GFLV replication in electroporated grapevine protoplasts
Laure Valat
a,b, Sandrine Toutain
b, Nadine Courtois
b, Fabien Gaire
c, Eric Decout
a,
Lothaire Pinck
c, Marie-Claude Mauro
b, Monique Burrus
a,*
aLaboratoire de Biologie et Physiologie Ve´ge´tales(URVVC,UPRES EA2069),URCA,BP1039,F-51687Reims,Cedex2,France bLaboratoire de Viticulture,Moe¨t & Chandon,6rue Croix de Bussy,F-51333Epernay,France
cInstitut de Biologie Mole´culaire des Plantes du C.N.R.S,12rue du Ge´ne´ral Zimmer,F-67084Strasbourg,Cedex,France Received 9 August 1999; received in revised form 3 December 1999; accepted 8 February 2000
Abstract
Grape6ine fanleaf6irus(GFLV), responsible for the economically important court-noue´ disease, is exclusively transmitted to its
natural host in the vineyards through Xiphinema nematodes. We have developed direct inoculation of GFLV into grapevine through protoplast electroporation. Protoplasts were isolated from mesophyll of in vitro-grown plants and from embryogenic cell suspensions. Permeation conditions were determined by monitoring calcein uptake. Low salt poration medium was selected. Electrical conditions leading to strong transient gene expression were also tested for GFLV inoculation (isolate F13). GFLV replication was detected with either virus particles (2 mg) or viral RNA (10 ng) in both protoplast populations, as shown by anti-P38 Western blotting. Direct inoculation and replication were also observed with Arabis mosaic 6irus (ArMV), a closely
related nepovirus, as well as with another GFLV isolate. These results will be valuable in grapevine biotechnology, for GFLV replication studies, transgenic plant screening for GFLV resistance, and biorisk evaluation. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Grapevine; Electroporation;Grape6ine fanleaf 6irus; Protoplasts; Virus inoculation
1. Introduction
Grape6ine fanleaf 6irus (GFLV) is a
nematode-transmitted virus infecting grapevine and causing fanleaf degeneration, thus leading to dramatic de-generescence of the whole plant and to yield losses in grape production. Belonging to the genus Nepo6irus within the family Comoviridae [1],
GFLV is characterized by polyhedral particles and by a genome constituted by two single positive-sense RNAs. RNA1 of the F13 isolate encodes a 253 kDa polyprotein (P1) involved in viral replica-tion and in polyprotein processing [2]. RNA2 en-codes a 122 kDa polyprotein (P2), required for viral spread in planta, which is cleaved into a 28
kDa N-terminal protein called 2A, a 38 kDa movement protein referred as 2BMPor P38 protein,
and a 56 kDa carboxy-terminal coat protein (CP) called 2CCP [3]. GFLV can only be controlled
through plant sanitary selection and soil disinfec-tion. Most chemicals used to kill the nematodes, however, are noxious to the environment and their use has been limited in several countries. Different new methods like gene transfer strategy [4] are currently under investigation in order to control GFLV spread.
Since Powell-Abel et al. [5] demonstrated that tobacco plants expressing theTobacco mosaic6irus
CP gene were protected against this virus, incorpo-ration and expression of different CP genes have provided resistance in several different virus groups [6 – 11]. Bertioli et al. [12] and Brault et al. [13] reported for the first time such protection in * Corresponding author. Tel.: +913318; fax:
33-326-913427.
theNepo6irus group. More recently, GFLV
inocu-lation of transgenic tobacco expressing the GFLV CP gene pointed out a delay in tobacco infection [14], suggesting that this strategy could be useful for the reduction of GFLV spread in grapevine. Thus, this gene has been introduced into several rootstocks and cultivars [15 – 18]. Tests are under progress in greenhouses and in vineyards, in order to evaluate the efficiency of the CP-mediated pro-tection strategy for grapevine [19]. Plant screening however, is slowed down by the fact that GFLV inoculation to grapevine is difficult to master [20], as sit requires its biological vector, Xiphinema index, for efficient transmission [19].
Several authors have shown that viral resistance of transgenic plants is functional at the single cell level [21,22]. Electroporation of virus into plant protoplasts constitutes a useful tool to understand the mechanism of its replication [23,24] and of its inhibition in transgenic plants [25 – 27], as well as to verify plant tolerance towards virus infection [28 – 30]. In order to evaluate and to compare the efficiency of the CP transgene and of other antivi-ral strategies in grapevine, we have developed a technique for direct inoculation of grapevine with GFLV based on protoplast electroporation. So far, electroporation of grapevine protoplasts has been described by Kolavenko et al. [31] for tran-sient gene expression only. In a first set of experi-ments, permeation conditions were determined for the rootstock 41B. We, then, established optimal uptake conditions for GFLV and ArMV, a related virus also involved in the fanleaf disease, using either particles or viral RNA. This work is the first case of direct inoculation of grapevine with GFLV and ArMV.
