254 (2000) 37–51
www.elsevier.nl / locate / jembe
Developing the options for managing marine pests:
specificity trials on the parasitic castrator, Sacculina carcini,
against the European crab, Carcinus maenas, and related
species
a ,* b c d c
R.E. Thresher , M. Werner , J.T. Høeg , I. Svane , H. Glenner ,
a e
N.E. Murphy , C. Wittwer
a
CSIRO Marine Research, G.P.O. Box 1538, Hobart, Tasmania 7001, Australia
b
¨ ¨
Goteborg University, Kristineberg Marine Research Station, S-450 34 Fiskebackskil, Sweden
c
Zoological Institute, University of Copenhagen, Universitetsparken 15, DK-2100, Copenhagen, Denmark
d
SARDI Aquatic Sciences, P.O. Box 120, Henley Beach, South Australia 5022, Australia
e
Department Parasitology, University of Queensland, St. Lucia, Queensland, Australia
Received 20 October 1999; received in revised form 10 April 2000; accepted 11 July 2000
Abstract
The impacts of introduced marine pests are becoming increasingly apparent, prompting interest in the possibility of their biological control. We undertook laboratory and field experiments on host selection of one potential control agent (the endoparasitic barnacle, Sacculina carcini ) against its natural host (the widely invasive European shore crab, Carcinus maenas) and several confamilial and more distantly related crustaceans. For comparison, we also tested host specificity in a related parasitic barnacle, Heterosaccus lunatus. The results confirm indistinct behavioral host selection in
S. carcini, indicate very different mechanisms for host selection by S. carcini and H. lunatus (which could be related to differences between the two species in attachment points), and suggest host specificity in S. carcini depends on interactions between the parasite and the host’s physiology. Development of convincing safety trials for marine parasites like S. carcini, in which the infective stage is a planktonic larva, will be more difficult than for many terrestrial parasites and will require detailed knowledge of the parasite’s behavior and physiological interaction with its hosts. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Host specificity; Rhizocephalan; Carcinus; Sacculina carcini; Biological control
*Corresponding author.
E-mail address: thresher@marine.csiro.au (R.E. Thresher).
1. Introduction
Non-indigenous marine species threaten both marine industries and biodiversity. Information on the numbers, diversity and impacts of exotic marine species is still sparse (Ruiz et al., 1999), but for a number of high profile ‘pests’ the impacts are so obvious that efforts are underway to reduce both the frequency of new introductions (Committee on Ships’ Ballast Water, 1996) and the impacts of existing pest populations. Examples of these high profile species include the Atlantic ctenophore, Mnemiopsis leideyi, intro-duced into the Black Sea and implicated in the decline in regional fisheries (Shiganova, 1998), the European seagrass, Spartina anglica, which is overgrowing mud flat communities along the coasts of North America, New Zealand and Australia, and the European shore crab, Carcinus maenas. C. maenas is an exceptionally successful emigrant, with established invasive populations in Australia, Japan, South Africa and North America (Cohen et al., 1995; Grosholz and Ruiz, 1995). The collapse of shellfish fisheries on the American east coast has been attributed to predation by C. maenas (Smith et al., 1955). Ecological studies of C. maenas in North America and Australia (Grosholz and Ruiz, 1996; papers in Thresher, 1997) indicate that it is a highly competitive generalist predator capable of radically altering the composition and dynamics of invaded assemblages (Grosholz et al., 2000).
Options for dealing with introduced marine pests parallel those for dealing with terrestrial pests. These range from physical removal (Walton, 1997) through to a variety of possible biological treatments and to environmental rehabilitation (the last based on the assumption that invasive species primarily affect degraded habitats). Environmental rehabilitation as a control strategy is an unlikely option for C. maenas, which has established dense populations in largely unaltered habitats (though see Janzen, 1998). The large numbers of the crabs and their high fecundity suggest that physical removal will also have only a minor effect on their impacts. These and other options available to deal with C. maenas were reviewed at an international workshop on managing the species (Thresher, 1997), which suggested two options that were likely to be effective. The first is a large-scale program of physical removal, possibly in the form of a subsidised fishery, which might reduce impacts temporarily or in small areas. The second was biological control.
Classical biological control involves the introduction of a predator, parasite or pathogen to reduce the impacts of the invasive species. The approach has a long history and mixed success rate for terrestrial pests (Van Driesche and Bellows, 1996). Success depends largely on the effectiveness of the control agent and its safety. The latter largely equates to issues of specificity: a ‘safe’ biological control agent is one that attacks only the target species. Detailed protocols for assessing the specificity of biological control agents against terrestrial pests have been developed (see Kaufman and Nechol, 1992). These protocols could be applied with minor modification to marine biological control agents (Kuris, 1997).
