Mycorrhization of vitroplants raised from somatic
embryos of cork oak (Quercus suber L.)
Jesús D´ıez
a,∗, José Luis Manjón
a, Gabor M. Kovács
b,
Cristina Celestino
c, Mariano Toribio
caDepartamento de Biolog´ıa Vegetal, Universidad de Alcalá, 28871 Alcalá de Henares, Spain bDepartment of Botany, University of Szeged, PO Box 657, 6701 Szeged, Hungary
cInstituto Madrileño de Investigación Agraria (IMIA), Finca “El Encin”, Apdo. 127, 28800 Alcalá de Henares, Spain
Received 31 May 1999; received in revised form 8 December 1999; accepted 23 March 2000
Abstract
The technique described herein allows in vitro ectomycorrhizal synthesis in Quercus suber vitroplants raised from somatic embryos with Pisolithus tinctorius and Scleroderma polyrhizum strains. Only strains of this species coming from fruit bodies collected in Quercus suber stands (strain QS241 and strain QS247) formed ectomycorrhizas, and hence these species seem to exhibit host adaptation. The in vitro mycorrhization facilitated the development of secondary roots and the ex vitro weaning of cork oak vitroplants. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Ectomycorrhizas; Somatic embryogenesis; Cork oak; Mycorrhization
1. Introduction
The cork oak (Quercus suber L.) is one of the most important trees of the Mediterranean forest and has economic value based on the production of cork. This is a natural product with multipurpose applications used mainly in floor coverings and wine bottling. The increasing demand for cork and the low natural regen-eration of this species justify intensive planting with improved material. Current tree improvement strate-gies in Quercus place great emphasis on breeding and cloning (Savill and Kanowski, 1993; Cuenca et al., 1999). One of our main goals is to develop the
mi-∗Corresponding author. Present address: Equipe Microbiologie Forestiere, INRA – Nancy 54280 Champenoux – France. Tel.:
+33-383-39-40-41; fax:+33-383-39-40-69. E-mail address: [email protected] (J. D´ıez)
cropropagation of cork oak based on somatic embryo-genesis.
Somatic embryogenesis is envisaged as one useful tool in cloning elite genotypes of forest trees (Hog-berg et al., 1998; Taber et al., 1998; Lelu et al., 1999; Radojevic et al., 1999; Svobodova et al., 1999). It is considered as a relatively stable way of micropropa-gation and hence prevents additive and non-additive genetic variation. Somatic embryos are similar to their zygotic counterparts, showing a vascular con-nection between root and shoot: therefore, they can reduce the risk of intraclonal variation displayed by rooted cuttings due to differences on efficiency among cuttings regarding vascular connection be-tween shoot and adventitious roots. Somatic embryo-genesis also offers high rates of multiplication based on the process of recurrent embryogenesis (Merkle, 1995).
Somatic embryogenic lines were obtained from leaves of mature trees. The somatic embryos can be obtained by a secondary embryogenesis which allows a high level of multiplication. We also achieved mod-erate percentages of germination of somatic embryos but regenerated plants transferred to substrate stopped growing and died (Fernández-Guijarro et al., 1995; Puigderrajols et al., 1996; Celestino et al., 1998).
In vitro mycorrhization of micropropagated plants can be used to increase survival and growth during ex vitro weaning (Nowak, 1998). For instance, in the case of fruit trees, the inoculation with arbuscular fungi facilitated vitroplants adaptation to ex vitro conditions (Sbrana et al., 1994). It has also been reported that in vitro ectomycorrhization can improve microcutting rooting (Normand et al., 1996) and enables vitroplants to acclimate more readily (Martins et al., 1996). The in vitro mycorrhization of micropropagated plants, e.g.
Helianthemum spp. (Morte et al., 1994) and Cistus
spp. (D´ıez and Manjón, 1996), has been obtained only in very few Mediterranean species.
2. Material and methods
Cork oak vitroplants were obtained from the som-atic embryogenic line M10 by secondary embryogen-esis as described by Fernández-Guijarro et al. (1995). This embryogenic line was obtained from a leaf of an elite mature tree (Toribio et al., 1998), and it is pre-served at the germoplasm collection of the IMIA In-stitute, Alcalá de Henares, Spain. In vitro culture was carried out in closed MagentaTM vessels (SigmaTM, 95×67 mm2, height×width, 60 ml medium) at 25±1◦C and 16 h photoperiod (mixed cool-white and grolux fluorescent lamps, 50mmol m−2s−1).
