Journal of Physics: Conference Series
PAPER • OPEN ACCESS
Influence Composition Fe
2
O
3
of Isotropic Magnet BaFe
12
O
19
on
Microstructure and Magnetic Properties.
To cite this article: Suprapedi et al 2018 J. Phys.: Conf. Ser.1091 012025
View the article online for updates and enhancements.
1234567890 ‘’“”
The 9th Seminar on Magnetic Materials (9th SMM) IOP Publishing IOP Conf. Series: Journal of Physics: Conf. Series 1091 (2018) 012025 doi :10.1088/1742-6596/1091/1/012025
Influence Composition Fe
2O
3of Isotropic Magnet BaFe
12O
19on Microstructure and Magnetic Properties.
Suprapedi 1*, Priyo Sardjono1, Muljadi1*, Kerista Sebayang2
1)Research Center for Physics- Indonesia Institute of Sciences Kawasan PUSPIPTEK
Serpong, Tangerang Selatan, Prov. Banten, Indonesia
2)
Physics-MIPA University of North Sumatera, Indonesia
*)
email : [email protected], [email protected]
Abstract. The isotropic magnets Barium Hexsaferit has been made by using a sintering process and the composition of Fe2O3 on barium hexaferrite was carried by the addition of
Fe2O3 (0, 0:25, and 0.5, % wt). The raw materials were used such as: commercial powder
BaFe12O19 and hematite Fe2O3 from e-Merck. Both the raw materials were weighed according
to the composition, and then refined for 48 hrs by using ball mill. After milling processes, the mixed powder was measured particle size distribution by using Laser Particle Size Analyzer, and it was obtained average particle size about 17,15 µm. After that, the powders were mixed with 2 % white. Celuona WE 518 as a binder, then the sample powders were formed by using cold pressing with a force about 5 tons becomes pellets with a diameter of 13.10 mm and thickness of 7.12 mm. Then samples pellet were sintered by using Electrical Thermolyne Furnace at temperature: 1150 ° C and holding time for 2 hours. The sintered samples were measured microstructure by using XRD, magnetic properties by using Permeagraph and Gausmeter. The variation of composition Fe2O3 on barium ferrite can influence significantly on
crystal structure and magnetic properties. It was found two phases such as: BaFe12O19 phase
and Fe2O3 phase on the samples with the addition of 0.5 % Fe2O3, but for sample without the
addition of Fe2O3 and with 0.24% Fe2O3 have only single phase BaFe12O19. The results of
measurement magnetic properties show that Magnet BaFe12O19, with addition 0.25% wt. Fe2O3 has high value for flux magnetic density and Br, but the Hc value of magnet BaFe12O19
becomes 2 times higher with the addition of 0.5% wt. Fe2O3.
Keywords: Barium Ferrite, hematite Fe2O3 , isotropic magnet, sintering, Flux magnetic
density, Permeagraph
1. Introduction
Barium hexa ferrite is a ferromagnetic oxides with a hexagonal crystal structure [1]. This magnetic material has been developed at the Philips laboratory in 1955. But it is still used until now in widely application, for example: as a component of motor, sensors, component speakers, magnetic recording media and so on [2,3]. The hexa ferrite is called permanent magnetic ferrites with basic formula MO.6Fe2O3 (M: Ba, Sr, Pb) [4]. Barium ferrite is scientifically and technologically attractive because
2
1234567890 ‘’“”
The 9th Seminar on Magnetic Materials (9th SMM) IOP Publishing IOP Conf. Series: Journal of Physics: Conf. Series 1091 (2018) 012025 doi :10.1088/1742-6596/1091/1/012025
The Curie temperature of ferrite magnets is 450°C. Table 1 shows the properties of magnetic materials as a permanent magnet.
Table 1. Magnetic Properties of Permanen Magnet Materials[ 1].