S. carcini is a common and well-studied parasite of C. maenas in the crab’s native ¨
range (Høeg and Lutzen, 1995). The life cycle of the parasite involves a mature female, which is situated in the abdominal brood chamber of the host, is fertilized by one or two cryptic dwarf males and which subsequently releases a series of broods of nauplii. The nauplii develop lecithotrophically, metamorphose into cypris larvae after about 5–6 days and become competent to settle after another 3–4 days in the plankton. Female cyprids settle on the exoskeleton of a host crab at the base of a plumose seta (Delage, 1884) and metamorphose into a special stage known as the kentrogon. The kentrogon penetrates the exoskeleton of the crab with a hollow stylet and injects the primordial parasite into the
¨
hemocoelic fluid. After an internal phase of a few months to 3 years (Lutzen, 1984), the parasite produces an external virginal reproductive body (externa) situated under the abdomen of the host. The externa attracts male cyprids, which implant as dwarf males (Høeg, 1987), remain with the female externa for the duration of the latter’s lifetime and fertilize all its broods. Externae failing to receive at least one male cannot mature and eventually perish.
S. carcini has severe and lasting effects on the growth, morphology, physiology, and behavior of the host crab. It arrests the moult cycle of the host, which therefore suffers increased fouling. More importantly, the parasite castrates both male and female crabs and feminises the males. The behavior of both sexes is altered such that both respond to the externa and parasite’s eggs as their own eggs. In effect, the sacculinized host becomes a parasite genotype with a crab phenotype.
These features suggest S. carcini could be a useful biological control agent against C. maenas (Lafferty and Kuris, 1996). The key issue, however, is the degree of host specifi-city. Host specificity in rhizocephalans is highly variable. Many attack only one or a few closely related host species, whereas others appear to be less host specific (Høeg and
¨
Lutzen, 1995). S. carcini appears to be in the latter group. In Europe, it occurs not only on C. maenas, but also on C. aestuarii, Liocarcinus depurator and some other Portunidae (Høeg, 1995) and is even known from Perimela denticulata (Perimelidae). Recent genetic work (Murphy and Goggin, 2000) appears to refute the hypothesis that S. carcini is a com-plex of cryptic species, each showing a high degree of host specificity (Høeg, 1995).
We undertook a series of experiments to assess the host specificity of the parasite when exposed to potential non-native hosts. These experiments address three issues. First, since there are few experimental tests of host specificity in rhizocephalans and
¨
2. General materials and methods
2.1. Sacculina carcini trials
Experiments were carried out at the Kristineberg Marine Research Station (KMRS), Fiskebackskil, Sweden, from 4 August 1996 to 7 April 1998. The infection trials took place between August and December, 1996; crabs were held in individual aquaria up to April 1998 to allow interna time to develop. During captivity, they were fed mussels, fish and shrimp.
The general experimental protocol was (1) expose potential hosts to healthy, appropriately staged cyprids, (2) examine potential hosts microscopically for attached cyprids, (3) transfer infected crabs to individual aquaria for rearing, followed by (4) sacrifice of the animals and microscopic and genetic examination for evidence of development of an interna. The experimental design encompassed two orthogonal hierarchical structures. First, the parasite and hosts were tested in three settings: (1) no choice of host (i.e. single species exposure), (2) choice of alternative hosts (i.e. simultaneous availability of natural and potential hosts) in the laboratory and (3) choice experiments in the field. The second hierarchy determined the host species tested. These were, in order of decreasing likelihood of infection: (1) the natural host (C. maenas) from Sweden and Australia, (2) other members of the same family (Portunidae), including a non-host species from Sweden and four potential hosts from Australia and (3) an out-group Australian species in an unrelated crab family (Grapsidae) never previously reported as attacked by Sacculina spp. All crab species were used in the choice experiments in the laboratory. Because of limited numbers of a few species, a smaller range was used in the no-choice and field trials. In order to prevent accidental introductions, only male crabs were used in the field trial. All were recovered and killed at the end of the experiment.
Visual inspection of the experimental crabs for attached cyprids was done by all of the authors, using dissection microscopes. Specimens were frequently reexamined by a second observer, to confirm counts. Morphological examination of the ethanol-preserved
¨
crabs for internae was done by M.W., J.T.H. and J. Lutzen at the Zoological Institute, University of Copenhagen, Denmark.