2. Materials and methods
2.1. Virus, RNA and DNA
ArMV and GFLV (isolates F13 and GH) were routinely propagated on Chenopodium quinoa. Virus and RNA extractions and purifications have been described previously [32].
Plasmidic DNA was propagated in Escherichia coliHB 101-p35S, purified by the Qiafilter plasmid maxi kit method (Qiagen, Germany), and resus-pended in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.3) at 1 mg/ml.
2.2. Plant material
Rootstock 41B (Vitis6inifera cv. Chasselas×V.
berlandieri) colone no. 233 was used.
2.3. Protoplast isolation
Protoplasts were prepared either from in vitro propagated plants or from embryogenic cell sus-pensions [33].
Well expanded leaves were sliced in 0.5 M man-nitol, 5 mM KCl, 2 mM CaCl2· 2H2O, 0.4 mM
MgCl2· 6H2O, 0.3 mM 2-[N-morpholino]
ethane-sulfonic acid (MES), pH 5.7 (osmotic pressure, 540 mosm/kg) and digested in 2% cellulase from Trichoderma 6iride(1 U/mg, Fluka) and 1%
pecti-nase from Rhizopus sp. (5 U/g, Fluka) for 16 h at 23°C in the dark. Mesophyll protoplasts were isolated and purified according to Chupeau et al. [34], cultured in liquid NN69 medium [35] with 0.6 M glucose and 2.5 mM MES, pH 5.8, at 23°C in the dark.
Embryonic cells (4 – 7 day old) were digested in 1% cellulase from Trichoderma 6iride (1 U/mg,
Fluka), 1% pectinase from Rhizopus sp. (5 U/g, Fluka), 0.5% Driselase fromBasidiomycetesssp. (1 U/mg, Sigma) in CPW salts [36] with 0.4 M man-nitol, 5 mM MES for 16 h at 25°C (osmotic pressure, 450 mosm/kg). Purified protoplasts (see above) were cultured in half-strength MS salts [37], MS vitamins, 0.5 M mannitol, 50 mM su-crose, 50 mg/1 casamino acids, 5.4 mM naphtoxy-acetic acid (NOA), 2.5 mM MES, pH 5.8, at 23°C in the dark.
Protoplast viability was estimated by mixing 40
ml of protoplast suspension and 10 ml of Ery-throsin B (0.4% w/v) and observation under a light microscope. Results were given as a percentage of total number protoplasts. In order to evaluate the effects of electroporation conditions on protoplast survival, viability was first established in both the electroporated samples and the non-electroporated control as mentioned above, and then, survival rate was calculated as a percent of the non-electro-porated control. A minimum of two independent repetitions was conducted for viability estimation.
2.4. Protoplast transfection
pH 5.6 [38]; ‘High Salt’ medium (HS), 10 mM HEPES, 150 mM NaCl, 5 mM CaCl2· 2H2O, pH
7.1 [39], both solutions being adjusted to the proper osmotic pressure with mannitol. Freshly isolated protoplasts were diluted in prechilled electroporation solution, at 7.5×105 p/ml unless
otherwise mentioned. Electroporation was per-formed with the Bio-Rad Gene Pulser II appara-tus set at different voltages and capacitances, using 400 ml of protoplasts and 400 ml of electro-poration mix per cuvette (final protoplast con-centration for electroporation, 3.75×105 per ml).
All electroporated samples were kept on ice for a further 30-min incubation and finally diluted in 2.5-ml culture medium. Negative controls con-sisted of completed samples that were not elec-troporated.
2.4.1. Calcein uptake
Protoplast suspension (400 ml) was mixed to 400 ml of 5 mM calcein in electroporation solu-tion and kept for 5 min on ice. After electropo-ration and the 30 min incubation, samples were rinsed twice in 14 mM CaCl2· 2H2O, 4 mM
MES, pH 5.7 adjusted with KCl to the proper osmotic pressure, and centrifuged 5 min at 100×g, and immediately observed under UV light. The frequency of permeation was deter-mined by counting the green fluorescent proto-plasts out of a minimum of 100 intact protoplasts. Only protoplasts exhibiting a bright intense fluorescence distributed throughout the cell were taken in account. A minimum of two independent repetitions was done for this study.
2.4.2. Plasmid DNA uptake
Two different plasmids were used, pVT-GUS containing the GUS gene under control of the enhanced CaMV ‘70S’ promoter, the non-trans-lated leader sequence of TMV and the NOS ter-minator [40]; pCK-GFP-S56C harboring the improved GFP S65C coding sequence under con-trol of the enhanced CaMV ‘70S’ promoter, the non-translated leader sequence of TMV and the CaMV 35S terminator [41].
Plasmid (20 mg), 30 mg of salmon sperm DNA as carrier were successively added to 400 ml of prechilled electroporation buffer and carefully mixed. Finally, 400 ml of protoplast suspension were added and a further 5 min incubation was done before electroporation.
