I
n spite of the observation that nitrogen (N) is often the nutrient that limits primary productivity in many oceanic environments, marine N2fixation has been largely overlooked as a source of fixed N in these systems. This is probably because reports in the early 1980s suggested that integrated rates of marine N2fixation were small compared with overall phytoplankton N demand. However, a range of new evidence indicates that marine N2fixation does provide a significant source of new N to tropical marine ecosystems. This evidence includes:• Recognition of a more widespread occurrence and higher den-sities of the cyanobacteria Trichodesmium spp. than previously reported1,2
.
• Deficits and imbalances in the marine N budget indicating unquantified N sources3–5
. • Particulate organic N and NO3
2
in surface waters with N iso-topic signatures close to that of atmospheric N2 (low d
15 N)6,7
. • Measurements of rates of N2fixation comparable to the flux and
uptake of fixed N (primarily NO3 2
) into the euphotic zone1,2,11 . Furthermore, evidence is accumulating for other N2fixers, which could contribute substantially to the ocean’s fixed N budget8
. N2fixation by Trichodesmium spp. is a significant source of new N to tropical and subtropical marine systems where they occur1,9–11
. Species of the non-heterocystous, diazotrophic, cyanobacterial genus Trichodesmium spp., are common throughout the N-depleted tropical and subtropical oligotrophic ocean, and rates of N2fixation by Trichodesmium spp. have been measured in a variety of lo-cations1. Although it is often assumed that Trichodesmium spp. depend primarily on N2 fixation to meet their nutritional needs
12 , they are also capable of taking up other nitrogenous compounds (e.g. NH4
1 , NO3
2
and some forms of organic N)13,14 .
Much of what we know about the physiology and ecology of Trichodesmium spp. derives from work on field populations be-cause attempts to culture these species were unsuccessful for many years. However, at least two Trichodesmium clones (IMS101 and NIBB1067) have now been isolated from natural populations and maintained for several years15,16
. Both clones have been tentatively identified as T. erythraeum based on their nifH gene sequence17–19.
Species-specific differences in N metabolism have not been broadly investigated. In natural assemblages, T. thiebautii fixed
N2 at about twice the rate of T. erythraeum at light intensities
.500mmol quanta m22 s21
and fixed N2at ~1.6 times higher rates at 300mmol quanta m22
s21
(Ref. 20). More rapid rates of N2fixation under high light intensities might explain the greater abundance of T. thiebautii relative to T. erythraeum under such conditions. Low light intensities were used for cultures during the isolation of strains from natural populations, and might have selected for T. erythraeum.
Ecology of marine N2fixation
Epiphytic, endosymbiotic and free-living unicellular and colonial cyanobacterial species, as well as bacteria, have been shown to fix N in pelagic marine systems21
. However, to date, Trichodesmium spp. appear to be the most quantitatively significant pelagic N2 fixers in marine systems. They are broadly distributed throughout the marine tropics and sub-tropics, and surface aggregations can occur over large expanses of ocean1,12
. Besides providing impor-tant N inputs, these species can dominate primary productivity and N cycling in the upper water column2,9,10
, particularly when they occur as episodic ‘blooms’1,4.
In the ocean, multi-cellular trichomes of Trichodesmium spp. often form spherical aggregates called ‘puffs’ and fusiform bundles called ‘tufts’, each containing up to several hundred trichomes20
. Free trichomes are also dispersed throughout the upper water col-umn in tropical and subtropical seas11
. Cultures of Trichodesmium IMS101 and NIBB1067 occur largely as free filaments, forming colonies only during stationary-phase growth13,15,16,22
. The precise causes of bundle formation, aggregation and the formation of so-called ‘blooms’ are unknown, but many investigators have suggested that oxygen protection of oxygen-labile nitrogenase, Fe or other nutrient limitation might be important. Rates of N2fixation by Trichodesmium spp. colonies vary widely between sample sites1,9,10,14
and this might be attributed to environmental and physi-ological factors affecting natural populations. Highly variable rates of combined N uptake have also been measured14
.
