et al./Plant Science151 (2000) 59 – 66
64
ing to 92% (Spd+AR, 3rd h), 84 and 88%
(Spm+AR, 3rd and 5th h), respectively, of the
variant AR.
4. Discussion
The results obtained from the present study show that after single AR spraying an increased
H2O2 content and MDA accumulation were
ob-served, i.e. a state of oxidative stress is induced related to membrane damage. The effect is dimin-ished after the 5th h that pointed to the develop-ment of recovery processes. As Foyer et al. [15]
stated H2O2 is a strong oxidant that can initiate
localized oxidative damage leading to disruption of metabolic function and losses of cellular
in-tegrity at sites where it accumulates. H2O2 and
other active oxygen species, such as OH, 1O
2and
O2−can be expected to be responsible for the lipid
peroxidation [16,17].
H2O2 can diffuse relatively long distances
caus-ing changes in the redox status of surroundcaus-ing cells and tissues where at relatively low concentra-tions it initiates an antioxidative response [15]. Rather than just the scavenging capacity, a
fine-tuning of H2O2levels is expected to be determining
for efficient stress control. The rationale of this
assumption is that H2O2, whilst deleterious to
some cellular components, is essential to plants in various biosynthetic reactions and, as suggested by some studies, possibly also in signal transduction pathways that could contribute to plant defense [18]. It may be supposed that the increased level of
H2O2 observed by us in AR-treated plants (Table
1) may also have signal functions; the reduced catalase activity (Fig. 1) may be one of the factors
of H2O2 accumulation. Chen et al. [19] and Du
and Klessig [20] proposed that catalase may be inactivated by binding to salicylic acid or by other cellular components, such as semidehydroascor-bate [21], reduced glutathione [22], superoxide and
hydroxyl radicals [23] and H2O2 [24], but the
rele-vance of these data towards physiological condi-tions is difficult to assess. In this study, during the
later terms after AR treatment the H2O2 content
was relatively reduced (Table 1), which could be explained by the increased catalase activity (Fig. 1). In this way the oxidative stress in plants may
be overcome. H2O2may also be involved in
perox-idase-mediated reactions of oxidative polymerisa-tion resulting in cell wall strengthening and formation of barrier anti-stress structures [25]. The increased activity of peroxidase in AR-treated plants (Fig. 1) suggests the protective role of the enzyme in this system.
The results are in accordance with other authors reporting similar patterns of peroxidase and cata-lase activities in different stress situations, such as Fe [26] and Al toxicity [27]. Catalase activity in spinach levels decreased by about 30% after 8 h
treatment with 0.5 ppm O3[28]. Visible symptoms
of injury and a significant activation of peroxidase during pH 2.2 treatment were found in two to-bacco cultivars [29].
In these experiments pretreatment with Spd or
Spm prevented the enhancement of H2O2 and
MDA caused by AR. Multiple properties of PAs may underly this protective effect. As bases PAs can neutralize the acid supplied to plants,
V.Veliko6a et al./Plant Science151 (2000) 59 – 66 65 ing’ cell pH. According to Tadolini [30] PAs act as
antioxidants by inhibiting lipid peroxidation; this is accounted for by the ability of PAs to form a ternary complex with iron and the phospholipid polar heads that may change the susceptibility of
Fe2+ to auto-oxidation and thus its ability to
generate free oxygen radicals. The results of Bor-rell et al. [7] suggest that inhibition of lipid perox-idation may be one of the mechanisms responsible for the anti-senescence effects of the PAs. Re-cently, an antioxidant effect of PAs was demon-strated in paraquat-treated plants [31]. Moreover, PAs are shown to stabilize membranes by associat-ing (as organic polycations) with negatively charged phospholipids. It was also demonstrated that treatment with Spd or Spm prevented the loss of chlorophyll, stabilized the molecular composi-tion of the thylakoid membranes and delayed
senescence [7,32]. Ultrastructural observations
showed that the integrity of the thylakoid mem-brane system is preserved with Spm [33]. Protec-tion of photosynthetic funcProtec-tions under acid stress and heat shock by PAs was demonstrated in ear-lier papers [34,35]. It was suggested that PAs kept a significant part of thylakoid membranes native by binding with them. The interaction of PAs with thylakoid membrane surface led to their stacking and to the association of light harvesting complex 2 with the PS 2 core complex. The assumption of the anti-stress effect of PAs is also supported by the reduced defensive peroxidase response to AR in the presence of Spm (3rd and 5th h) reported in the present work.