Transient gene expression was analysed in a minimum of two independent repetitions. GUS fluorimetric assay was done 48 h after electropo-ration [42] with total protein extracts (5 mg of proteins per assay). GFP analysis was performed 72 h after electroporation: samples were ob-served under blue light (450 – 490 nm). The fre-quency of transient expression was determined by counting the green fluorescent protoplasts out of a minimum of 100 intact protoplasts, in at least four repetitions.
2.4.3. Virus and RNA inoculation
Protoplasts (400 ml) at different densities were carefully mixed with 400 ml of electroporation buffer containing 2 mg of virions, unless other-wise mentioned, or 10 mg of viral RNA. After electroporation and incubation on ice, proto-plasts were diluted in their respective culture medium and incubated as described above, for 72 h, unless otherwise specified, before im-munoblotting. Experiments were repeated at least three times.
2.5. Western blotting
Protoplasts were harvested and centrifuged 3 min at 100×g. The pellet was resuspended in 15
ml of loading buffer (2% SDS, 0.025% Bro-mophenol Blue, 10% glycerol, 0.06 M Tris – HCl, pH 6.8). Proteins were denatured at 100°C for 5 min. Total proteins from 3×105 protoplasts
3. Results and discussion
3.1. Grape6ine protoplast characteristics
The average diameter of leaf protoplasts freshly isolated from 2-month-old plants was 20910 mm. Viability was estimated to 7796% and protoplast yield was between 50 and 60×106 per g of fresh
leaves. After 6 days of culture, protoplast viability was, on average, 6594.5% (mean of five different experiments).
Embryogenic cell suspension protoplasts were smaller (592 mm) and they contained lots of starch grains. Protoplast yield was between 22 and 30×106 per ml packed cell volume and their
viability after isolation was estimated to 8496% (mean of four independent replicates).
3.2. Setting optimal permeation conditions
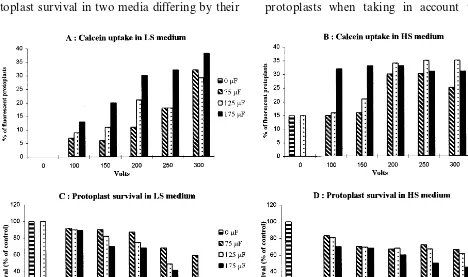
Grapevine protoplast electroporation was stud-ied using a two-step approach. First, we selected appropriate conditions for plasma membrane per-meabilization by monitoring calcein uptake and protoplast survival in two media differing by their
ionic strength. Indeed, chemical properties of the poration medium are known to affect electrical membrane breakdown [45]. Second, within these experimental conditions, we selected those yielding the highest levels of transient gene expression after plasmid uptake.
When considering leaf protoplasts, without elec-troporation, in LS medium (Fig. 1A), no fluores-cence was observed, whereas in HS medium (Fig. 1B), 15% of the protoplasts contained calcein, due to natural passive uptake. When applying an elec-trical field, fluorescence increased with both the voltage and the capacitance (Fig. 1A and B). In LS medium, maximal calcein uptake was observed at 300 V – 175 mF, 38% of the intact protoplasts were bright fluorescent. Not every protoplast of the population, however, was transfected under these electrical conditions as protoplasts varied in size and shape, as well as in the conductive proper-ties of cellular components (data not shown). Sur-vival after treatment at 300 V – 175 mF was about 1/4 of the non-electroporated control (Fig. 1C). In HS medium, best permeation conditions were 250 or 300 V – 125 mF, with 20% electropermeabilized protoplasts when taking in account the strong
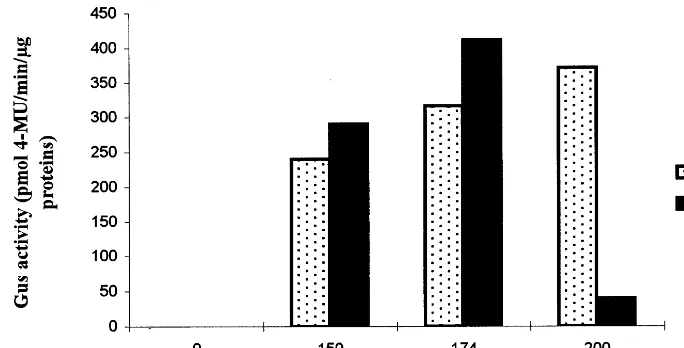
Fig. 2. GUS activity in leaf protoplasts electroporated with 20mg of pVT-GUS plasmid and 30mg of salmon sperm DNA in LS medium. GUS fluorimetric assay was done 48 h after electroporation, using 5mg of total proteins. Substrate for glucuronidase 4-MU, 4 methylumbelliferylb-D-glucuronide.
fluorescent background of the non-electroporated controls. Survival was above 65% of the negative controls (Fig. 1D).
With embryogenic cell suspension protoplasts, best electroporation conditions were the same for both media: 200 V – 350 mF, yielding 50% of to-tally fluorescent protoplasts in LS medium and 37% in HS medium. No passive calcein uptake was observed.