Nitrogenase synthesis and activity in natural23,24and cultured22,25 populations of Trichodesmium spp. exhibits a daily cycle, and nitrogenase activity is confined to the light portion of the day. This daily cycle of N2fixation corresponds with the pattern of photo-synthetic C fixation. In natural populations of T. thiebautii, new
The nitrogen physiology of the
marine N
2
-fixing cyanobacteria
Trichodesmium spp.
Margaret R. Mulholland and Douglas G. Capone
Trichodesmium spp. have proved to be enigmatic organisms, and their ecology and physiology
are unusual among diazotrophs. Recent research shows that they can simultaneously fix N2
and take up combined nitrogen. The co-occurrence of these two processes is thought to be incompatible, but they could be obligatory in Trichodesmium spp. if only a small fraction of
cells within a colony or along a filament are capable of N2fixation. Combined nitrogen is
released from cells during periods of active growth and N2fixation, and concomitantly taken up
nitrogenase is synthesized each morning23,26
, the enzyme is modi-fied and thereby inactivated during the afternoon and subse-quently degraded through the night24
. Peak N2 fixation occurs around midday23, suggesting that nitrogenase activity might be regulated by light. However, nifH (the gene encoding the nitroge-nase protein) mRNA abundance in cells collected from natural populations is highest before dawn, indicating that light itself does not directly promote nitrogenase synthesis26. The metabolic signals causing the modification and inactivation of nitrogenase have not been identified23,24. An endogenous rhythm for N
2 fixation and the transcription, translation and activity of nitrogenase in Trichodesmium IMS101 has been confirmed22,27.
Recently, it has been observed that not all cells within a colony or along a filament of Trichodesmium spp. contain nitrogenase. Immunological studies suggest that ,10–15% of cells clustered along regions of filaments contain nitrogenase and are therefore capable of fixing N2 (Refs 21,28–31). Cells within these clusters have been named diazocytes. Although they have some ultrastructural simi-larities to heterocysts, they do not have thickened cell walls and re-tain many of the photosynthetic characteristics of vegetative cells21,30. However, what is unclear is whether particular cells permanently dif-ferentiate to perform N2fixation or if all cells retain the capacity but do not simultaneously differentiate and fix N2. It follows that cells that do not fix N2require fixed N for growth. The observation that all Trichodesmium spp. cells do not simultaneously have the ability to fix N2has important implications with regard to N utilization and N metabolism in these species: some of these will be discussed here.
Nitrogen nutrition of Trichodesmium spp.
Low rates of 15N incorporation of combined N, which have been reported previously, and low d15
N of Trichodesmium spp. led to the general conclusion that natural populations of Trichodesmium spp. growing in N-depleted oligotrophic waters use N2to meet the majority of their cellular N requirements7. In this regard, estimates
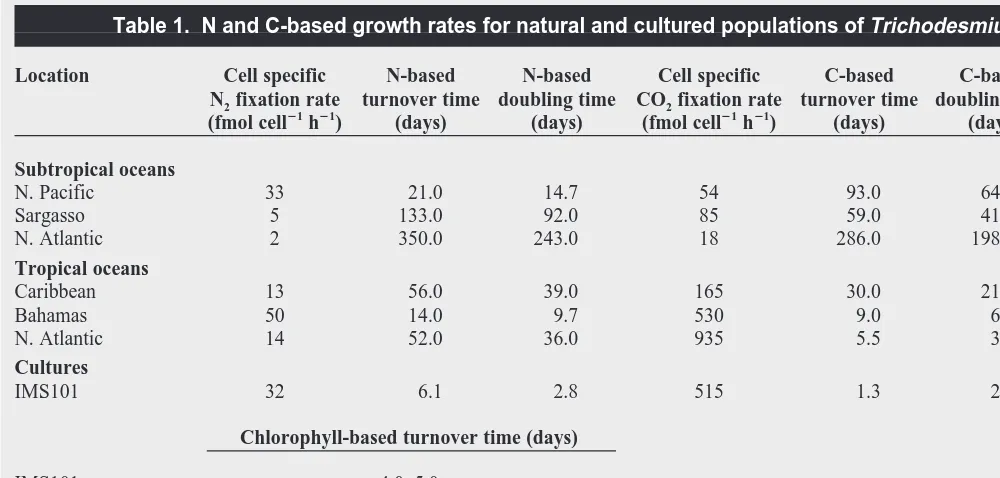
of Trichodesmium spp. growth rates for natural populations vary widely among and even within studies depending on whether they are based on CO2 fixation (Table 1) or on N2 fixation (Table 1; M.R. Mulholland and D.G. Capone, unpublished). In-terestingly, doubling times are ~2–14 days for cultures of Trichodesmium NIBB1067 and IMS101 growing exponentially on seawater or on artificial media13–16,22
, which is close to the C-based doubling times for field populations (Table 1). The higher growth rates estimated from C relative to N in natural populations suggests that there are other sources of N. Alternatively, C-based growth rates might be artifactually high (M.R. Mulholland and D.G. Capone, unpublished).