Prism 377 DNA Sequencer). Nucleotide sequence data was collected from the 39region of the SSU, ITS1 and partial 5.8S rRNA using two forward (SB2 and SAC1) primers located in the 39 region of the SSU and one reverse (SAC3) primer located in the 59
region of the 5.8S rRNA. Amplification of S. carcini was verified by alignment of rRNA nucleotide sequence data from interna, externa and larvae collected in Sweden, which were identical. Genetic screening used a species diagnostic primer designed from the ITS1 rRNA nucleotide sequence of S. carcini, which amplifies a 350-bp product from infected crabs.
Details about the crabs tested are given in Table 1. We used juveniles wherever possible, on the basis that they might be more susceptible than adults to attack (Høeg
¨
and Lutzen, 1995). Swedish C. maenas and a portunid not used as a host by S. carcini in Swedish waters, Liocarcinus depurator, were obtained using fyke nets in the Ellosfjor-den, Strommarna and adjacent areas. Individuals with no evident externa were held for several days prior to the experiments in 20–40-l open-flow seawater aquaria. Specimens of five species of Australian native crabs plus specimens of the introduced population of C. maenas were collected by hand at sites along the southeast coast of Australia and overnight air-freighted to Sweden. All of the shipped crabs except for Charybdis callianassa were very active and apparently healthy on arrival; all but one of the Charybdis (the smallest) died within 6 days of arrival. The animals were held in screened slow-drip open aquaria for 5 days prior to the experiments, during which time they were generally active and fed vigorously on fish and live mussels. In one test, we also included as an extreme out-group a native Swedish thallassinid (ghost shrimp), collected from the Ellosfjorden.
Sacculina carcini cyprids were obtained by rearing larvae hatched from externa-bearing C. maenas. Individual crabs, collected as described above, were placed in 3-l aquaria. The crabs were checked daily for hatching. After hatching, each brood was siphoned into an individual rearing chamber, which consisted of a 1-l glass beaker half submerged in a constant temperature (15.98C) waterbath. The design of the culture system is based on Strathmann (1987). Seawater in the beakers was changed when the larvae were about 4 days old (i.e. precyprid stage). Water in each beaker was stirred slowly via a small paddle, to minimise the chances of larvae being trapped by surface
Table 1
Characteristics of host crabs tested for susceptibility to Sacculina carcini settlement. Note carapace width of Portunus pelagicus includes prominent lateral spines, which constitutes about 30%
Species Family Carapace width Sex ratio Source location (mm) (male / female)
tension. In most cases, we used larvae from a single brood for each experiment, in order to avoid confounding effects of variable sex ratio (which can differ between broods from all male to all female, with only the females parasitic) and larval condition (Walker, 1985). In all cases, the only broods used were either predominantly or entirely female (based on morphological examination of a sample of cyprids) and in good health, as indicated by vigorous swimming activity.
2.2. Heterosaccus lunatus trials
Experiments were conducted at the University of Queensland, Brisbane, Australia, in July /August, 1996. Protocols generally follow those described above. Heterosaccus lunatus cyprids were obtained by rearing larvae from externa-bearing Charybdis callianassa, collected by trawling in Moreton Bay, Queensland. The crabs were held in closed-circulation aquaria, fed once or twice weekly and inspected daily for darkening of the externa, which indicates imminent hatching. Once darkening had occurred, the parasitised crabs were isolated in small aerated aquaria and inspected hourly. Newly hatched nauplii were collected by attracting them to a light and pipetting them out. One hundred larvae from each batch were measured to determine sex ratio. Only broods of
.50% female were used in the trials. The experiments were conducted using juvenile, non-externa-bearing crabs, ranging in carapace width from 2.5 to 3.5 cm (Charybdis callianassa) and 1.5–2.5 cm (C. maenas). Charybdis callianassa were collected by trawling in Moreton Bay; the juvenile C. maenas were collected by hand in Tasmania and air-freighted to Brisbane.
2.3. Statistical analysis
Significance in experiments involving comparisons among multiple host species was tested using analysis of variance. Pair-wise comparisons were done using t-tests. Procedures follow Sokal and Rohlf (1981).
3. Results
3.1. Sacculina carcini: experiment No. 1 — no-choice exposure in the laboratory
3.1.1. Methods
that afternoon (1600 h) and again at noon the next day. Afterwards, all five crabs were transferred to the long-term rearing facilities, a second set of juveniles placed into the beakers (which still were conspicuously full of swimming cyprids), and the experiment repeated.
3.1.2. Results
Cyprids were found attached to three of the five taxa tested, but attachment rates were extremely low. Among the ten crabs tested and the thousands of cyprids present, we found only five attached cyprids: all four of the C. maenas (two Swedish, two Australian) tested each had one attached cyprid on them and one cyprid was found attached to one of the P. gaimardii.