Maturation of embryos was achieved by nutrient de-pletion in 12G medium (Gamborg, 1966) for 30 days, which synchronizes shoot and root embryo develop-ment, followed by a storage at 4◦C for 30 days to
switch embryos from embryogenic pathway to germi-nation (Fernández-Guijarro et al., 1995).
One embryo per vessel was germinated in MagentaTM vessels with 30 ml liquid SH medium (Schenk and Hildebrandt, 1972) and perlite filling up to 4 cm height of the vessels. Two months later, the SH medium was taken out with the aid of a pipette, the perlite was washed twice with sterile water before
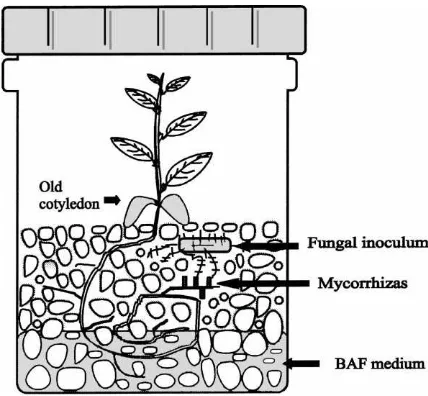
Fig. 1. System for in vitro mycorrhization of cork oak vitroplants raised from somatic embryos.
adding 30 ml of liquid BAF medium (Moser, 1960) at one-half of the phosphate concentration (Fig. 1). Then, each vessel was inoculated with a 0.7×0.7 cm2 piece of a fungal colony of a mycorrhizal fungus grown on solid BAF medium. Several strains avail-able in our laboratory were used, mainly strains of
P. tinctorius (Pers.) Coker & Couch and Scleroderma
(S. meridionale Demoulin & Malç. and S. polyrhizum G.F. Gmel.). These strains were obtained from fruit bodies collected: (a) in a Pinus pinaster Aiton stand (Segovia, Spain) (S. meridionalis strain PP10); (b) in a Eucalyptus camaldulensis Dehnh. plantation (Mi-ravete, Cáceres, Spain) (P. tinctorius strain EC204); (c) in a Q. suber stand in Aracena Mountains (Huelva, Spain) (P. tinctorius strain QS241, S. polyrhizum G.F. Gmel. strain QS247). The strains are deposited at the Departamento de Biolog´ıa Vegetal of Alcala Uni-versity, and fruit body samples at Alcalá Herbarium (AH). One month after inoculation, morphological (dissecting microscope) and anatomical (light micro-scope) examinations were carried out in order to con-firm the mycorrhizal formation. The ectomycorrhizas were described according to Agerer (1987–1993).
3. Results
Plants raised from somatic embryos inoculated with strains coming from a Pinus, Cistus or Eucalyptus vegetation did not form mycorrhizas. In some cases, the mycelium of these strains did not grow. In other cases they grew covering completely the plantlets which finally died. In contrast, ca. 85% of vitroplants inoculated with P. tinctorius QS241 and ca. 90% of those inoculated with S. polyrhizum G.F. Gmel. QS247 formed ectomycorrhizas. One month after inoculation, inoculation percentages of inoculated vit-roplants ranged from 50–75% to 60–70% of the tips of the secondary roots, respectively. Whereas mycor-rhizated plants displayed a very ramified root system, the non-inoculated plants lacked secondary roots.
Both strains coming from fruit bodies collected in Q. suber stands (strain QS241 and strain QS247) formed ectomycorrhizas. The perlite substrate was full of mycelium growth associated to the roots.
The P. tinctorius–Q. suber mycorrhizas were
golden-yellow. Although they were young, their ram-ification system was monopodial-pinnate. There were young rhizomorphs which were yellow and not dif-ferentiated yet. Mycelium grew mainly associated to the root system. Despite the mantle being sometimes young and thin, it was often well-developed. The man-tle was plectenchymatic. The outer layer of the manman-tle was formed by yellow running hyphae, some of them with crystals on their surface. The inner layer was formed by compact and sometimes parallel-running hyphae; this structure was the same at the young parts of the mantle. At the well-developed parts of the mantle, it was possible to observe the structure of the Hartig net.
The S. polyrhizum–Q. suber mycorrhizas were
brown with abundant mycelium giving them a white colour. Their ramification system was monopodial-pinnate. Mycelium grows on the substrate. There were rhizomorphs which were from hyaline to light-yellow in light microscope. The rhizomorphs were highly differentiated with centrally arranged thicker hyphae. Mantle was often well-developed, though it was sometimes very young and thin. At both parts, the mantle was plectenchymatic. Its outer layer showed a ring-like structure according to the terminology of Agerer (1995). The inner layer was plectenchymatic too with more or less the same structure. The Hartig
net could be observed under the well-developed parts of the mantle.