Because of the less permeation efficiency of HS medium, further study was conducted with LS medium only. It is characterized by low salt con-tent, and thus higher resistivity. It is less concen-trated in salts than the pulse medium used by Kovalenko et al. [31] which contained 20 mM KCl and 6 mM MgCl2 in 0.6 M sorbitol, pH 6.0.
Furthermore, their electroporation apparatus was different and multiple pulses were applied to the samples, making direct comparisons difficult.
We, then, evaluated transient gene expression in both protoplast populations electroporated with plasmidic DNA. We selected, from the previous study, a range of experimental conditions combining good levels of protoplast permeation (ca. 30%) and moderate mortality (ca. 50% sur-vival), both compatible with gene expression: for leaf protoplasts, one pulse at 150, 174 and 200 V – 150 or 175 mF; for embryogenic protoplasts, one pulse at 200 V – 100 or 150 mF.
In leaf protoplasts, study of plasmid uptake confirmed that cell viability after electric treatment under most conditions was sufficient for gene ex-pression. In all experiments done with pVT-GUS
plasmid, GUS activity increased with both the voltage and the capacitance as shown on Fig. 2, excepted at 200 V – 175 mF. Highest GUS activity was observed at 174 V – 175 mF and 200 V – 150
mF. Using the GFP gene, we showed that 72 h after electroporation at 174 V – 175 mF, 1693.5% of the remaining protoplasts expressed the GFP gene intensively, and 18.593% at 200 V – 150 mF. GFP activity was also observed in both electri-cal conditions selected for embryogenic cell sus-pension protoplasts, with 1492% fluorescent protoplasts. Protoplast viability was estimated to 6098% of the intact protoplasts.
3.3. Inoculation with GFLV particles
Analysis of transient protein expression indi-cated that, for several electrical conditions, mem-brane breakdown was sufficient for plasmid uptake without irreversibly altering protoplast sur-vival and protein synthesis. Similar study was then conducted with GFLV particles, in order to evalu-ate optimal conditions of virus inoculation and multiplication.
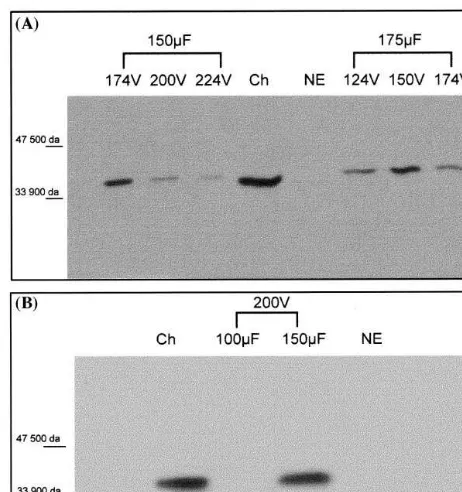
visible in five out of six electrical conditions tested, at about 38 kDa. Because no protein re-acted in the negative control, the product de-tected after electroporation indicates that virus penetration and replication had occurred. Proto-plast viability estimated 72 h after GFLV inocu-lation i.e. 4098% of the remaining protoplasts after electroporation at 174 V – 175mF, was suffi-cient to allow virus multiplication. For further work, we selected two conditions for which a significant amount of P38 protein was detected in the protoplast extracts, 274 V – 175 mF and 200 V – 150 mF. This is the first time that a Nepo6irus is successfully inoculated to grapevine
through electroporation. This experiment also
in-dicates that, in grapevine protoplasts, conditions for plasmid and viral particle uptake are almost similar, but not identical, thus justifying the pro-gressive approach of our study. This shift which remained consistent over the repetitions should be explained by different physical characteristics (size, shape, electrical charges) of virus particles and plasmidic DNA molecules.