3.2. S. carcini: experiment No. 2 — choice exposure in the laboratory
3.2.1. Methods
Individual crabs were placed in 1-cm square-mesh green plastic bags, along with a small granite rock to make the bags sink. Each bag was about 15 cm long39 cm wide36 cm high, large enough to allow the crabs a small amount of mobility. The captive crabs were placed into a recirculating flume tank, in one or more rows perpendicular to the current flow. Positions were randomized among samples, and recorded to check for position effects. The flume tank was 300 cm long348 cm wide and filled to a depth of 22 cm with clean seawater (32 ppt) at the beginning of the experiment. Current speed was set at 1 cm / s.
In the first trial, five specimens were tested (a Swedish and an Australian C. maenas, plus one each O. australiensis, Portunus pelagicus and Paragrapsus gaimardii) and checked daily for cyprid attachment. At the beginning of the experiment (day 1), several thousand, very actively swimming 7-day post-hatch cyprids were added. Two broods were used, both estimated to be .95% female. When after 3 days the first attached cyprids were found, two more specimens of each crab taxa were added, along with our only remaining specimens of N. integrifrons and Charybdis calliannassa, and two L. depurator. We also added several thousand more cyprids, 10-days post-hatch. All crabs were checked daily for the next 2 days, by which time the number of active cyprids in the tank was declining sharply and the trial was terminated (day 6).
A second trial was started immediately, with fresh, 9-day post-hatch larvae. As the specimens of N. integrifrons, C. callianassa and L. depurator had no attached cyprids from the first trial, they were placed back into the flume, along with fresh specimens of the other samples. We also added a small thallassinid (ghost shrimp) as an extreme out-group sample. The second trial was run for 24 h.
All Carcinus and Australian crabs were transferred to holding facilities at the end of the trials.
3.2.2. Results
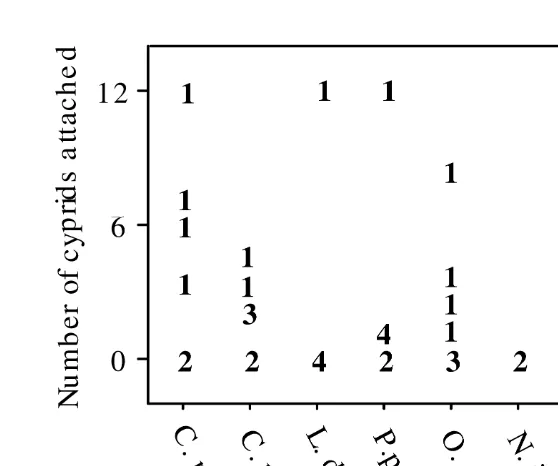
Fig. 1. Number of cyprids found attached to individual crabs in a single night in the flume tank, compiled over the four nights of the experiment. Values specify the number of individuals at the indicated level of infestation.
Swedish C. maenas, and the lowest (no cyprids over 2 days trialed) for the Australian N. integrifrons. However, overall the number of attached cyprids did not differ significantly among crab species (Anova F7,3550.52, NS). Reflecting this, maximum attachment rates were also similar between C. maenas and other species: up to 12 attached cyprids attached in a single night to specimens of C. maenas, Portunus pelagicus and L. depurator, 10 on a single Paragrapsus gaimardii, and 8 on O. australiensis. Eight cyprids were also found attached to the thallassinid.
The distribution of attached cyprids among crabs was extremely patchy. The number of attached cyprids could not be related to the sex, size (carapace width) or location of the crab in the flume tank (statistics for all individual parameters not significant). The number of attaching cyprids varied with time / age of the cyprids in the first trial, with no settlement among cyprids less than 10 days post-hatch. The host range was identical at the beginning and end of the settlement period, indicating no reduction in specificity as the cyprids neared the end of their competent period.
3.3. S. carcini: experiment No. 3 — choice exposure in the field
3.3.1. Methods
weeks into the experiment, the crabs were individually confined in nylon mesh bags (123634 cm) inside each cylinder, to prevent cannibalism. A few specimens lost before then were replaced.
The cages were suspended just above the bottom in water 3 m deep, and separated from one another by 0.5–1 m. There is little discernible tide in the area, so current flow under the bridge was slight. The experiment was conducted from 17 September to 31 October, during which time the crabs were checked for cyprids at 3–4 day intervals. They were fed blue mussels (Mytilus edulis) weekly. The natural infection rate by S. carcini at four localities close to the test site, based on the frequency of scarred and feminized crabs, ranged from 3.4 to 29.5% with the highest rates in inshore waters.