Whereas non-inoculated vitroplants died during ac-climatization, ca. 80% of mycorrhizated vitroplants survived; thus, mycorrhization seems to facilitate the weaning process of vitroplants raised from somatic embryos.
4. Discussion and conclusion
We observed that strains collected under other species in vitro did not establish mycorrhizas with
Q. suber. For instance, P. tinctorius strains
associ-ated to Eucalyptus failed to form mycorrhizas with
Q. suber plantlets raised from somatic embryos.
These findings led us to undertake the isolation of strains from basidiocarps collected in cork oak stands. We inoculated vitroplants raised from somatic em-bryos with strains collected associated with Q. suber woods. These strains belonged to P. tinctorius and
S. polyrhizum taxa and were collected in a cork oak
stand in Aracena Mountains (southern Spain). In vitro these strains formed mycorrhizas with plants raised from somatic embryos. Therefore, it seems to be a different ability of the strains of P. tinctorius to form mycorrhizas with cork oak, depending on the host from which they were isolated. This could indi-cate that a host specificity exists among the different strains of Pisolithus as molecular ecology studies had also previously indicated (Anta et al., 1998).
Regarding in vitro mycorrhizal establishment, some authors use an agar medium to synthesize in vitro mycorrhizas (Malajczuk and Hartney, 1986). Others use perlite or perlite–peat. In our case, synthesis was carried out in a hydroponic medium using perlite as substrate. We did not obtain synthesis in agar medium. This indicates that in our case hydroponic medium is more suitable than agar-based mediums.
The in vitro synthesized mycorrhizas showed the typical ectomycorrhizal morphology and anatomy. They had Hartig net and well-developed mantle. The morphology and anatomy of these mycorrhizas are in agreement with the mycorrhizas previously described in other wood species.
seedlings (Anta et al., unpublished). This description is also congruent with the previous descriptions in other hosts such as Picea abies (Weiss, 1990, 1991), Betula
alleghaniensis (Massicotte et al., 1990) or eucalyptus
(Rose et al., 1981). This description is in agreement with the description of the in vitro synthesised mycor-rhizas of P. tinctorius with eucalyptus (Tonkin et al., 1989).
According to the literature, there is no description of the mycorrhiza formed by S. polyrhizum in any host species. The synthesized mycorrhizas showed the main anatomical characteristics of the mycorrhizas of the genus Scleroderma, such as the characteristic structure of the mantle and rhizomorphs described in the case of S. citrinum (Pers.:Pers.), S. bovista and S.
verrucosum (Waller and Agerer, 1993; Agerer, 1995).
This is the first time that the morphology of the ecto-mycorrhiza of S. polyrhizum has been studied.
Boutekrabt et al. (1990) and Boutekrabt and Pargeny (1991) investigated the ultrastructure of the in vitro synthesized mycorrhizas of Tuber
melanospo-rum Vitt. with microcuttings of Q. robur L. and Q. pubescens Willd. These authors concluded that there
were no differences between the mycorrhizas formed on microcuttings and seedlings. We can suppose that in the case of plants of cork oak raised from so-matic embryos the mycorrhizas are similar to the mycorrhizas of seedlings. To confirm this fact we compared the in vitro synthesized mycorrhizas with those obtained ex vitro by spore inoculation of cork oak seedlings. In the case of P. tinctorius both myc-orrhizas showed similar anatomy and morphology.
Although in the literature there are works on in vitro mycorrhization (Burgess et al., 1994; Wiemken, 1995; Sudhakara and Satyanarayana, 1998a,b), it must be remarked that few of them used vitroplants raised from somatic embryos. When micropropagated plants have been used, most cases involved microcut-tings (Strullu et al., 1986; Tonkin et al., 1989) rather than plants raised from somatic embryos. Sasa and Krogstrup (1991) achieved ex vitro formation of my-corrhizas in P. sitchensis plantlets raised from somatic embryos. Piola et al. (1995) observed that in vitro ectomycorrhization improved the root development of
Larix somatic embryos. Moreover, the benefit of the
“biotization” of micropropagated plants with micro-bial inoculants is well known (Nowak, 1998). In our case, in vitro mycorrhization increased the formation
of secondary roots and the survival after acclimatiza-tion of vitroplants raised from somatic embryos. Thus, this approach is likely to leave behind the bottleneck so far encountered by Q. suber micropropagation by somatic embryogenesis.
