A model for molecular mechanisms of synaptic competition
for a finite resource
Hiroshi Okamoto *, Kazuhisa Ichikawa
Corporate Research Labs.,Fuji Xerox Co.Ltd.,430Sakai,Nakai-machi,Ashigarakami-gun,Kanagawa259-0157,Japan
Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) undergoes Ca2+/calmodulin-dependent autophosphoryla-tion of threonine-286/287 (Thr286/287). Extremely high concentration of CaMKII in the postsynaptic spine indicates
that increase in the Ca2+concentration in the spine induced by synaptic activation results in Thr286/287
autophospho-rylation of this enzyme. It has recently been shown that theKdvalue of CaMKII for Ca2+/calmodulin (Ca2+/CaM)
drastically decreases after Thr286/287autophosphorylation. Therefore, Ca2+/CaM associated with CaMKII becomes tightly bound to this kinase after Thr286/287 autophosphorylation. This has been called ‘Ca2+/CaM trapping’. We discussed the functional significance of Ca2+/CaM trapping in the neuronal system by a mathematical-modelling approach. We considered neighbouring synapses formed on the same dendrite and different increase in the Ca2+ concentration in each spine. CaMKII undergoing Thr286/287autophosphorylation in each spine is eager to recruit
nearby calmodulin in the dendrite for Ca2+/CaM trapping. Since the amount of calmodulin is limited, recruiting calmodulin to each spine causes competition among synapses for this finite resource. The results of our computer simulation show that this competition leads to ‘winner-take-all’: almost all calmodulin is taken by a few synapses to which relatively large increases in the Ca2+concentration are assigned. These results suggest a novel form of synaptic encoding of information. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ca2+/calmodulin-dependent protein kinase II; Thr286/287autophosphorylation; Calmodulin trapping; Synaptic
competi-tion; Winner-take-all
www.elsevier.com/locate/biosystems
1. Introduction
Ca2+/calmodulin-dependent protein kinase II
(CaMKII) is ubiquitously distributed in all cell types and participates in a variety of cellular processes triggered by Ca2+ signalling (for
re-view, see Hanson and Schulman, 1992; Soderling,
1993; Braun and Schulman, 1995). In particular, it is noteworthy that the CaMKII concentration is extremely high in the postsynaptic spine compart-ment. This suggests crucial involvement of the enzyme in synaptic regulation. Indeed, there have been accumulating lines of evidence indicating that CaMKII plays essential roles in several forms of synaptic plasticity (Kennedy, 1994; Lisman, 1994; Stevens et al., 1994).
CaMKII is a multimeric enzyme consisting of eight to 12 almost identical subunits. In the
ab-* Corresponding author. Tel.: +81-465-802015.
E-mail address: [email protected] (H. Okamoto)
sence of Ca2+ and calmodulin complex (Ca2+ /
CaM), each CaMKII subunit is inactive. The subunit acquires Ca2+
/CaM-dependent activity when Ca2+/CaM associates with this subunit. In
the presence of associating Ca2+/CaM, the
sub-unit undergoes autophosphorylation at threonine-286/287 (Thr286/287) of the a/b subunit, which endows the enzyme with Ca2+/CaM-independent
activity. Thus, the Thr286/287-phosphorylated sub-unit remains active even if Ca2+/CaM dissociates.
It has recently been verified that autophosphory-lation of Thr286/287
is an intersubunit reaction (Hanson et al., 1994; Mukherji and Soderling, 1994). This implies that Thr286/287
autophosphory-lation is an autocatalytic process (Okamoto and Ichikawa, 2000).
Besides generation of Ca2+
/CaM-independent activity, Thr286/287 autophosphorylation has an additional effect (Meyer et al., 1992; Putkey and Waxham, 1996). Among Ca2+/CaM binding
proteins, theKdvalue of (Thr286/287 -dephosphory-lated) CaMKII for Ca2+/CaM is rather small.
However,Kd, or, more precisely, the off rate koff decreases more than a thousand fold after Thr286/287 autophosphorylation. Hence Ca2+/CaM
associ-ated with CaMKII subunit becomes tightly bound to this subunit if it is phosphorylated at Thr286/287. This is called ‘Ca2+
/CaM trapping’. The functional significance of Ca2+
/CaM trap-ping in intracellular processes still remains to be elucidated. Several authors have proposed that Ca2+/CaM trapping endows CaMKII with
capa-bility of frequency detection of Ca2+ oscillations
(Hanson et al., 1994; De Koninck and Schulman, 1998; Dupont and Goldbeter, 1998). This func-tion may be attributed to those played within a
single cellular compartment such as a postsynap-tic spine. The present study has been devoted to another possibility. We have show that Ca2+
/
CaM trapping is likely to be participating also in a function played across multiple compartments.