3.3.2. Results
We found one attached cyprid on each of two out of the four Australian C. maenas and on two out of the four P. gaimardii tested. This equates to an overall attack rate of 16.7%.
3.4. S. carcini: development of interna
The sole Charybdis callianassa and all Portunus pelagicus died by February, 1997. Both are subtropical species and appeared to be unable to tolerate the low seawater temperatures in Sweden, even in the laboratory. As well, two C. maenas (one Swedish and one Australian specimen), two O. australiensis and the last remaining N. integrif-rons also died between 6 and 12 months after the onset of the trials. The remaining specimens (27 individuals in three species), all of which fed and molted regularly, were killed in April 1998 (19 months after attachment). Specimens were frozen after death and then transferred to ethanol, or preserved immediately in ethanol. All were examined morphologically for evidence of interna. Genetic tests were subsequently conducted on all but the C. callianassa and P. pelagicus.
Externae developed on three reared crabs — one Swedish and two Australian C. maenas — approximately 12 months after the experiments. Morphological examination of these and the other specimens found S. carcini interna in only these three individuals. There was no sign of roots in any of the Australian native species, irrespective of attack rates by rhizocephalan cyprids in the laboratory. None of the crabs exposed to S. carcini in the field developed visible externa or interna.
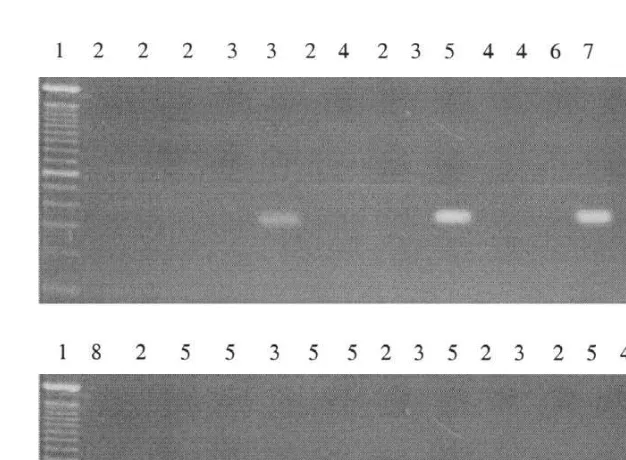
Fig. 2. Electrophoretic banding for experimentally infected crabs. For details, see text. Key to symbols: 1, 100-bp DNA ladder (Gibco BRL); 2, Paragrapsus gaimardii; 3, Ovalipes australiensis; 4, Swedish Carcinus maenas; 5, Experimental Australian C. maenas; 6, uninfected Australian C. maenas (control); 7, positive control (Sacculina carcini ); 8, negative control (no DNA).
Of the 24 specimens attached to S. carcini in the laboratory, the genetic test indicated 6 developed infections: 2 of 5 Australian C. maenas, 2 of 6 Swedish C. maenas, 2 of 6 O. australiensis, and none of either the 6 Paragrapsus gaimardii or the single N. integrifrons trialed. In the field, the only specimen infected was a Swedish C. maenas. Overall the rate of infection of C. maenas (4 out of 15 specimens examined genetically, both source locations pooled) did not differ significantly from that of portunids other than Carcinus (2 out of 9) and the only non-portunid tested, Paragrapsus gaimardii (0
2
out of 10) (x 54.21, df52, NS). The infections of Swedish C. maenas might reflect infestation prior to the experimental work, but even if so, deletion of those specimens from the analysis does not change the conclusions drawn.
Among the laboratory-exposed specimens, the number of attached cyprids was a poor predictor of whether or not the crabs developed interna (differences in attachment rate between infected and un-infected crabs, unpaired t51.31, NS). Restricting the com-parison to only the two species infected (C. maenas and O. australiensis) and further restricting it to only crabs on which we found cyprids did not improve the relationship (t51.12, NS). There were also no differences between infected and un-infected crabs in
2
3.5. Heterosaccus lunatus
3.5.1. Methods
Two sets of no-choice experiments were conducted. In the first, whole juvenile crabs (3 Charybdis callianassa and 4 C. maenas) were placed for 1–2 h in individual beakers (200–500 ml.) containing several hundred day-2 cyprids. Unlike S. carcini, female cyprids of H. lunatus attack their hosts via the gills (Walker, 1999). Consequently, after the experimental exposure, the crabs were immediately dissected, their gills removed to individual cavity blocks containing clean seawater and attached cyprids counted using a dissecting microscope. Cyprids were considered attached if they could not be easily brushed off the gill. The second experiment was similar to the first, but the cyprids were exposed to only fresh gill filaments (five each C. callianassa and C. maenas) suspended in the beakers for approximately 1 h.