Although surface waters in the tropical oligotrophic ocean gyres are generally depleted in combined N – NH4
1
and dissolved organic N (DON) are rapidly recycled to support much of the apparent N demand of primary production by the phytoplankton in these systems32,33
. Dissolved pools of N (NH4 1
, NO3 2
1NO2 2
and DON) and PO4 23
can become enriched in and around blooms, presumably because of N inputs from cells fixing N2(Refs 4,34).
Trichodesmium spp. appear to simultaneously fix N2and take up combined N when it is available at low concentrations13,14
. Although low rates of NH4
1 , NO3
2
and urea-uptake have been reported in some studies using populations from the N Atlantic Ocean and from the Caribbean Sea, there is considerable evidence that Trichodesmium spp. are capable of taking up combined nitro-gen (e.g. NH4
1 , NO3
2
and DON)14. Natural populations of Tri-chodesmium spp. from the NW Pacific, the tropical N Atlantic, the Caribbean Sea and the tropical Atlantic, as well as cultured popu-lations, all have a capacity to take up NH4
1
at high rates14 . High rates of NH4
1
uptake have been correlated with NH4 1
concentrations in exponentially growing cultured populations of Trichodesmium NIBB1067 and IMS101 (Refs 13,14). A capacity for glutamate (Glu) and glutamine (Gln) uptake has also been demonstrated in natural and cultured populations of Trichodesmium spp.13,14,35,36.
Table 1. N and C-based growth rates for natural and cultured populations of Trichodesmium spp.
Location Cell specific N-based N-based Cell specific C-based C-based Notes Ref. N2fixation rate turnover time doubling time CO2fixation rate turnover time doubling time
(fmol cell21h21) (days) (days) (fmol cell21h21) (days) (days)
Subtropical oceans
N. Pacific 33 21.0 14.7 54 93.0 64.0 a,b 50
Sargasso 5 133.0 92.0 85 59.0 41.0 a,b,c 51
N. Atlantic 2 350.0 243.0 18 286.0 198.0 a,b 52 Tropical oceans
Caribbean 13 56.0 39.0 165 30.0 21.0 a,b,c
51
Bahamas 50 14.0 9.7 530 9.0 6.5 a,b d
N. Atlantic 14 52.0 36.0 935 5.5 3.8 a,b e Cultures
IMS101 32 6.1 2.8 515 1.3 2.2 f
Chlorophyll-based turnover time (days)
IMS101 4.0–5.0 IMS101 2.0–4.0 NIBB1067 12.5–14.5 NIBB1067 3.0–4.0
aAssumed C biomass of 1 mmol C col21. bAssumed N biomass of 0.14 mmol N col21. c
Data recalculated using 3:1 ratio of C2H2reduction:N2fixation. d
D.G. Capone, unpublished.
There are several possible explanations for the lack of N uptake in some of the earlier studies. For example, when natural popu-lations of Trichodesmium spp. are encountered in the field, there is no way of knowing the prior nutritional history or of knowing what stage of growth they are in at the time of sampling. In culture systems, NH4
1
can accumulate to several mM in the growth
medium over the growth cycle and consequently cells take up this recycled N (which has been measured using 15NH
4 1
tracer addi-tions) at rates comparable to rates of N2fixation
13,14
. The relative proportion of N utilization, derived from N2fixation and N uptake,
changes over the course of the growth cycle as more N is fixed into the system and becomes available through recycling33(M.R. Mulholland and D.G. Capone, unpublished). Because Trichodesmium spp. must first fix N2into the system to grow, rates of N regeneration might be lower during the early growth stages. If natural populations are encountered early in the growth cycle, when the proportion of N2 fixation is a higher fraction of the total N uptake, then measure-ments of NH4
1
uptake might be low. Alternatively, higher mea-sured rates of N uptake and lower rates of N2 fixation might be expected in populations sampled during late stationary phase growth, when new biomass production is low (Fig. 1).