2. Theory
2.1. Model
We considered neighbouring synapses formed on the same dendrite (Fig. 1). In each spine, the following biochemical reactions were assumed: Ca2+
/CaM-dependent Thr286/287 autophosphory-lation of CaMKII followed by Ca2+
/CaM trap-ping versus dephosphorylation of the enzyme followed by Ca2+/CaM dissociation. We further
assumed diffusional transportation of free calmodulin among the spines via the dendrite (Fig. 1).
We considered the situation that each synapse was simultaneously stimulated for a certain dura-tion. In the hippocampal CA1 region, synaptic stimulation results in activation of postsynaptic NMDA receptors (if postsynaptic membrane is sufficiently depolarized). The NMDA receptor ac-tivation causes postsynaptic increase in the Ca2+
concentration, which is necessary for the induc-tion of long-term potentiainduc-tion in the hippocampal CA1 region, the most extensively studied form of synaptic plasticity thought to be underlying lean-ing and memory in the brain (Bliss and Collingridge, 1993). With this evidence in mind, we assumed Ca2+ increase in each spine during
the synaptic stimulation.
Increase in Ca2+ in each spine promotes Ca2+/
CaM-dependent Thr286/287 autophosphorylation of CaMKII, which is accompanied by Ca2+/CaM
trapping to this enzyme. Since Thr286/287 au-tophosphorylation is autocatalytic (Okamoto and Ichikawa, 2000), Ca2+
/CaM trapping to a por-tion of CaMKII in each spine promotes further Ca2+
/CaM trapping. Thus, recruiting nearby calmodulin from the dendrite to each spine for Ca2+/CaM trapping tends to proceed in a
feed-forward way. However, the amount of calmodulin in a cell is limited. Hence recruiting calmodulin to
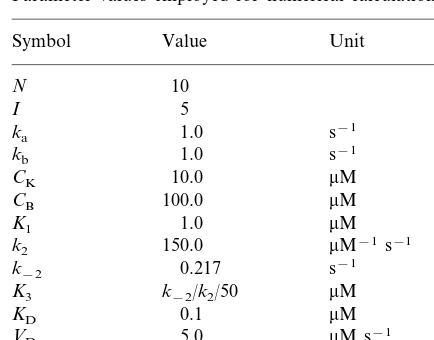
Table 1
Parameter values employed for numerical calculations
Symbol Value Unit
the concentration of X in the [X]d
dendritic shaft
the total concentration of
CCaM
calmodulin
CK the total concentration of the CaMKII holoenzyme
CB the total concentration of B
I the number of neighbouring synapses
2.3. Reaction scheme
The calmodulin molecule becomes active if its four Ca2+-binding sites are fully occupied:
4Ca2++CaM K1 Ca2+/CaM (1)
The active calmodulin Ca2+
/CaM interacts with effector proteins, such as S and B:
Ca2+/CaM+S
It has long been known that autophosphoryla-tion of CaMKII is an intraholoenzyme reacautophosphoryla-tion. Hence Thr286/287
autophosphorylation of the CaMKII holoenzyme in the i-th spine can be described by one-step ascending processes
K(n)
Gi(n)
K(n+1) (n=0,…,N−1) (4)
where Gi(n) is the transition probability per unit
time fromnton+1. As mentioned in the preced-ing section, Thr286/287 autophosphorylation is an intersubunit reaction. It has also been shown that Ca2+/CaM binding to the ‘substrate’ subunit is
prerequisite for this intersubunit reaction (Hanson et al., 1994; Mukherji et al., 1994). Ca2+
/CaM bound to the ‘substrate’ subunit becomes ‘trapped’ after Thr286/287 autophosphorylation of this subunit. This means that the Thr286/287 -phos-phorylated subunit S* is necessarily bound by Ca2+/CaM. Therefore, either Ca2+/CaM-S or
Ca2+/CaM-S* can be the ‘kinase’ subunit for this
intersubunit reaction, whereas only Ca2+/CaM-S
each spine must result in some kind of competi-tion among the synapses for this finite resource. We investigated the consequence of such competi-tion by a mathematical-modelling approach.
2.2. Symbols
Symbols used in the present paper are listed as below.