3.5.2. Results
Two hundred and forty-three cyprids were found attached to the gills of the three C. callianassa; none were found attached to the gills of C. maenas. The difference between species is highly significant at P,0.01 (t54.19, df55). Cyprids were most often found attached to the more posterior gills. In the trials using excised gills, 29 cyprids were found attached to the five C. callianassa gills, whereas none were found on the five C. maenas gills.
4. Discussion
Host specificity by rhizocephalans could involve at least three mechanisms: (1) the free-swimming female larvae attack only the preferred host; (2) the larvae attack a range of targets, but non-host species defend themselves behaviorally, e.g. cleaning; and (3) the larvae attack a range of targets, but physical (shell thickness) or physiological mechanisms (incompatibility) prevent penetration by the kentrogon and / or development or maturation of the interna (Ritchie and Høeg, 1981).
Our experiments indicate that the mechanisms underlying specificity differ fundamen-tally in the two rhizocephalan species we tested. In H. lunatus, cyprids strongly selected their native host over a non-native confamial, even when exposed only to the excised gills of the two species and even when offered no alternative. The implication is that selection in this species is an active process, based on parasite behavior and mediated by properties inherent to the host and specifically to its gills, the preferred point of attachment. The nature of these properties is uncertain, but the very well developed chemosensory organs of rhizocephalan cyprids (Glenner et al., 1989; Walker, 1999) suggest they are chemical.
not differ significantly among the crab species tested, and the highest level of attack against a single C. maenas, 12 cyprids in a single night, was equaled by those against L. depurator and Portunus pelagicus. Cyprids even attached to a thalinassid shrimp. In the field, although overall attack rates were much lower, parasites attached not only to the natural host, but also to unrelated crabs in the family Grapsidae (a family absent in Sweden). Although the laboratory studies appeared to have suffered from substantial artifacts, the laboratory and field data in conjunction make it difficult to reject an hypothesis of low behavioral host specificity in S. carcini. We conclude that there is no ‘lock-and-key’ mechanism in S. carcini larvae that underlies its choice of hosts, though such a mechanism remains a possibility in H. lunatus.
Two further observations also suggest major differences between H. lunatus and S. carcini in their mechanisms of host choice. First, the overall rate of attack differed spectacularly between them in the laboratory. In just 2 h, an average of 81 H. lunatus cyprids attached to each Charybdis callianassa in no-choice, small beaker experiments. In the equivalent no-choice experiment for S. carcini, no cyprids attached to any host, including Carcinus maenas, in the first hour of exposure and after 21 h, the average was still less than 1 cyprid per host. Similarly, in the flume tank experiments, the average number of attached cyprids for S. carcini was less than three per target over a 24-h period. These low settlement levels are not unique to our particular experiments, as we have observed similarly very low levels of attachment in this species previously (Høeg, 1984).
The two parasites also differed in the variability of their host preferences. In H. lunatus, all native hosts exposed were attacked at similar levels, and consistent patterns were observed in, for example, increasing attachment rates on the posterior-most gills. In contrast, settlement by S. carcini was inconsistent and patchy, and could not be related to any parameters we could measure (e.g. sex and size of the crab or its location in the tank). Again, our prior work supports this inconsistent responsiveness by S. carcini larvae. In one case, we observed more than 50 S. carcini cyprids attached to a single C. maenas after one night in the laboratory, while another C. maenas, in the same container carried no attached cyprids at all (J. Høeg, unpublished data). One variable we did not measure in our experiments, stage in the molt cycle, could affect susceptibility, a possibility we verified in a subsequent experiment (Glenner and Werner, 1998). Although molt stage may contribute to the patchiness of attachment in the present study, we think it does not account for all or even most of it; nor does an effect of molt stage invalidate our general conclusion of low host specificity in S. carcini.
Weak host selection in S. carcini appears contradictory to its low attachment rates in the laboratory. Like H. lunatus, female S. carcini cyprids have well developed olfactory organs, on which basis we speculate that the cyprids require a very specific chemical cue to trigger attachment, which is presumably common to a number of potential hosts (based on the range of hosts naturally attacked by S. carcini). The exact nature of this cue, why it was not available in the laboratory in any experiment, and how it differs from that used by H. lunatus, is not yet clear.
species that attached to its host’s gill filaments: Lernaeodiscus porcellanae attacking the porcellanid crab Petrolistes cabrilloi (Ritchie and Høeg, 1981). There are several similarities between that work and our results for H. lunatus, despite the fact that the parasites are in different rhizocephalan families. Both are known from only a single natural host; both readily attach to these hosts in the laboratory; and both show a high degree of host specificity in the laboratory. Although the larvae of L. porcellanae readily attach to crabs congeneric, but allopatric with its natural host, they only occasionally attached to another porcellanid offered, and did not attach at all, even in no-choice experiments, to any of seven non-porcellanid crustacean species offered (Ritchie and
¨
Høeg, 1981; Høeg and Lutzen, 1995).