The results presented in this paper clearly indi-cate that single AR treatment induced oxidative stress related to membrane damage but did not cause irreversible changes. Catalase and peroxi-dase are involved in overcoming of oxidative stress. Exogenous PAs (Spd and Spm) applied before AR are suggested to prevent bean plants in this stress situation. For the period (24 h) between PA and AR supply, i.e. before AR stress, PAs may ‘prepare’ the cell to meet and combat stress by stabilizing membranes and forming a potential of higher ‘buffering’ and antioxidant capacity. After the stress, all protective properties of PAs may be involved in plant responses; the ‘buffering’ ability would be probably of primary importance. The more pronounced protective effect of Spm in com-parison with Spd could be accounted for by its longer chain and greater number of positive
charges which allows more important neutralizing and membrane stabilizing ability.
Acknowledgements
This research was supported by the National Fund of ‘Scientific Investigations’ (Project
MU-BAV-7/1995) at the Ministry of Education,
Sci-ence and Technology.
References
[1] R.W. Ferenbaugh, Effects of simulated acid rain on
Phaseolus6ulgarisL. (Fabaceae), Am. J. Bot. 63 (1976)
283 – 288.
[2] L.C. Evans, Biological effects of acidity in precipitation on vegetation: a review, Environ. Exp. Bot. 22 (1) (1982) 155 – 169.
[3] V. Velikova, I. Yordanov, M. Kurteva, T. Tsonev, Ef-fects of simulated acid rain on the photosynthetic charac-teristics ofPhaseolus6ulgarisL., Photosynthetica 34 (4)
(1997) 523 – 535.
[4] H. Willekens, D. Inze, M. van Montagu, W. van Camp, Catalases in plants, Mol. Breeding 1 (1995) 207 – 228. [5] A.W. Galston, R. Kaur-Sawhney, Polyamines as
endoge-nous growth regulators, in: P.J. Davies (Ed.), Plant Hormones: Physiology, Biochemistry and Molecular Bi-ology, Kluwer, Dordrecht, 1995, pp. 158 – 178.
[6] R. Kaur-Sawhney, A.W. Galston, Physiological and bio-chemical studies on the anti-senescence properties of polyamines in plants, in: R.D. Slocum, H.E. Floress (Eds.), Biochemistry and Physiology of Polyamines in Plants, CRC Press, Boca Raton, FL, 1991, pp. 201 – 211. [7] A. Borrell, L. Carbonell, R. Farra`s, P. Puig-Parellada, A.F. Tiburcio, Polyamines inhibit lipid peroxidation in senescing oat leaves, Physiol. Plant. 99 (1997) 385 – 390. [8] G. Berta, M.M. Altamura, A. Fusconi, F. Cerruti, F. Capitani, N. Bagni, The plant cell wall is altered by inhibition of polyamine biosynthesis, New Phytol. 137 (1997) 569 – 577.
[9] G. Seufert, V. Hoyer, H. Wo¨llmer, U. Arndt, The Ho-henheim long-term experiment. General methods and materials, Environ. Pollut. 68 (1990) 205 – 229.
[10] I. Sergiev, V. Alexieva, E. Karanov, Effect of spermine, atrazine and combination between them on some en-dogenous protective systems and stress markers in plants, Compt. Rend. Acad. Bulg. Sci. 51 (1997) 121 – 124. [11] R.L. Heath, L. Packer, Photoperoxidarion in isolated
chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation, Arch. Biochem. Biophys. 125 (1968) 189 – 198.
V.Veliko6a et al./Plant Science151 (2000) 59 – 66
66
[13] M.A. Dias, M.M. Costa, Effect of low salt concentra-tions on nitrate reductase and peroxidase of sugar beet leaves, J. Exp. Bot. 34 (1983) 537 – 543.
[14] M. Kato, S. Shimizu, Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing to-bacco leaves; phenolic-dependent peroxidative degrada-tion, Can. J. Bot. 65 (1987) 729 – 735.