Another possible explanation for the variability in reported NH4
1
-uptake rates is that NH4 1
concentrations in the water in interfilamental spaces of colonies can be higher relative to concen-trations measured in surrounding waters. In field studies, although ambient water column NH4
1
concentrations have always been at the limits of analytical detection, Glu, Gln, NH4
1
and urea concen-trations are enriched in the interfilamental spaces of colonies14,36. Consequently, cells within or adjacent to these microenvironments are exposed to higher ambient nutrient concentrations. Calcu-lations of NH4
1
uptake using stable isotopes are sensitive to the ambient concentrations of nutrients used in the calculations. If, in actuality, cells are exposed to higher concentrations of N substrates because of elevated N within the intrafilamental spaces, 15 N-labelled substrates diffusing in would be diluted relative to the enrichment factor used in the calculation based on ambient seawater concentrations. Using the extracolonial or ‘ambient’ concentrations in making uptake-rate calculations might thereby result in an underestimation of the true rate of uptake.
With respect to the natural abundance data, although low d15N sig-natures in aquatic communities typically indicate a N2N source, this does not preclude the utilization of other isotopically light N sources. For instance, the NH4
1
and DON pools derived directly from the release of recently fixed N2 would be isotopically lighter than NH4
1
or DON derived from recycled material derived from non-atmospheric N sources. Rapid reassimilation of this isotopically light pool of regenerated N would allow Trichodesmium spp. to retain their light isotopic signature while using regenerated N sources.
If only a small fraction of Trichodesmium spp. cells contain nitro-genase, a high capacity for NH4
1
uptake and an ability to concur-rently take up nitrogenous substrates and fix N2 during the light period might be an important adaptation for the extracellular distrib-ution of N among cells and filaments13,14,35
. Examination of Tri-chodesmium spp. filaments has not produced evidence of structural mechanisms for along-filament transport of fixed N (Ref. 30). Con-sequently, it is unlikely that fixed N is transferred between cells along filaments. An extracellular mechanism for the transfer of fixed N among Trichodesmium spp. cells that are fixing N2and among those that are not has been proposed. It is suggested that fixed N, pri-marily amino acids, are released by cells fixing N2 and these are available for uptake by cells that are not fixing N2 (Ref. 35). High rates of amino acid36, DON (Ref. 37) and NH
4 1
(Ref. 13) release, and a simultaneous uptake capacity for amino acids and NH4
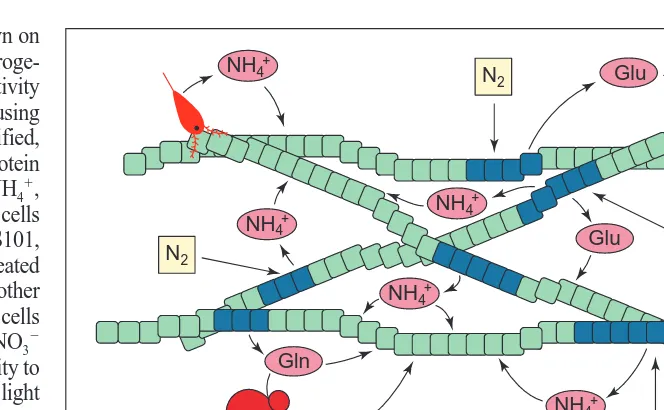
1 by Trichodesmium spp. colonies supports this contention (Fig. 2).
In cultures of Trichodesmium NIBB1067 and IMS101, grow-ing exponentially on a defined seawater medium without added N sources, N2fixation accounts for ~15% whereas the sum of NH4
1
and glu uptake accounts for ~85% of the total-measured daily N uptake13,14
. This is also consistent with the idea that 85% of Trichodesmium spp. cells along a filament or within a colony do not fix N2, but instead must rely on the uptake of combined N.