Acknowledgements
We wish to thank Instituto Nacional de Investi-gación Agraria (INIA) of Spain (Project SC98-030) for financial support.
References
Agerer, R. (Ed.), 1987–1993. Colour Atlas of Ectomycorrhizas. Einhorn, Schwäbisch, Gmünd.
Agerer, R., 1995. Anatomical characteristics of identified ectomycorrhizas. An attempt towards a natural classification. In: Varma, A., Hock, B. (Eds.), Mycorrhiza. Springer, Berlin, pp. 685–734.
Anta, B., D´ıez, J., Manjón, J.L., 1998. Genetic variability of ITS sequence among Pisolithus strains associated with native hosts and exotic Eucalyptus in the Mediterranean forests. In: Abstract Book of Second International Conference on Mycorrhiza, Uppsala, Sweden, pp. 19–20.
Boutekrabt, A., Pargeny, J.C., 1991. Étude ultrastructurale de Tuber melanosporum Vitt. en culture isolée et en association avec des vitroplants de Quercus (Q. robur et Q. pubescens). Cryptogr. Mycol. 12 (1), 25–45.
Boutekrabt, A., Chevalier, G., Pargney, J.C., Dexheimer, J., 1990. Mycorrhization par Tuber melanosporum Vitt. de vitroplants de Quercus robur L. et Quercus pubescens. Agronomie 10, 127– 132.
Burgess, T., Dell, B., Malajckzuk, N., 1994. Variation in mycorrhizal development and growth stimulation of 20 Pisolithus isolates inoculated onto Eucalyptus grandis W. Hill ex Maiden. New Phytol. 127, 731–739.
Celestino, C., Picazo, M.L., Toribio, M., Álvarez-Ude, J.A., Bardasano, J.L., 1998. Influence of 50 Hz electromagnetic fields on recurrent embryogenesis and germination of cork oak somatic embryos. Plant Cell Tissue Organ. Cult. 54, 65–69. Cuenca, B., San-José, M.C., Mart´ınez, M.T., Ballester, A., Vieitez,
A.M., 1999. Somatic embryogenesis from stem and leaf explants of Quercus robur L. Plant Cell Rep. 18, 538–543. D´ıez, J., Manjón, J.L., 1996. Mycorrhizal formation by vitroplants
of Cistus albidus L. and C. salvifolius L. and its interest for truffle cultivation in poor soils. In: Azcón-Aguilar, B., Barea, J.M. (Eds.), Mycorrhizas in Integrated Systems from Genes to Plant Development. European Commission, Brussels, pp. 528–530.
Gamborg, O.L., 1966. Aromatic metabolism in plants. II. Enzymes of the shikimate pathway in suspensions of plants cells. Can. J. Biochem. 44, 791–799.
Hogberg, K.A., Ekberg, I., Norell, L., Von Arnold, S., 1998. Inte-gration of somatic embryogenesis in a tree breeding programme: a case study with Picea abies. Can. J. For. Res. 28, 1536–1545. Lelu, M.A., Bastien, C., Drugeault, A., Gouez, M.L., Klimaszewska, K., 1999. Somatic embryogenesis and plantlet development in Pinus sylvestris and Pinus pinaster on medium with and without growth regulators. Physiol. Plant. 105, 719– 728.
Malajczuk, N., Hartney, V.J., 1986. Procedure for inoculation of micropropagated plantlets of Eucalyptus camaldulensis with ectomycorrhizal fungi, and comparison with seedlings inocu-lation using inoculum contained in a peat/vermiculite carrier. Aust. For. Res. 16, 199–206.
Martins, A., Barroso, J., Pais, M.S., 1996. Effect of ectomyco-rrhizal fungi on survival and growth of micropropa-gated plants and seedlings of Castanea sativa Mill. Mycorrhiza 6, 265–270.
Massicotte, H.B., Peterson, R.L., Ackerley, C.A., Melville, H., 1990. Structure and ontogeny of Betula alleghaniensis– Pisolithus tinctorius ectomycorrhizae. Can. J. Bot. 68, 579–593. Merkle, S.A., 1995. Strategies for dealing with limitations of somatic embryogenesis in hardwood trees. Plant Tissue Cult. Biotechnol. 1, 112–121.
Morte, M.A., Cano, A., Honrubia, M., Torres, P., 1994. In vitro mycorrhization of micropropagated Helianthemum almeriense plantlets with Terfezia claveryi (desert truffle). Agric. Sci. Finland 3, 309–314.
Moser, M., 1960. Die gattung Phlegmacium (Schleimköpfe). J. Klinkhardt, Bad Helbrunn, Austria.
