Blackwell Science, Ltd
Extramatrical ectomycorrhizal mycelium contributes
one-third of microbial biomass and produces, together
with associated roots, half the dissolved organic carbon
in a forest soil
Mona N. Högberg and Peter Högberg
Department of Forest Ecology, Section of Soil Science, Swedish University of Agricultural Sciences, SE−901 83 Umeå, Sweden
Summary
• A large-scale tree-girdling experiment enabled estimates in the field of the contribution of extramatrical mycelium of ectomycorrhizal (ECM) fungi to soil microbial biomass and by ECM roots and fungi to production of dissolved organic carbon (DOC). • Tree-girdling was made early (EG) or late (LG) during the summer to terminate the flow of photosynthate to roots and ECM fungi. Determination of microbial C (Cmicr) and microbial N in root-free organic soil was performed by using the fumigation– extraction technique; extractable DOC was determined on unfumigated soil. • Soil Cmicr was 41% lower on LG than on control plots 1 month after LG, whereas at the same time (that is, 3 months after EG), the Cmicr was 23% lower on EG than on control plots. Extractable DOC was 45% lower on girdled plots than control plots. • Our results, which are of particular interest as they were obtained directly in the field, clearly demonstrate the important contribution by extramatrical ECM mycelium to soil microbial biomass and by ECM roots to the production of DOC, a carbon source for other microbes.
Key words: ectomycorrhizal fungi, extractable dissolved organic carbon (DOC), ectomycorrhizal mycelium, forest soil, fumigation–extraction, microbial N, soil microbial biomass.
© New Phytologist (2002) 154: 791–795
Author for correspondence: Mona N. Högberg
Fax: + 46 90 7867750
Email: [email protected]
Received: 11 January 2002 Accepted: 12 February 2002
Introduction
Biogeochemists, ecologists and soil microbiologists can be divided into those that neglect and those that recognize the role of mycorrhizal fungi in ecosystems. A reason for this division is the problem of assessing the contribution of mycorrhizal fungi to the soil microbial community, especially in the field. More precise estimates are available on the carbon (C) cost of ectomycorrhizal (ECM) symbiosis in laboratory model systems (Rygiewicz & Anderson, 1994) but only rough calculations are available for forest ecosystems (Söderström, 1992; Smith & Read, 1997). To date, it has not been possible to quantify the contribution made by ECM fungi to total microbial biomass in the soil, despite the need to distinguish these symbiotic fungi from other mycorrhizal fungi and from saprotrophic fungi and other decomposers. However, the
phospholipid fatty acid (PLFA) 18 : 2ω6,9 and ergosterol
fungal biomarkers were monitored inside and outside cores
(2.0 dm2) that were root isolated (Wallander et al., 2001).
The root isolation killed ECM fungi inside trenches and
Wallander et al. calculated the ECM biomass by subtracting
the amount of fungi outside, from the values obtained from inside trenched cores. The ECM biomass, including that of
fungal mantels, was calculated to be approx. 800 kg ha−1
. The amount of ECM mycelium produced in mesh bags filled with quartz sand, an inert substrate mainly colonized by
mycorrhizal fungi, was approx. 160 kg ha−1
. It is unclear if this estimate applies to the situation in the organic mor-layer, which is the horizon of greatest biological activity in the boreal forests.
(Garbaye, 1994; Timonen et al., 1998), and several studies report that ECM fungi and roots produce large amounts of
certain organic acids (Griffiths et al., 1994; Wallander et al.,
1997). The contribution made by ECM roots and ECM fungi to the production of dissolved organic C (DOC) in the mor-layer of boreal forests, where concentrations of DOC are
high (van Hees et al., 2000), is not known. This gap in our
knowledge is particularly serious given that DOC affects rates
of weathering of soil minerals (Lundström et al., 2000) and
represents important sources of C for microbes.
In a recent large-scale tree-girdling experiment (Högberg
et al., 2001) a 50% loss of soil respiration was interpreted as a loss of activity by ECM roots and their extramatrical mycelium. Here, we make use of this unique field experi-ment to quantify the contribution by the extramatrical mycelium of ECM fungi to total soil microbial biomass in the organic mor-layer. We also used this experiment to estim-ate the production of extractable DOC by ECM roots and fungi.