Thr286/287-dephosphorylated S
subunit
S* Thr286/287-phosphorylated subunit
B Ca2+/CaM-binding protein
other than CaMKII
N the number of subunits in the CaMKII holoenzyme K(n) CaMKII holoenzyme
compris-ingn Thr286/287
/CaM CaM bound by four calcium ions
Ca2+
/CaM-X substance X binding Ca2+ /
CaM
the concentration of X in the [X]i
can be the ‘substrate’ subunit. Transition from n
ton+1 hence occurs through either of the follow-ing intersubunit reactions: Ca2+
/CaM-S phos-phorylates Thr286/287 of another Ca2+/CaM-S;
Ca2+/CaM-S* phosphorylates Thr286/287of Ca2+/
CaM-S. By simple statistical consideration (Okamoto and Ichikawa, 2000), we have
Gi(n)=kagi2(N−n)(N−n−1)+kbgin(N−n)
(5)
where
gi=
[Ca2+/CaM−S]
%N n=0
(N−n)[K(n)]i
(6)
Thr286/287 dephosphorylation of the CaMKII holoenzyme in the i-th spine can be described by one-step descending processes
K(n) Ri(n)
K(n−1) (n=1,…,N) (7)
where Ri(n) is the transition probability per unit
time from n to n−1 and defined in the same manner with Okamoto and Ichikawa (2000) as
Ri(n)=
nVD
KD+ %
N
n=0
n[K(n)]i
(8)
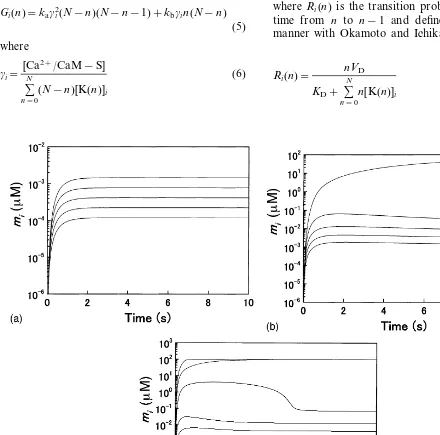
Fig. 2. Time courses of mi(i=1,…,5) numerically calculated for different CCaM’s. a, CCaM=10 mM; b, CCaM=30 mM; c,
CCaM=50 mM. In all calculations, the values for [Ca2+]i(I=1,…,5) are set as follows: [Ca2+]1=1.8 mM, [Ca2+]2=1.7 mM, [Ca2+]
Fig. 3. Time course of mi(i=1,…,5) calculated for CCaM=
and Ei represents the total concentration of
non-trapped calmodulin in the i-th spine:
Ei=[Ca2+/CaM-S]i+[Ca2+/CaM-B]i B]i, whose dynamical equations are absent, are
algebraically determined by [K(n)]i (n=0,…,N),
ical variable but as an external force which drives Ca2+
/CaM-dependent Thr286/287 autophosphory-lation in the i-th spine. We considered the situa-tion that each synapse was activated by different stimulus intensity. This was represented by differ-ent Ca2+ concentration at each synapse. For
simplicity, we assumed that [Ca2+]
1, [Ca2+]2, … and [Ca2+]
Iwere kept constant with time during
the synaptic stimulation. We focused upon the dynamical behaviour of the model during the synaptic stimulation and examined the conse-quences that would result from differences in the values for [Ca2+
2.4. Mathematical formulation of the model
The mathematical formulation of the model is given by the following set of equations describing the time course of the enzyme concentrations in the spines and dendrite:
trapping in each spine was calculated as an out-put. It is appropriate to quantify the degree of Ca2+
/CaM trapping in the i-th spine by mi, the
concentration of Thr286/287 phosphorylated sub-unit in the i-th spine defined by
mi=%
N
n
n[K(n)]i (21)
The time course ofmiwas examined by
numer-ically solving Eqs. (9) – (12). The parameter values fixed for all calculations done in the present study are listed in Table 1. The values for CCaM and [Ca2+
present in this table are mentioned in the text below.
In the first set of calculations, the value for
CCaMwas varied while those for [Ca 2+] competition among synapses resulting in striking phenomena which would most appropriately be expressed by the metaphor ‘winner-take-all’ (Fig. 2B): high level of Ca2+/CaM-trapping was
achieved only in the spine at which the largest increase in the Ca2+
concentration was assigned (Fig. 2B). Calmodulin is the resource for which synapses compete. Hence we also examined how the results of the competition were altered if this resource was increased or reduced. If CCaM, the total concentration of calmodulin, was increased to 50mM, the number of winners was increased to two (Fig. 2C). In contrast, ifCCaMwas reduced to
10 mM, there were no longer winners (Fig. 2A).
Next we confirmed that the fate of each synapse, whether it would become a winner or a loser, was determined not by the absolute value of the Ca2+ concentration assigned to this synapse
but by its relative value. Fig. 3 shows the results for [Ca2+]
1=1.9 mM, [Ca2+]2=1.8 mM, [Ca2+ ]3=1.7 mM, [Ca2+]4=1.6 mM and [Ca2+]5= 1.5 mM. For CCaM, we set the same value with Fig. 2B. In Fig. 2B, the synapse with the Ca2+
concentration of 1.8 mM is the winner because the largest Ca2+ concentration is assigned to
this synapse. In Fig. 3, in contrast, the synapse with the same Ca2+
concentration is a loser because there is now a stronger competitor (i.e. the synapse with the Ca2+ concentration of 1.9
mM).