Because we confined the targets to small beakers and mesh bags, restricting their freedom of movement, we cannot comment on the hypothesis that host behavior affects infection rate. However, our results do appear to support the hypothesis that specificity could involve physiological incompatibility. Although differences in infection rates, as indicated by the genetic screening, did not differ significantly between C. maenas and the other species tested, experienced observers saw no evidence of interna in either of the infected O. australiensis. This contrasts strongly with observations for C. maenas, in which all infected specimens examined morphologically clearly had developing interna. This difference is consistent with the weak genetic signal of the parasite in O. australiensis, and implies an infection that was much less further developed than in the natural host, and may even have stopped altogether. The genetic test is sensitive enough that even dead remnants of the parasite would have been detected. Physiological defenses are also suggested by the overall low rate of infection among crabs exposed in the laboratory (25%), despite often high attachment levels.
The results have two implications for the use of S. carcini as a biological control agent against C. maenas in Australia. First, they indicate strongly that, given the opportunity, S. carcini cyprids will attach to a range of Australian hosts, including at least some non-portunids (i.e. Paragrapsus gaimardii ). However, second, limited and possibly incomplete development of the interna in species other than C. maenas suggests a specificity mechanism that could render it non-viable in anything other than the target species. Our experiments indicate that safety tests for potential native Australian hosts need to move to this level.
studied in the rhizocephalan except under very artificial and constrained conditions. As a result, designing more natural experiments for the marine parasite will take longer and is more likely to involve trial and error than would be the case for a terrestrial parasitoid. And third, uncertainties about larval dispersal in the ocean and social sensitivities about environmental impacts of escaped larvae or non-native hosts make it very difficult to design and have approved field enclosure experiments in a marine environment. In contrast, the technologies for safe confinement in terrestrial environments are much better developed and more widely acceptable. For these reasons, the emphasis for marine biological control agents will need to be on developing convincing safety tests in the laboratory. For species like S. carcini, this will require a much better understanding of parasite behavior and physiology than we currently have.
Acknowledgements
We thank Craig Proctor for collecting and shipping to us the Australian crabs used in the Swedish trials, the family Royson for providing the Swedish crabs used in the
¨
experiments, J. Lutzen for his assistance in examining infected crabs for interna, A. Kuris, G. Ruiz and the participants in the 1997 workshop on Carcinus management for valuable discussions regarding the project and its results, and A. Kuris and two anonymous reviewers for commenting on the manuscript. We also thank the Kristineberg Marine Biological station for providing the facilities in which the work was conducted. This project was supported in part by a grant from the Australian Department of Industry, Science and Technology. [SS]
References
Cohen, A.N., Carlton, J.T., Fountain, M.C., 1995. Introduction, dispersal, and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar. Biol. 122, 225–238.
Committee on Ships’ Ballast Water, 1996. Stemming the Tide. National Acad. Press, Washington, DC. Delage, Y., 1884. Evolution de la Sacculine (Sacculina carcini Thomps.). Crustace endoparasite de l’ordre
nouveau des Kentrogonides. Arch. Zool. Exp. Gen. 2, 417–736.
Glenner, H., Høeg, J.T., Klysner, A., Brodin Larsen, B., 1989. Cypris ultrastructure, metamorphosis and sex in seven families of parasitic barnacles (Crustacea: Cirripedia: Rhizocephala). Acta Zool. (Stockholm) 70, 229–242.
Glenner, H., Werner, M., 1998. Increased susceptibility of recently moulted Carcinus maenas (L.) to attack by the parasitic barnacle Sacculina carcini Thompson 1836. J. Exp. Mar. Biol. Ecol. 228, 29–33.
Goggin, C.L., 1997. Parasites (excluding Sacculina) which could regulate populations of the European green crab Carcinus maenas. In: Thresher, R.E. (Ed.), Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European crab, Carcinus maenas. Centre for Research on Introduced Marine Pests Tech. Rpt. (11), pp. 87–91.
Grewe, P.M., Krueger, C.C., Aquadro, C.F., Bermingham, E., Kincaid, H.L., May, B., 1993. Mitochondrial DNA variation among lake trout (Salvelinus namaycush) strains stocked into Lake Ontario. Can. J. Fish. Aquat. Sci. 50, 2379–2403.