Materials and Methods
Field site, experimental design and soil sampling
The forest was a naturally regenerated 45- to 55-year-old
Scots pine (Pinus sylvestris L.) located on a weakly podzolized
sandy silt sediment at Åheden, northern Sweden (64°14′ N,
19°46′ E, 175 m above sea level). The climate is cold with a
mean annual temperature of 1.0°C, and a mean annual
precipitation of 600 mm. There is usually snow cover for 6 months between late October and early May. There was a
sparse understorey of Calluna vulgaris L. and Vaccinium
vitis-idaea L. The bottom layer consisted of mainly Cladonia
spp. lichens and Pleurozium schreberi moss. The organic
mor-layer (F + H horizons) was 2 cm thick and had the
follow-ing characteristics (n = 9, mean ± SD): bulk density 0.16 ±
The experiment comprised nine quadratic plots of 900 m2
each (with c. 120 trees each) and was divided into three blocks
(Fig. 1). Girdling was performed in early June 2000 (early girdling, EG) and in mid-August 2000 (late girdling, LG) on three plots at a time leaving three plots as control plots. Girdling had no effects on soil temperature and moisture
(Högberg et al., 2001). Seventy-two days after girdling, the
number of sporocarps and their biomass were reduced by
98.4% and 99.4%, respectively, on the central 100 m2 of EG
plots compared with control plots.
On 12 September 2000, soil from the F and H horizons was sampled by use of a 0.1-m diameter corer. Sampling was
performed along the border of the central 100 m2 of each
plot. Five composite samples made up from 10 cores each were taken from each plot.
Fumigation and determinations of Cmicr and DOC
Soil samples were stored at 4°C overnight. Roots were
thereafter sorted out by hand. After a day at 16°C, microbial
C, Cmicr, and microbial N, Nmicr, were determined by the
fumigation–extraction (FE) method (modified from Brookes,
1985a,b; Vance et al., 1987). Approximately 12 g (w : w)
root-free soil was put into each of 45 50 ml glass beakers,
which were placed in a desiccator (18 dm3 volume). Forty-five
millilitres of ethanol-free CHCl3 (Lichrosolv, Merck no. 2444,
Merck KGaA, Darmstadt, Germany) was used as fumigant
(22°C, 20 h). At the same time as the fumigation process was
started, the nonfumigated soil was shaken (150 rev min−1) for
30 min with 50 ml 0.5 M K2SO4 (mean soil : solution ratio =
1 : 13, w : v) and filtered (Munktell 00H filters (equivalent to Whatman no. 42), Munktell Filter AB, Grycksbo, Sweden). The fumigated soil was extracted as described above after
removal of the CHCl3 from the soil by repeated evacuations.
The extracts were kept frozen at −30°C before analysis.
Extracts were analysed for total organic C on a TOC-5000 (Shimadzu Corporation, Kyoto, Japan): the organic C
com-ponent was combusted to CO2 at 680°C and detected on
an infrared gas analyser. Extractable DOC was determined as total organic C in extracts from nonfumigated soil. The sum of
organic N and NH4-N in the K2SO4 extracts was determined
as NH4-N at 590 nm by flow injection analysis (FIAstar,
FOSS TECATOR, Höganäs, Sweden) after preincubation
and micro-Kjeldahl digestion (Wyland et al., 1994). The Cmicr
and Nmicr were obtained after correcting for the efficiency of
extraction of microbial biomass C and N, respectively. Cmicr
was calculated as Cmicr = Cf/kEC, where Cf is (organic C
extracted from fumigated soil) − (organic C extracted from
unfumigated soil) and kEC is 0.4. The Nmicr was calculated
as Nmicr = Nf/kEN, where Nf is (organic N extracted from
fumigated soil) − (organic N extracted from unfumigated
soil) and kEN is 0.4. These values of kEC and kEN were from a
similar soil in a Finnish Pinus sylvestris forest of C. vulgaris type
and were calibrated by microscopic counting (Martikainen & Palojärvi, 1990). Soil dry weight was determined after drying
at 105°C for 24 h. The organic matter content was
deter-mined by loss on ignition (600°C, 4 h) and pH was measured
in water (soil : solution ratio = 1 : 5, v : v).
Statistical analyses
Statistical analyses were performed using SIGMASTAT 2.0
(SPSS Science, Chicago, IL, USA). Effects of treatments and
blocks on Cmicr, Nmicr, microbial C : N and DOC were tested
by two-way ANOVA using the mean values for each plot. If a
significant effect (P < 0.05) was found, Tukey’s post hoc test
was performed to test for significant differences among treatments and blocks.