Materials Magnetic Properties
AlNiCo 0,85-0,90 115-127 121-136 35,8-63-3 860
The behaviour of ferrite magnets in different temperatures is different than that of other kinds of permanent magnets. With elevated temperature coercivity increases, but remanence decreases [6,7]. The disadvantages of ferrite magnets are their brittleness and high shrinkage after sintering [8]. They need machining after sintering process. Ferrite magnets can be prepared as sintered or bonded magnets and isotropic or anisotropic in character. Permanent magnets of this type are the most popular, mainly due to their low price. Ferrite magnets are nevertheless still produced and applied up to this point, because raw materials cost, the production costs and product prices are cheaper than raw materials based on rare earth metal. The production of permanent magnet based on barium ferrite consists of several stages, the first stage is powder preparation to produce powder barium ferrite with a hexagonal structure (BaFe12O19), the second stage is forming process and third stage is a densification process at
high temperature or the sintering process, and the final stages are a finishing process and the process of magnetization [1]. The milling process of Fe2O3 and BaCO3 mixture leads to increase the content of
Fe2O3 phase and decrease the content of BaCO3, and milling process causes enriching of mixed
powder particles by Fe2O3 [5,9]. The effect of Fe2O3 composition on magnetic properties and
microstructure have been studied [5]. The increasing of Fe2O3 content in material Ba-Ferrite can
influence magnetic properties, the remanence (Br) will decrease and coercivity (Hc) will increase [5]. The manufacturing of Ba-ferrite by milling and heat treatment process or solid-solid reaction should pay attention to factors of increased impurity materials (Fe2O3) during the process of milling [10]. The
increasing of Fe2O3 content during milling process can affect the characteristics of the permanent
magnet [7,10,11]. In this paper, the effect of variation of Fe2O3 composition on manufacturing
Ba-Ferrrite to the microstructure, physical properties and magnetic properties were investigated.
2. Experimental Method
The sample preparation was used a commercial powder of barium ferrite and hematite (Fe2O3)
1234567890 ‘’“”
The 9th Seminar on Magnetic Materials (9th SMM) IOP Publishing IOP Conf. Series: Journal of Physics: Conf. Series 1091 (2018) 012025 doi :10.1088/1742-6596/1091/1/012025
3. Results and Discussion
Figure 1 shows curves of particle size distribution for 48 hrs milled powder, and figure1 shows that its obtained average particle size about 17.55 µm after milling process of commercial BaFe12O19 powder
for 48 hrs. .
Figure 1.Particle Size Distribution Curve for Powder After Milling 48 h
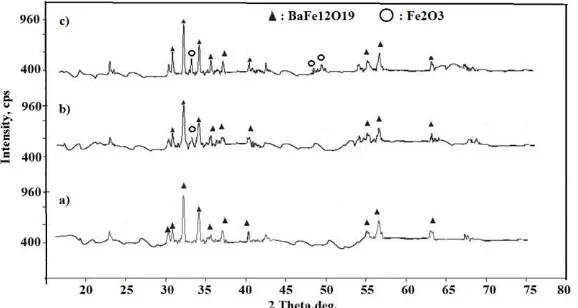
The results of XRD analysis for sample milled powder with a variation of Fe2O3 composition are
shown in figure 2. Figure 2 indicates that sample without additive Fe2O3 (original sample) and after
sintering 1150oC has one phase of BaFe12O19. The XRD patterns of sample with 0.25% Fe2O3 is
similar with the original sample, but for sample with 0.5% Fe2O3 is different and it has 2 phases
(BaFe12O19 and Fe2O3). This experiment shows that the additive of Fe2O3, with content 0.5 % gives
effect on the crystal structure
Figure 2XRD patterns of sample after sintering 1150 oC for (a) sampel with 0% Fe2O3, (b) sample with 0.25 % Fe2O3 and (c) sample with 0.5% Fe2O3
Flux magnetic density is a value of magnetic field on the surface of the sample and it was measured by using Gaussmeter. Table 2 shows the value of flux magnetic density for all samples, and value of flux magnetic density decrease with increasing of percentage of Fe2O3, but value of flux magnetic
4
1234567890 ‘’“”
The 9th Seminar on Magnetic Materials (9th SMM) IOP Publishing IOP Conf. Series: Journal of Physics: Conf. Series 1091 (2018) 012025 doi :10.1088/1742-6596/1091/1/012025
Table 2. Value of flux magnetic density
% wt. Fe2O3 Flux magnetic density, Gauss
0 595.3
0.25 589.7
0.5 495.5
Decreasing of flux magnetic density is due to changing of crystal structure or the existing of Fe2O3
phase. This is because the presence of Fe2O3 phase which is paramagnetic in the sample will reduce
the value of flux magnetic density. Samples with 0.25% and 0.5%wt. Fe2O3 were measured hysteresis
curve by using Permeagraph to know the value of Remanence (Br), Coercivity (Hc), and Energy Product (BHmax). The results of measurement are shown in figure 3.