[15] C.H. Foyer, H. Lopez-Delgado, J.F. Dat, I.M. Scott, Hydrogen peroxide- and glutathione-associated mecha-nisms of acclimatory stress tolerance and signalling, Physiol. Plant 100 (1997) 241 – 254.
[16] C.J. Douglas, Phenylpropanoid metabolism and lignin biosynthesis: from weeds to trees, Trends Plant Sci. 1 (1996) 171 – 178.
[17] R. Whetten, R. Sederoff, Lignin biosynthesis, Plant Cell 7 (1995) 1001 – 1013.
[18] R. Schreck, P.A. Baeuerle, The role of oxygen radicals as second messengers, Trends Cell Biol. 1 (1991) 39 – 42. [19] Z. Chen, H. Silva, D.F. Klessig, Active oxygen species in
the induction of plant systemic acquired resistance by salicylic acid, Science 262 (1993) 1883 – 1886.
[20] H. Du, D.F. Klessig, Identification of a soluble, high-affinity salicylic acid-binding protein in tobacco, Plant Physiol. 113 (1997) 1319 – 1327.
[21] A.J. Davison, A.J. Kettle, D.J. Fatur, Mechanism of the inhibition of catalase by ascorbate. Roles of active oxy-gen species, copper and semidehydroascorbate, J. Biol. Chem. 261 (1986) 1193 – 1200.
[22] Y. Sun, L.W. Oberley, The inhibition of catalase by glutathione, Free Radic. Biol. Med. 7 (1989) 595 – 602. [23] Y. Kono, I. Fridovich, Superoxide radical inhibits
cata-lase, J. Biol. Chem. 257 (1982) 5751 – 5754.
[24] H.N. Kirkman, S. Galiano, G.F. Gaetani, The function of catalase-bound NADPH, J. Biol. Chem. 262 (1987) 660 – 666.
[25] D.J. Colgrove, Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement, Plant Cell 9 (1998) 1031 – 1041.
[26] G.A.F. Hendry, K.J. Brocklebank, Iron-induced oxygen radical metabolism in waterlogged plants, New Phytol. 101 (1985) 199 – 206.
[27] I. Cakmak, W.J. Horst, Effects of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxi-dase activities in root of soybean (Glycine max), Physiol. Plant 83 (1991) 463 – 468.
[28] K. Tanaka, Y. Suda, N. Kondo, K. Sugahara, O3
toler-ance and the ascorbate-dependent H2O2 decomposing
system in chloroplasts, Plant Cell Physiol. 26 (1985) 1425 – 1431.
[29] F. Manes, R. Federico, F. Bruno, Peroxidase activity in
Nicotiana tabacumL. leaves treated with simulated acid rain, Phytopath. Medit. 25 (1986) 76 – 79.
[30] B. Tadolini, Polyamine inhibition of lipo-peroxidation. The influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads, Biochem. J. 249 (1988) 33 – 36.
[31] B. Ye, H.H. Muller, J. Zhang, J. Gressel, Constitutively elevated levels of putrescine and putrescine-generating enzymes correlated with oxidant stress resistance in
Conyza bonariensisand wheat, Plant Physiol. 115 (1997) 1443 – 1451.
[32] R.T. Besford, C.M. Richardson, J.L. Campos, A.F. Tiburcio, Effect of polyamines on stabilization of molec-ular complexes in thylakoid membranes of osmotically-stressed oat leaves, Planta 189 (1993) 201 – 206.
[33] A.F. Tiburcio, R.T. Besford, T. Capell, A. Borrell, P.S. Testillano, M.C. Risuen˜o, Mechanisms of polyamine ac-tion during senescence responses induced by osmotic stress, J. Exp. Bot. 45 (1994) 1780 – 1789.
[34] V.B. Velikova, I.T. Yordanov, K.M. Georgieva, T.D. Tsonev, V. Goltsev, Effects of exogenous polyamines applied separately and in combination with simulated acid rain on functional activity of photosynthetic appara-tus, J. Plant Physiol. 153 (1998) 299 – 307.
[35] I.T. Yordanov, V. Goltsev, The protective effect of some polyamines on thylakoid membrane functioning, Plant Physiol. (Sofia) 4 (1990) 42 – 51.