Results and Discussion
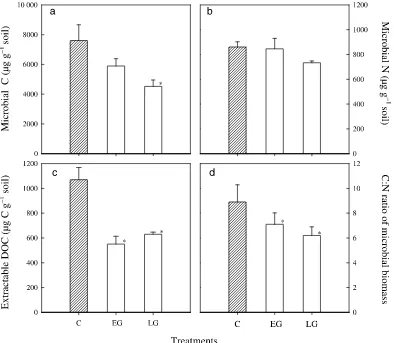
Microbial C and N contributed 1.6% to soil organic C and 6.6% to soil organic N, respectively, on control plots. These
figures are in agreement with the mean values of 1.8% for C and 8.5% for N given for a similar Finnish forest soil (Martikainen & Palojärvi, 1990).
Microbial C was 41% lower on LG plots than on control
plots (P < 0.05), while on EG plots it was 23% lower than on
control plots (difference was nonsignificant) (Fig. 2a). For
Nmicr, there were no differences among treatments (Fig. 2b).
However, the C : N ratios of the soil microbial biomass were
significantly lower (P < 0.05) on both EG and LG than on
control plots (Fig. 2d). In this case, there was also a significant block effect.
In the girdling experiment, in which up to 56% of soil res-piration was lost during the first year after girdling (Högberg
et al., 2001), measured total soil respiratory activity on con-trol plots included that of ECM roots and their fungal sheaths in addition to that of the free soil studied here. In root-free soil, there should be respiratory activity by the extra-matrical ECM mycelium, extraextra-matrical ericoid mycorrhizal mycelium, saprophytic fungi, bacteria and other soil organisms. In this study, the extramatrical ECM mycelium is the
major functional component, along with mycorrhizosphere organisms, that could be negatively affected by the girdling. Conversely, the activity and growth of the other organisms could be enhanced because they could use the dying ECM mycelium as a substrate and/or benefit from a release from competition for space and nutrients. Thus, based on the
aver-age loss of Cmicr in the treatments EG and LG, at least 32%
of the soil microbial biomass was contributed by extramatrical ECM mycelium. This contribution was calculated to be
equivalent of 145 kg ha−1
, corresponding to 58 kg C ha−1
at a fungal biomass carbon content of 40% by dry weight.
Potential changes in microbial respiratory activity, growth and community composition could have been going on for 3 months in EG plots compared with only 1 month in LG plots. This difference in time since girdling may help to
explain the more drastic decline in Cmicr in LG plots.
There-fore, the 41% loss of Cmicr in the LG treatment may be the
more relevant estimate of ECM biomass. The difference in biomass between EG and LG plots could also relate to season-ality. For respiration, the highest calculated contribution by ECM roots and mycelium was found in late summer
(Högberg et al., 2001), which is in line with observations on
C allocation patterns in temperate conifers (Hansen et al.,
1997). This means that the LG treatment was conducted when the ECM fungal biomass would be expected to be greatest. Several studies show a seasonal pattern in fungal biomass in forest soils. High values for fluorescein diacetate (FDA)-active fungi were found in early spring and autumn (Söderström, 1979; Bååth & Söderström, 1982) and growth of extramatrical mycelium of ECM fungi was highest in the autumn (Wallander
et al., 2001). At the time of this study, the respiratory activity was 37–39% lower in EG and LG plots than in control plots, which suggests a rough correlation between biomass and respiration.
The lower microbial C : N ratios of 7.1 and 6.2 in the EG and LG soils, respectively, compared with 8.9 for the control soil, may reflect a lower abundance of ECM fungi (lower fungi : bacteria ratio), since the C : N ratio in bacteria is mostly lower than in fungi (Paul & Clark, 1996). Alternat-ively, there are no changes in the microbial community with respect to the abundance of fungi and bacteria. Thus, the lower C : N ratios are simply the results of the same amounts of N being associated with smaller amounts of C.
Levels of extractable DOC were 49% and 41% lower on EG and LG plots, respectively, than on control plots (Fig. 2c). At the same time, there were no differences in extractable dissolved organic nitrogen (DON) among treat-ments (data not shown). This suggests the loss of organic DOC with a high C : N ratio of 51. It would be of considerable interest to know the molecular composition of the DOC in the different treatments, since low molecular weight organic acids are thought to play a major role in the mineral
weather-ing by ECM fungi (van Breemen et al., 2000) and as DOC
comprises potentially important C sources for other microbes.
We suggest that our estimate of a 32% contribution by extramatrical ECM mycelium to the total microbial biomass is a conservative one, primarily because the dead ECM mycelium could be used as a substrate by other organisms. The particular strength of our estimates of the contribution of ECM fungi to microbial biomass and by ECM roots and fungi to the production of DOC is that they relate to an organic soil in a field setting, in which the only major manip-ulation of the studied system is the removal of the C source
of the functional group of interest. Högberg et al. (2001)
demonstrated the vital importance of the flux of current photosynthates to ECM roots for soil respiratory activity. Our results show that this flux similarly directly supports a considerable portion of the soil microbial biomass and is of utmost importance for the production of DOC.