Figure 3. Hysterisis Curve of Sample With a) 0.25 %wt. Fe2O3 and b) 0.5% wt. Fe2O3
The addition of Fe2O3 can influence magnetic properties, where the value of Br decreases and the
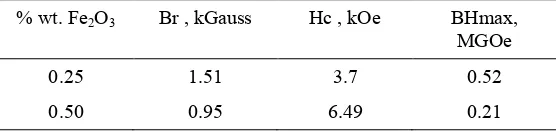
value of Hc increases in sample with higher Fe2O3 composition. Table 3 shows value of Br, Hc and
BHmax. Increasing of Fe2O3 composition from 0.25 % to 0.5 % can increase value of Hc two times
and decrease value of Br from 1.51 kGauss to 0.95 kGauss. The decline in the value of Br with the existing of Fe2O3 phase due to material properties of Fe2O3, that is paramagnetic Fe2O3.
Table 3 shows value of Br, Hc and BHmax.
% wt. Fe2O3 Br , kGauss Hc , kOe BHmax,
MGOe
0.25 1.51 3.7 0.52
0.50 0.95 6.49 0.21
A novelty of the results of this research is to add 0.25% of Fe2O3 being 0.5% can increase the value of
Hc reach almost twice. Surely it is the resilience of the demagnetization BaFe12O19 samples with the
1234567890 ‘’“”
The 9th Seminar on Magnetic Materials (9th SMM) IOP Publishing IOP Conf. Series: Journal of Physics: Conf. Series 1091 (2018) 012025 doi :10.1088/1742-6596/1091/1/012025
4. Conclussion
The percentage of addition Fe2O3 can give significantly influence to crystal structure (microstructure)
and magnetic properties. Magnet BaFe12O19, with addition 0.25% wt. Fe2O3 has high value for flux
magnetic density and Br, but the Hc value of magnet BaFe12O19 becomes 2 times higher with the
addition of 0.5% wt. Fe2O3
References
[1]. Buschow K H J 2009 Handbook of Magnetic Materials 18 1 15-28. [2]. Bahadur R D, Kumar S R and Kumar A 2006 J.Chem.Sci.118 1 15-21.
[3]. Moosa I S 2013International Journal of Advanced Research in Engineering and Technology [IJARET] 4 6 127-141.
[4]. Tang X, Yang Y G 2009 Materials Science-Poland27 2.
[5]
Nowosielski R, Babilas R, Wrona J 2007 Achievements in Materials and Manufacturing Engineering20 1-2 307-309.[6]. Mallick K K, Shepherd P, Green R J 2007 Journal of Magnetism and Magnetic Materials 312, 418–429.
[7] Hshiang H I, Yao R Q 2007 Materials Chemistry and Physics104 1-4. [8]. Jančárik V, Grusková A, Sláma J, Dosoudil R 2006 Journal Of Electrical Engineering57 8/S 163-166.
[9]. Shah I, Abbas T, Ullah Z, Hassan N, Rauf A, Ullah K and Naseem S 2015 Armenian Journal of Physics8 4 185-190.
![Table 1. Magnetic Properties of Permanen Magnet Materials[ 1].](https://thumb-ap.123doks.com/thumbv2/123dok/3916728.1863808/3.595.101.492.156.289/table-magnetic-properties-permanen-magnet-materials.webp)