Trichodesmium NIBB1067, an isolate from the Kuroshio current near Japan, can grow on NH4
1 , NO3
2
and urea-enriched media in the laboratory38,39. However, nitrogenase synthesis and activity are
Fig. 1. Pattern of NH4
1
uptake (light gray) and N2fixation (dark gray)
over diel cycles during (a) early exponential or lag phase growth, (b) exponential phase growth and (c) stationary phase growth. Note that the total N uptake varies with the growth stage as does the proportion of the total N turnover as a result of N2fixation. N2fixation accounts
for most of the N turnover during early exponential growth, less than half the total N turnover during exponential growth and less than a
T
rends in Plant Science
Time (h)
N Utilization (nmol N l
–1
N Utilization (nmol N l
–1
h
–1
)
06.00 12.00 18.00 24.00
00.00
N Utilization (nmol N l
–1
h
–1
)
06.00 12.00 18.00 24.00
affected by the N source. Cultures grown on NO3
2 , NH4
1
and urea do not exhibit nitroge-nase activity or reduced nitrogenitroge-nase activity during light periods13,22,38. In a study using Trichodesmium NIBB1067, the modified, inactive form of the nitrogenase Fe-protein was found in cells grown on NO3
2 or NH4
1,
but nitrogenase was absent entirely from cells grown on urea39
. In Trichodesmium IMS101, the Fe-protein was absent from cells treated with 20 mMof either NH4
1
or urea. In another study of Trichodesmium NIBB1067, cells cultured on medium containing excess NO3
2
or urea retained nitrogenase and a capacity to fix N2during at least a portion of the light period. However, they did so at rates lower than those measured in cultures maintained on medium without added N substrates13. Cultures growing in medium with excess urea turned over their cell N much more rapidly than cultures growing on NO3
2 -enriched medium or on medium without added N sources13
. However, growth rates were similar among cultures grown with or without combined N sources13,38
. The vari-ation in N turnover rates between cultures growing on different N sources could occur because N2fixation is confined to the light period regardless of the N regime in natural and cultured populations of Trichodesmium spp. By contrast urea and NH4
1
are taken up
at comparable rates during both the dark and the light periods. Uptake of NO3
2
occurs primarily during the light period13
, and probably competes with N2fixation for photosynthetic energy and reductant. The absolute growth rate (e.g. doubling of biomass) might be limited intrinsically.
Clearly, additional studies investigating the effect of combined N on growth and the competitive advantage of Trichodesmium spp. need to be pursued. Although Trichodesmium NIBB1067 and IMS101 appear to be able to take up combined N and fix N2 simul-taneously or alternately in cultures, little is known about the growth characteristics of Trichodesmium spp. under different N regimes in nature. Trichodesmium spp. can alleviate N limitation and therefore out-compete other species in N-limiting environments in nature, although blooms persist even after N concentrations become elevated. It is unclear how increasing availability of combined N species affects the growth of Trichodesmium spp. and their competitive success in natural systems.
The modification and expression of nitrogenase might be at least partially regulated by the N regime. NH4
1
, Glu and Gln additions have been shown to reduce N2fixation over time, probably because of feedback inhibition of nitrogenase by metabolite pools23 (M.R. Mulholland et al. unpublished). From studies using metabolic inhibitors, it appears that metabolites that accumulate during N assimilation, are important in regulating nitrogenase activity and synthesis in natural and cultured populations of Trichodesmium spp.39
. However, the presence of low concentrations of NH4 1
and amino acids in the culture medium during growth do not inhibit N2 fixation. Additions of combined N of 5–10mMor more might be necessary before nitrogenase synthesis or activity is affected. Such high concentrations are not typical of the oligotrophic regions in which these species occur.
Although N2fixation might supply the ‘new’ N necessary for alle-viating N limitation of net growth, much of the gross N turnover
might be satisfied by NH4 1
uptake supplied by N recycled within the upper water column community13,14
. Because Trichodesmium spp. are able to take up combined N, C-based productivity might not be a mea-sure of new production in systems where allochthonous N is imported.