4. Discussion
We examined competition among neighbouring synapses formed on the same dendrite for calmod-ulin as a finite resource (Fig. 1). The results of investigation by mathematical modelling show that this competition leads to ‘winner-take-all’ (Fig. 2B). The number of winners increases as the amount of the resource is increased (Fig. 2). The fate of each synapse, whether it becomes a winner or a loser, is determined not by the absolute value of the Ca2+ concentration assigned to this
synapse by its relative value (Fig. 2B and Fig. 3). The results obtained in the present study sug-gest a novel form of synaptic encoding of infor-mation. LetSibe a graded number assigned to the i-th synapse. Permute S1, S2, … and SI in
de-scending order: Sp1\Sp2\···\SpI. LetOi
repre-sent ‘all-or-none’; i.e. Oi=1 or 0. Through
competition among synapses for a finite resource,
S1,S2, … andSIare converted to O1,O2, … and
OI in such a way that: Opi=1 for pi5pw and
Opi=0 forpi\pw(Fig. 4). As the amount of the resource is reduced, pw, the number of winners, decreases.
For modelling development of a receptive field of a single cell, it has long been found necessary to add mechanisms of competition among synapses to the simple correlation-based Hebbian process (Miller, 1996). The findings of the present
study provide a possible explanation for a molec-ular – mechanical basis of such synaptic competition.
References
Bliss, T.V.P., Collingridge, G.L., 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Na-ture 361, 31 – 39.
Braun, A.P., Schulman, H., 1995. The multifunctional cal-cium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 57, 417 – 445.
Dupont, G., Goldbeter, A., 1998. CaM kinase II as frequency decoder of Ca2+ oscillations. Bioessays 20, 607 – 610. Hanson, P.I., Meyer, T., Stryer, L., Schulman, H., 1994. Dual
role of calmodulin in autophosphorylation of multifunc-tional CaM kinase may underlie decoding of calcium sig-nals. Neuron 12, 943 – 956.
Hanson, P.I., Schulman, H., 1992. Neuronal Ca2+/ calmod-ulin-dependent protein kinases. Annu. Rev. Biochem. 61, 559 – 601.
Kennedy, M.B., 1994. The biochemistry of synaptic regulation in the central nervous system. Annu. Rev. Biochem. 63, 571 – 600.
De Koninck, P., Schulman, H., 1998. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227 – 230.
Lisman, J., 1994. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 17, 406 – 412.
Meyer, T., Hanson, P.I., Stryer, L., Schulman, H., 1992. Calmodulin trapping by calcium – calmodulin-dependent protein kinase. Science 256, 1199 – 1202.
Miller, K.D., 1996. Synaptic economics: competition and co-operation in synaptic plasticity. Neuron 17, 371 – 374. Mukherji, S., Brickey, D.A., Soderling, T.R., 1994. Mutational
analysis of secondary structure in the autoinhibitory and autophosphorylation domains of calmodulin kinase II. J. Biol. Chem. 269, 20733 – 20738.
Mukherji, S., Soderling, T.R., 1994. Regulation of Ca2+/ calmodulin-dependent protein kinase II by inter- and intra-subunit-catalyzed autophosphorylations. J. Biol. Chem. 269, 13744 – 13747.
Okamoto, H., Ichikawa, K., 2000. Switching characteristics of a model for biochemical-reaction networks describing au-tophosphorylation versus dephosphorylation of Ca2+/ calmodulin-dependent protein kinase II. Biol. Cybern. 82, 35 – 47.
Putkey, J.A., Waxham, M.N., 1996. A peptide model for calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 271, 29619 – 29623. Soderling, T.R., 1993. Calcium/calmodulin-dependent protein
kinase II: role in learning and memory. Mol. Cell. Biochem. 127/128, 93 – 101.
Stevens, C.F., Tonegawa, S., Wang, Y., 1994. The role of calcium – calmodulin kinase II in three forms of synaptic plasticity. Curr. Biol. 4, 687 – 693.
![Fig. 3. Time course of m]i(i=1,…,5) calculated for CCaM=30 �M and [Ca2+]1=1.9 �M, [Ca2+]2=1.8 �M, [Ca2+3=1.7 �M, [Ca2+]4=1.6 �M and [Ca2+]5=1.5 �M.](https://thumb-ap.123doks.com/thumbv2/123dok/3142117.1383271/5.612.44.257.38.200/fig-time-course-calculated-ccam-ca-ca-ca.webp)