Next, we studied virus multiplication over the time, by assaying P38 accumulation in electropo-rated protoplasts after different periods of cul-ture, 24, 48, 72 h and 6 days. Virus multiplication could be occasionally detected as soon as 24 h after electroporation in both se-lected electrical conditions but, in general, a more intense and consistent reaction of the anti-body was visible after 48 or 72 h. Therefore, this later period of culture was preferred for further studies. Six days after electroporation, P38 could not be detected any more, probably because of the high mortality observed in the protoplast populations several days after virus inoculation. In Chenopodium protoplasts electroporated with GFLV virus, P38 concentration increased, in a similar way, from 18 h after electroporation until 96 h [43], and CP detectable after 24 h following electroporation also accumulated [38]. The effect of ‘protoplast quality’ had a strong effect on virus multiplication. Plants of different ages (30, 50, 60 and 90 days after the last subculture) were used as protoplast sources that were elec-troporated under normal conditions, 174 V – 175
mF and 200 V – 150 mF. We clearly observed an effect of the age of the mother-plants: in all cases, virus replication was detected but band intensity decreased with plant ageing, although viability of freshly isolated protoplasts before electroporation was not significantly affected. With 90-day-old plants, replication was barely observed. Plants not older than 2 months after subculture were most amenable for virus intro-duction and multiplication. Virus concentration has often been reported to be a limiting factor for virus replication in plant protoplasts. Nishiguchi et al. [46] reported a linear relation-ship between infection rate and virus concentra-tion up to 50 mg/ml. Okada et al. [23] noticed that concentrations of TMV particles needed for tobacco protoplast infection were very high (500
mg/ml), compared to those needed with RNA (10
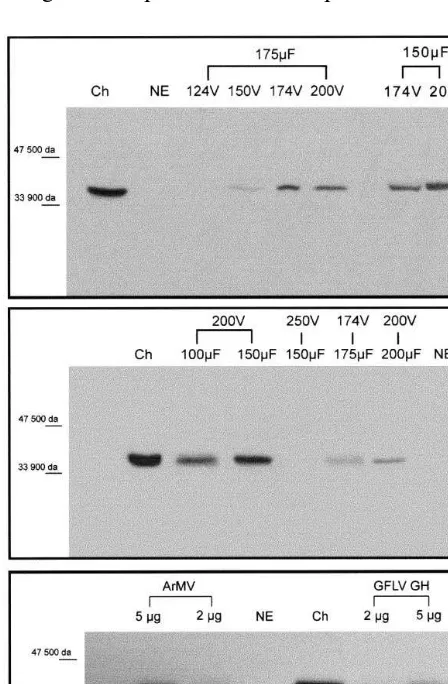
Fig. 4. Viral RNA inoculation and replication in electropo-rated grapevine protoplasts, effects of electrical parameters, (A) Leaf protoplasts (2 mg of virus particles); (B) Embryo-genic cell suspension protoplasts (2 mg of virus particles). Western blotting with anti P38 antibody, 72 h after electropo-ration; Ch, GFLV-infected leaves ofChenopodium; NE, non-electroporated protoplasts incubated in electroporation mix.
most conditions, but at 200 V – 100 and 150 mF, P38 detection was maximal. This condition was selected for further work.
3.4. Inoculation with ArMV and GFLV–GH particles
In order to verify that the protocol here devel-oped was not specific of the GFLV-F13 strain, we electroporated embryogenic cell suspension proto-plasts with 2 and 5mg of ArMV and of GH strain of GFLV, at 200 V – 150 mF. Western blot, done 72 h after electroporation with anti-P38 antibody as described above, is shown in Fig. 3C. Two bands were clearly visible in all four conditions, a major band at 38 kDa, also found in infected Chenopodium leaves, and corresponding to P38; a second smaller band probably corresponding to proteolytic processing of P38, as observed in other work [43]. These results show that the protocol set up in this paper is not specific of a given strain of GFLV.
3.5. Inoculation with 6iral RNA
Electroporation has been widely used to intro-duce viral RNA into protoplasts [47 – 51]. Nishiguchi et al. [46] however, showed that inocu-lation conditions suitable for RNA were different from those for viral particles. Based on these observations, best conditions for viral RNA up-take in leaf and cell suspension protoplasts were determined. Results concerning leaf protoplasts are shown in Fig. 4A. For all six electrical condi-tions examined, one band of 38 kDa was visible as in infected Chenopodium leaf extract, whereas no product was observed in the negative controls. The best signals, however, were obtained with 174 V – 150 mF and 150 V – 175 mF, which were then chosen for further work. These conditions are quite similar to those required for particle inocula-tion. This is quite different from the results of Nishiguchi et al. [46] which showed that a longer pulse was necessary at the same voltage to induce TMV particle entry in tobacco protoplasts, com-pared with RNA uptake.
For inoculation of cell suspension protoplasts with RNA, not all electrical conditions were suit-able as shown on Fig. 4B. With 10 ng of RNA in the electroporation mix, P38 protein was detected only at 200 V – 150mF but never at 200 V – 100mF.
mg/ml) We tested different amounts of GFLV viral particles: 0.5, 1, 2, and 5 mg. No P38 protein was detected with 0.5 mg of particles. With 1 mg, the corresponding band was clearly visible. Increasing virus concentration over 2 mg did not increase band intensity. Viral particles (2mg) were used for further analysis.
The effect of protoplast concentration on repli-cation and detection of P38 was studied, using the same preparation of protoplasts at different densi-ties, 3.75×105, 7.5×105 and 1.125×106
proto-plasts per ml, and 2 mg of GFLV particles. Our results indicated that the lower the concentration of protoplasts, the better the detection of P38 was on the Western blot. We selected 3.75×105
proto-plasts per ml for further work.