Acknowledgements
We thank Birgitta Olsson and Bengt Andersson for conducting the FIA and TOC analysis, respectively. This study was supported by grants from the Swedish Council for Forestry and Agricultural Research (SJFR), the EU (project FORCAST) and the Swedish Natural Sciences Research Council (NFR).
References
Bååth E, Söderström B. 1982. Seasonal and spatial variations in fungal biomass in a forest soil. Soil Biology and Biochemistry 14: 353–358.
van Breemen N, Finlay R, Lundström U, Jongmans AG, Giesler R, Olsson M. 2000. Mycorrhizal weathering: a true case of mineral plant nutrition? Biogeochemistry 49: 53–67.
Brookes PC. 1985a. Chloroform fumigation and the release of soil nitrogen: the effect of fumigation time and temperature. Soil Biology and Biochemistry 17: 831–835.
Brookes PC. 1985b. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil: the effect of fumigation time and temperature. Soil Biology and Biochemistry 17: 837–842.
Garbaye J. 1994. Helper bacteria: a new dimension to the mycorrhizal symbiosis. Tansley Review, 76. New Phytologist 128: 197–210. Griffiths RP, Baham JE, Caldwell BA. 1994. Soil solution chemistry of
ectomycorrhizal mats in forest soil. Soil Biology and Biochemistry 26: 331–337.
Hansen J, Türk R, Vogg G, Heim R, Beck E. 1997. Conifer carbohydrate physiology: updating classical views. In: Rennenberg H, Eschrich W, Ziegler H, eds. Trees – contributions to modern tree physiology. Leiden, The Netherlands: Backhuys, 97–108.
van Hees PAW, Lundström US, Giesler R. 2000. Low molecular weight organic acids and their Al-complexes in soil solution-composition, distribution and seasonal variation in three podzolized soils. Geoderma 94: 173–200.
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ. 2001. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411: 789–792.
Martikainen PJ, Palojärvi A. 1990. Evaluation of the fumigation–extraction method for the determination of microbial C and N in a range of forest soils. Soil Biology and Biochemistry 22: 797–802.
Paul EA, Clark FE. 1996. Ammonification and nitrification. In: Paul EA, Clark FE, eds. Soil microbiology and biochemistry. San Diego, CA, USA: Academic Press, 131–146.
Rygiewicz PT, Anderson CP. 1994. Mycorrhizae alter quality and quantity of carbon allocated belowground. Nature 369: 58–60.
Smith SE, Read DJ. 1997. The roles of mycorrhizas in ecosystems. In: Smith SE, Read DJ, eds. Mycorrhizal symbiosis. London, UK: Academic Press, 409–452.
Söderström B. 1979. Seasonal fluctuations of active fungal biomass in horizons of a podzolized pine-forest soil in central Sweden. Soil Biology and Biochemistry 11: 149–154.
Söderström B. 1992. The ecological potential of the ectomycorrhizal mycelium. In: Read D, Lewis DH, Fitter AH, Alexander IJ, eds. Mycor-rhizas in ecosystems. Cambridge, UK: CAB International, 77–83.
Timonen S, Jørgensen KS, Haatela K, Sen R. 1998. Bacterial community structure at defined locations of Pinus sylvestris–Suillus bovinus and
Pinus sylvestris–Paxillus involutus mycorrhizospheres in dry pine forest humus and nursery peat. Canadian Journal of Microbiology 44: 499–513.
Vance ED, Brookes PC, Jenkinson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19: 703–707.
Wallander H, Nilsson LO, Hagerberg D, Bååth E. 2001. Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytologist 151: 753–760.
Wallander H, Wickman T, Jacks G. 1997. Apatite as a P source in mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Plant and Soil
196: 123–131.
Wyland LJ, Jackson LE, Brooks PD. 1994. Eliminating nitrate interference during Kjeldahl digestion of soil extracts for microbial biomass determination. Soil Science Society of America Journal 58: 357–360.
About New Phytologist
• New Phytologist is owned by a non-profit-making charitable trust dedicated to the promotion of plant science. Regular papers, Letters, Research reviews, Rapid reports and Methods papers are encouraged. Complete information is available at
www.newphytologist.com
• All the following are free – essential colour costs, 100 offprints for each article, online summaries and ToC alerts (go to the website and click on Synergy)
• You can take out a personal subscription to the journal for a fraction of the institutional price. Rates start at £83 in Europe/$133 in the USA & Canada for the online edition (go to the website and click on Subscriptions)