Normand, L., Bärtschi, H., Debaud, J.C., Gay, G., 1996. Root-ing and acclimatization of micropropagated cuttRoot-ings of Pinus pinaster and Pinus sylvestris are enhanced by the ectomyco-rrhizal fungus Hebeloma cylindrosporum. Physiol. Plant. 98, 759–766.
Nowak, J., 1998. Benefits of in vitro “biotization” of plant tissue cultures with microbial inoculants. In Vitro Cell. Dev. Biol. Plant. 34, 122–130.
Piola, F., Rohr, R., von Aderkas, P., 1995. Controlled mycorrhizal initiation as a means to improve root development in somatic embryo plantlets of hybrid larch (Larix×eurolepis). Physiol. Plant. 95, 575–580.
Puigderrajols, P., Fernández-Guijarro, B., Toribio, M., Molinas, M., 1996. Origin and early development of secondary embryos in Quercus suber L. Int. J. Plant. Sci. 157 (6), 674–684. Radojevic, L., Álvarez, C., Fraga, M.F., Rodr´ıguez, R., 1999.
Somatic embryogenic tissue establishment from mature Pinus nigra Arn. ssp. Salzmannii embryos. In Vitro Cell. Dev. Biol. Plant. 35, 206–209.
Rose Jr., R.W., Van Dyke, C.G., Davey, C.B., 1981. Scanning electron microscopy of three types of ectomycorrhizae formed
on Eucalyptus nova-anglica in southern United States. Can. J. Bot. 59, 683–688.
Sasa, M., Krogstrup, P., 1991. Ectomycorrhizal formation in plantlets derived from somatic embryos of sitka spruce. Scand. J. For. Res. 6, 129–136.
Savill, P.S., Kanowski, P.J., 1993. Tree improvement programs for European oaks: goals and strategies. Ann. Sci. For. 50, 368S– 383S.
Sbrana, C., Giovannetti, M., Vitagliano, C., 1994. The effect of mycorrhizal infection on survival and growth renewal of micropropagated fruit rootstocks. Mycorrhiza 5, 153–156. Schenk, R.U., Hildebrandt, A.C., 1972. Medium and techniques for
induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50, 199–204.
Strullu, D.G., Grellier, B., Marciniak, D., Letouzé, R., 1986. Micropropagation of chestnut and conditions of mycorrhizal syntheses in vitro. New Phytol. 102, 95–101.
Sudhakara, R.M., Satyanarayana, T., 1998a. Ectomycorrhizal formation in micropropagated plantlets of Populus deltoides. Symbiosis 25, 343–348.
Sudhakara, R.M., Satyanarayana, T., 1998b. Inoculation of micro-propagated plantlets of Eucalyptus tereticornis with ectomy-corrhizal fungi. New For. 16, 273–279.
Svobodova, H., Albrechtova, J., Kumstyrova, L., Lipavska, H., Vagner, M., Vondrakova, Z., 1999. Somatic embryogenesis in Norway spruce: anatomical study of embryo development and influence of polyethylene glycol on maturation process. Plant Physiol. Biochem. 37, 209–221.
Taber, R.P., Zhang, C., Hu, W.S., 1998. Kinetics of Douglas-fir (Pseudotsuga menziesii) somatic embryo development. Can. J. Bot. 76, 863–871.
Tonkin, C.M., Malajczuk, N., McComb, J.A., 1989. Ectomy-corrhizal formation by micropropagated clones of Eucalyptus marginata inoculated with isolates of Pisolithus tinctorius. New Phytol. 111, 209–214.
Toribio, M., Celestino, C., Gallego, J., Mart´ınez I., 1998. Induction of somatic embryogenesis in tissues from mature oak trees. In: Proceedings of the Annual Meeting of the Working Group I on “Physiology and Control of Plant Propagation in vitro”. European COST Action 8.22. Lisse, the Netherlands. Waller, K., Agerer, R., 1993. Scleroderma citrinum. In: Agerer,
R. (Ed.), Colour Atlas of Ectomycorrhizae. Plate 63. Einhorn, Schwäbisch, Gmünd.
Weiss, M., 1990. Studien an ektomykorrhyzen. XXV. Unter-suchungen zur ontogenie von ectomykorrhizen an Picea abies. Nova Hedwigia 50, 361–392.
Weiss, M., 1991. Pisolithus tinctorius. In: Agerer, R. (Ed.), Colour Atlas of Ectomycorrhizae. Plate 63. Einhorn, Schwäbisch, Gmünd.