Concerning cell suspension protoplasts, differ-ent electrical conditions were tested for virus inoc-ulation, with 2 mg of particles and 7.5×105
4. Discussion
In this work, different molecules were introduced into grapevine protoplasts isolated from either mes-ophyll or embryogenic cell suspension; calcein, a non-permeant dye; plasmidic DNA leading to tran-sient gene expression; viral RNA and virus parti-cles. For the first time, direct inoculation and replication of two nepoviruses, GFLV and ArMV, were made possible into Vitis without the help of their biological vectors.
Direct inoculation of nepoviruses will constitute a new useful tool for grapevine technology. First, whereas until now, GFLV replication has been studied only in tobacco BY2 cells as an experimen-tal model [52], our results will extend these studies to its natural host, Vitis. Second, screening of transgenic grapevine plants for resistance to the fanleaf disease can be attempted at the cell level: protoplasts expressing diverse virus-derived se-quences or different gene copies will be challenged with the virus, replication studied, and, thus, effi-ciencies of the regenerants established. Such ap-proach has been successfully applied to tobacco and Medicago[28,29]. Comparison between viral parti-cles and RNA infection of transgenic plant material can contribute to a better understanding of GFLV resistance in transgenic cells, as already done by Carr et al. [53] withCucumber mosaic6irus. Finally,
protoplast inoculation with nepoviruses could be-come a useful way of evaluating biorisks, i.e. the possible transcapsidation or recombination events between genomic ArMV RNA and the GFLV – CP transgene expressed in grapevine plants. Indeed, as such experiments in the vineyards are difficult and require several years, the new tool developed in the present paper will bring quickly a first answer to these important questions.
Acknowledgements
We wish to thank Dr Bernard Walter (INRA Colmar, France) for providing us GFLV and ArMV, and Dr Gunther Hahne (IBMP, Stras-bourg, France) for reading the manuscript.
References
[1] F.A. Murphy, C.M. Fauquet, D.H.L. Bishop, S.A.
Ghabrial, G.P. Martelli, M.A. Mayo, M.D. Summers, Virus Taxonomy, Springer, Berlin, 1995.
[2] C. Ritzenthaler, M. Viry, M. Pinck, R. Margis, M. Fuchs, L. Pinck, Complete sequence and genetic organisation of grapevine fanleaf nepovirus RNA 1, J. Gen. Virol. 72 (1991) 2357 – 2365.
[3] M.A. Serghini, M. Fuchs, M. Pinck, J. Reinbolt, B. Walter, L. Pinck, RNA2 of grapevine fanleaf virus: sequence analysis and coat protein cistron location, J. Gen. Virol. 71 (1990) 1433 – 1441.
[4] B. Walter, Le transfert de ge`nes:un outil pour I’ ame´liora-tion de la vigne du IIIe´me mille´naire. Journal Internaame´liora-tional des Sciences de la Vigne et du Vin. Vigne et Vin Publica-tions Internationales, (1996) Martillac.
[5] P. Powell-Abel, R.S. Nelson, B. De, N. Hoffman, S.G. Rogers, R.T. Fraley, R.N. Beachy, Delay of disease development in transgenic plants that express theTobacco mosaic6irus coat protein gene, Science 232 (1986) 738 –
743.
[6] R.N. Beachy, Coat protein mediated resistance against virus infection, Annu. Rev. Phytopathol. 28 (1990) 451 – 474.
[7] T.M.A. Wilson, Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms, Proc. Natl. Acad. Sci. USA 90 (1993) 3134 – 3141.
[8] H. Barker, B. Reavy, K.D. Mc Geachy, High level of resistance in potato toPotato mop-top6irusinduced by
transformation with the coat protein gene, Eur. J. Plant Pathol. 104 (1998) 737 – 740.
[9] M. Ravelonandro, R. Scorza, J.C. Bachelier, G. Labonne, L. Levy, V. Damsteegt, A.M. Callahan, J. Dunez, Resis-tance of transgenic Prunus domesticato Plum pox 6irus
infection, Plant Dis. 81 (1997) 1231 – 1235.
[10] S. Lius, R.M. Manshardt, M.M.M. Fitch, J.L. Slightom, J.C. Sanford, D. Gonsalves, Pathogen-derived resistance provides papaya with effective protection againstPapaya ringspot6irus, Mol. Breeding 3 (1997) 161 – 168.
[11] G.H. Clought, P.B. Hamm, Coat protein transgenic resis-tance toWatermelon mosaicandZucchini yellows mosaic
6irusesin squash and cantaloupe, Plant Dis. 79 (11) (1995)
1107 – 1109.
[12] D.J. Bertioloi, J.I. Cooper, M.L. Edwards, W.S. Hawes,
Arabis mosaic nepo6iruscoat protein in transgenic tobacco
lessens disease severity and virus replication, Ann. Appl. Biol. 120 (1992) 47 – 54.
[13] V. Brault, T. Candresse, O. Le Gall, R.P. Delbos, M. Lanneau, J. Dunez, Genetically engineered resistance against Grape6ine chrome mosaic nepo6irus, Plant Mol.
Biol. 21 (1993) 89 – 97.