Grosholz, E.D., Ruiz, G.M., 1996. Predicting the impact of introduced marine species: lessons from the multiple invasions of the European green crab. Biol. Cons. 78, 59–66.
Grosholz, E.D., Ruiz, G.M., Dean, C.D., Shirley, K.A., Maron, J.L., Connors, P.G., 2000. The impacts of a nonindigenous marine predator on multiple trophic levels. Ecol. 81, 1206–1224.
Hillis, D.M., Dixon, M.T., 1991. Ribosomal DNA molecular evolution and phylogenetic inference. Quart. Rev. Mar. Biol. 66, 411–453.
Høeg, J.T., 1984. Size and settling behaviour in male and female cypris larvae of the parasitic barnacle Sacculina carcini Thompson (Crustacea: Cirripedia: Rhizocephala). J. Exp. Mar. Biol. Ecol. 76, 145–156. Høeg, J.T., 1987. Male cypris metamorphosis and a new male larval form, the trichogon, in the parasitic barnacle Sacculina carcini (Crustacea: Cirripedia: Rhizocephala). Phil. Trans. R. Soc. Lond. 317B, 47–63. Høeg, J.T., 1995. The biology and life cycle of the Rhizocephala (Cirripedia). J. Mar. Biol. Assoc. UK 75,
517–550. ¨
Høeg, J.T., Lutzen, J., 1995. Life cycle and reproduction in the Cirripedia Rhizocephala. Ann. Rev. Oceanog. Mar. Biol. 33, 427–485.
Janzen, D., 1998. Gardenification of wildland nature and the human footprint. Science 279, 1312–1313. Kaufman, W.C., Nechol, J.E. (Eds.), 1992. Selection Criteria and Ecological Consequences of Importing
Natural Enemies. Entomological Soc. America, Lanham, MD.
Kuris, A.M., 1997. Conceptual framework for biocontrol of introduced marine pests. In: Thresher, R.E. (Ed.), Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European crab, Carcinus maenas. Centre for Research on Introduced Marine Pests Tech. Rpt. (11), pp. 66–68.
Lafferty, K.D., Kuris, A.M., 1996. Biological control of marine pests. Ecology 77, 1989–2000. ¨
Lutzen, J., 1984. Growth, reproduction and life span in Sacculina carcini Thompson (Cirripedia: Rhizocephala) in the Isefjord, Denmark. Sarsia 69, 91–106.
Murphy, N.E., Goggin, C.L., 2000. Genetic discrimination of sacculinid parasites (Cirripedia: Rhizocephala): implication for control of introduced green crabs. J. Crust. Biol. 20, 153–157.
Ritchie, L.A., Høeg, J.T., 1981. The life history of Lernaeodiscus porcellanae (Cirripedia: Rhizocephala) and its co-evolution with its porcellanid host. J. Crust. Biol. 1, 334–347.
Ruiz, G.M., Fofonoff, P., Hines, A., Grosholz, E.D., 1999. Non-indigenous species as stressors in estuarine and marine communities: assessing invasion impacts and interactions. Limnol. Oceanogr. 44, 950–972. Shiganova, T.A., 1998. Invasion of the Black sea by the ctenophore Mnemiopsis leidyi and recent changes in
pelagic community structure. Fish. Oceanogr. 7, 305–310.
Smith, O.R., Baptist, J.P., Chin, E. 1955. Experimental farming of the soft-shell clam, Mya arenaria, in Massachusetts, 1949–1953. Comm. Fish. Rev., 1–16.
Sokal, R.R., Rohlf, F.J., 1981. Biometry. Freeman, San Francisco.
Stephenson, W., Hudson, J.J., Campbell, B.B., 1957. The Australian portunids (Crustacea: Potunidae) II. The genus Charybdis. Aust. J. Mar. Freshwater Res. 8, 491–507.
Strathmann, M.F., 1987. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast. Data and Methods For the Study of Eggs, Embryos and Larvae. Univ. Wash. Press, Seattle. Thresher, R.E. (Ed.), 1997. Proceedings of the First International Workshop on the Demography, Impacts and
Management of Introduced Populations of the European crab, Carcinus maenas. Centre for Research on Introduced Marine Pests Tech. Rpt. (11).
Van Driesche, R.G., Bellows, Jr. T.S., 1996. Biological Control. Chapman and Hall, New York.
Walker, G., 1985. The cypris larvae of Sacculina carcini Thompson (Crustacea: Cirripedia: Rhizocephala). J. Exp. Mar. Biol. Ecol. 93, 131–145.
Walker, G., 1999. The cypris larvae of the rhizocephalan barnacle Heterosaccus lunatus with particular reference to antennular morphology. Acta Zool. 80, 209–217.