[14] N. Bardonnet, F. Hans, M.A. Serghini, L. Pinck, Protec-tion against virus infecProtec-tion in tobacco plants expressing the coat protein ofGrape6ine fanleaf nepo6irus, Plant Cell Rep.
13 (1994) 357 – 360.
[15] M.C. Mauro, S. Toutain, B. Walter, L. Pinck, L. Otten, P. Coutos Thevenot, A. Deloire, P. Barbier, High effi-ciency regeneration of grapevine plants transformed with the GFLV coat protein gene, Plant Sci. 112 (1995) 97 – 106. [16] S. Krastanova, M. Perrin, P. Barbier, G. Demangeat, P. Cornuet, N. Bardonnet, L. Otten, B. Walter, Transforma-tion of grapevine rootstocks with the coat protein gene of
Grape6ine fanleaf nepo6irus, Plant Cell Rep. 14 (1995)
[17] B. Xue, S. Krastanova, S.V. Ling, M.E. Sekiya, H.Y. Zhu, N. Petrovic, C.L. Reid, I. Velazquez, T.J. Burr, D. Gonsalves, Transformation of grapevine rootstocks con-taining genes fromGrape6ine fanleaf6irusandGrape6ine
leafroll associated clostero6irus 2 and 3, in: Extended
Abstract of the 12th Meeting of the ICVG, 28 Septem-ber – 2 OctoSeptem-ber 1997, Lisbon, Portugal, p. 137.
[18] R. Golles, A. De Camara Machado, A Minafra, R. Moser, H. Katinger, M. Laimer De Camara Machado, Regeneration of Vitis sp. transformed with the coat protein gene sequences of four different grapevine viruses, in: Extended Abstract of the 12th Meeting of the ICVG, 28 September – 2 October 1997, Lisbon, Portugal, pp. 139 – 140.
[19] P. Barbier, G. Demangeat, M. Perrin, P. Cobanov, C. Jacquet, B. Walter, Grapevine genetically transformed with the coat protein gene ofGrape6ine fanleaf6irus: an
analysis of transformants, in: Extended Abstract of the 12th Meeting of the ICVG, 28 September – 2 October 1997, Lisbon, Portugal, pp. 131 – 132.
[20] M.F. Nysterakis, Sur quelques tentatives de communi-quer a` des vignes saines l’agent pathoge´ne du court-noue´ contagieux, Bull. O.I.V. 20 (1947) 9 – 13.
[21] J.C. Register III, R.N. Beachy, Resistance to TMV in transgenic plants results from interference with an early event in infection, Virology 166 (1988) 524 – 532. [22] J.A. Lindbo, L. Silva-Rosales, W.M. Proebsting, W.G.
Dougherty, Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance, Plant Cell 5 (1993) 1749 – 1759.
[23] K. Okada, T. Nagata, I. Takebe, Expression and integra-tion of genes introduced into highly synchronized plant protoplasts, Plant Cell Physiol. 27 (1986) 619 – 626. [24] J.W. Watts, J.M. King, N.J. Stacey, Inoculation of
pro-toplasts with viruses by electroporation, Virology 157 (1987) 40 – 46.
[25] R.W. Jones, A.O. Jackson, T.J. Morris, Defective-inter-fering RNAs and elevated temperatures inhibit replica-tion of Tomato bushy stunt 6irus in inoculated
protoplasts, Virology 176 (1990) 539 – 545.
[26] C.C. Huntley, T.C. Hall, Interference withBrome mosaic
6irus replication in transgenic rice, Mol. Plant – Microb.
Interact. 9 (3) (1996) 164 – 170.
[27] A. Bendahmane, B.A. Kohm, C. Dedi, D.C. Baulcombe, The coat protein of Potato 6irus X is a strain-specific
elicitor of Rx1-mediated virus resistance in potato, Plant J. 8 (1995) 933 – 941.
[28] K.K. Hill, N. Jarvis-Eagan, E.L. Halk, K.J. Krahn, L.W. Liao, R.S. Mathewson, D.J. Merlo, S.E. Nelson, K.E. Rashka, L.S. Loesch-Fries, The development of virus-re-sistant alfalfa, Medicago sati6a, Biotechnology 9 (1991)
373 – 377.
[29] J.A. Lindbo, W.G. Dougherty, Untranslatable tran-scripts of the Tobacco etch 6irus coat protein gene
se-quence can interfere with Tobacco etch 6irusreplication
in transgenic plants and protoplasts, Virology 189 (1992) 725 – 733.
[30] M. Brooks, G. Bruening, A subgenomic RNA associated
withCherry leafroll6irusinfections, Virology 208 (1995)
132 – 141.
[31] P.G. Kovalenko, N.V. Schuman, S.P. Ponomarenko, Biotechnological advances of eletroporation of grapevine and sugar beet cells, Bioelectrochem. Bioenerg. 43 (1997) 165 – 168.
[32] M. Pinck, J. Reinbolt, A.M. Loudes, M. Loret, L. Pinck, Primary structure and location of the genome-linked protein (VPg) ofGrape6ine fanleaf nepo6irus, FEBS Lett.
284 (1991) 117 – 119.
[33] P. Coutos-Thevenot, O. Maes, T. Jouenne, M.C. Mauro, M. Boulay, A. Deloire, J. Guern, Extracellular protein patterns of grapevine cell suspensions in embryogenic and non-embryogenic situations, Plant Sci. 86 (1992) 137 – 145.
[34] M.C. Chupeau, M. Lemoine, Y. Chupeau, Requirement of thidiazuron of healthy protoplasts development to efficient tree regeneration of hybrid popular (Populus tremualasp.alba), J. Plant Physiol. 141 (1993) 601 – 609. [35] J.P. Nitsch, C. Nitsch, Haploid plants from pollen
grains, Science 163 (1969) 85 – 87.
[36] E.M. Frearson, J.B. Power, E.G. Cocking, The isolation culture and regeneration of Petunia leaf protoplasts, Dev. Biol. 33 (1973) 130 – 137.
[37] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 473 – 497.
[38] F. Hans, Re´plication du RNA satellite du virus du court-noue´ de la vigne (GFLV) dans les protoplastes de
Chenopodium quinoa, action antivirale de la prote´ine de capside du GFLV exprime´e par des tabacs transge´niques. Ph.D. Thesis, University Louis-Pasteur, Strasbourg I (1992).
[39] M. Fromm, L.P. Taylor, V. Walbot, Expression of genes transferred into monocot and dicot plant cells by electro-poration, Proc. Natl. Acad. Sci. USA 82 (1985) 5824 – 5828.
[40] R. Hunold, M. Burrus, R. Bronner, J.P. Duret, G. Hahne, Transient gene expression in sunflower (He
-lianthus annus L.) following microprojectile bombard-ment, Plant Sci. 105 (1995) 95 – 109.
[41] R. Heim, A.B. Cubitt, R.Y. Tsien, Improved green fluorescent, Nature 373 (1995) 663 – 664.
[42] R.A. Jefferson, Assaying chimeric genes in plants: the GUS gene fusion system, Plant Mol. Biol. Rep. 5 (1987) 387 – 405.
[43] C. Ritzenthaler, M. Pinck, L. Pinck, Grape6ine fanleaf
nepo6irus P38 putative movement protein is not
tran-siently expressed and is a stable final maturation product in vivo, J. Gen. Virol. 76 (1995) 907 – 915.
[44] M. Longstaff, H.S. Edmonds, C.A. Newell, An improved method for the detection and quantification of recombi-nant protein in transgenic plants, Plant Mol. Biol. Rep. 4 (1995) 362 – 368.
[45] D.C. Chang, Structure and dynamics of electric field-in-duced membrane pores as revealed by rapid-freezing electron microscopy, in: Guide to Electroporation and Electrofusion, Saunders, San Diego, 1992, pp. 9 – 28. [46] M. Nishiguchi, T. Sato, F. Motoyoshi, An improved
method for electroporation in plant protoplasts: infection of tobacco protoplasts byTobacco mosaic6irusparticles,
[47] M. Nishiguchi, W.H.R. Langridge, A.A. Szalay, M. Zaitlin, Electroporation mediated infection tobacco leaf protoplasts withTobacco mosaic6irusRNA andCucumber
mosaic6irusRNA, Plant Cell Rep. 5 (1986) 57 – 60.
[48] Y. Watanabe, T. Meshi, Y. Okada, Infection of tobacco protoplasts with in vitro transcribed tobacco mosaic virus RNA using an improved electroporation method, FEBS Lett. 219 (1987) 65 – 69.
[49] H. Morikawa, A. Iida, C. Matsui, M. Ikegami, Y. Ya-mada, Gene transfer into intact plant cells by electroinjec-tion through cell walls and membranes, Gene 41 (1986) 121 – 124.
[50] J.A. Saunders, C.R. Smith, J.M. Kaper, Effects of electro-poration pulses wave on the incorelectro-poration of viral RNA
into tobacco protoplasts, Res. Rep. 7 (10) (1989) 1124 – 1131.
[51] M. Tsukada, T. Kusano, Y. Kitagawa, Introduction of foreign genes into tomato protoplasts by electroporation, Plant Cell Physiol. 30 (1989) 599 – 603.
[52] F. Gaire, Implication du syste`me endomembranaire dans la re´plication du virus du court-noue´ de la vigne (GFLV): roˆle de la prote´ine 2A dans la re´plication du RNA2. Ph.D. Thesis, University Louis Pasteur Strasbourg I (1998). [53] J.P. Carr, A. Gal-on, P. Palukaitis, M. Zaitlin,
Replicase-mediated resistance toCucumber mosaic6irusin transgenic
plants involves suppression of both virus replication in the inoculated leaves and long-distance movement, Virology 1999 (1994) 439 – 447.