Nitrogen metabolism
Nitrogen assimilation in natural populations of Trichodesmium spp. proceeds via the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway35. GS is necessary for NH
4 1
assimilation regardless of the primary form of N being used. High rates of GS transferase activity (a reverse reaction assay that provides an approximation of total GS active sites) relative to rates of total N uptake have been observed in natural and cultured populations of Trichodesmium spp. (Refs 13,14). Rates of both GS transferase and GS biosynthetic activity (which approximates in vivo forward reaction activity) in Trichodesmium spp. increase in the afternoon during the period when rates of N2fixation are highest. The ratio of GS transferase:biosynthetic activity decreases during the period of maximum N2fixation, indicating that the proportion of the GS pool that is biosynthetically active increases during the day.
The biosynthetic capacity of GS is sufficient to allow Tri-chodesmium spp. colonies to turnover their cell N at least three times per day, suggesting that N assimilation does not limit the rate of N uti-lization by cells, even during midday when N2fixation rates are high-est13,14. Cells appear to have sufficient capacity to assimilate all of the intracellular N substrates derived from N2 fixation and N uptake in cultures growing on media with or without added N (Ref. 13).
Excess GS activity is characteristic of cells limited by N or using N2as their N source
40. A positive correlation between GS and
nitrogenase enzyme abundance and distribution has been observed in a variety of heterocystous41,42and non-heterocystous43,44 cyano-bacteria including Trichodesmium spp35
. The GS enzyme is twice as abundant in Trichodesmium spp. cells that contain nitrogenase
Fig. 2. Conceptual diagram of nitrogen cycling within a colony of Trichodesmium. Four
fila-ments are shown, with green cells representing vegetative or ‘non-N2-fixing’ cells. Blue cells
represent N2-fixing cells clustered in regions along filaments. Dinitrogen diffuses into N2-fixing
cells, where it is fixed in the form of NH4
1
, Gln and Glu. Some of these compounds are released directly into the growth medium where they are available for uptake by Trichodesmium cells or other organisms and other non-assimilatory reactions (pink ovals). Organisms mediating other possible fates of fixed N are depicted in red. Associated organisms with cell-surface amino acid oxidases might oxidize the amino acids, thereby liberating NH4
1
(depicted as red circles, lower left). Additionally, NH4
1
might be released during grazing (red copepod in upper left).
Trends in Plant Science
+ Keto acid N2
N2
N2
N2
Glu
Gln
NH4
Glu
+
NH4+
NH4+
NH4+
NH4+
than in those that do not35
. Although different types of GS might be present in cells, different promoters for transcribing the gene encoding GS during growth on fixed N and molecular N2 have also been observed40. Thus, there might be both a constitutive pool of GS, regulated for the general assimilation of N derived from various N sources, and a nitrogenase-linked pool co-regulated specifically with nitrogenase under low N conditions.
In support of two separately regulated GS pools in Trichodesmium spp., glnA transcript abundance is elevated both during the pre-dawn period and again in the late afternoon45. The early morning peak in glnA transcript abundance corresponds with peak nifH (a gene encod-ing the Fe-protein of nitrogenase) transcript abundance26, whereas the afternoon peak does not. In Trichodesmium NIBB1067 grown on medium without added N, biosynthetic GS activity increased before the onset of the light period and then again in the late afternoon (M.R. Mulholland et al. unpublished), consistent with the pattern of glnA mRNA abundance in natural populations45
.
The absence of a strong diel cycle of GS activity might reflect the observation that Trichodesmium spp. colonies and filaments take up regenerated N sources in addition to fixing N2 during the day. It might also reflect the observation that only a fraction of cells along a filament or within a colony may contain nitrogenase and are, therefore, capable of N2fixation. If two pools of GS are present in Trichodesmium spp., the small changes in total filament or colony GS activity over a diel cycle might be significant in terms of the capacity for N assimilation in cells fixing N2. The ratio of GS trans-ferase:biosynthetic activity in natural and cultured populations of Trichodesmium spp. varies by ~20 to 30% over a diel cycle13,14. The magnitude of this change is consistent with the suggestion that nitrogenase is confined to ,10–15% of cells along a filament or within a colony29,31
, that GS is twice as abundant in cells containing nitrogenase35, and that a portion of the GS pool is regulated in con-junction with nitrogenase.
Based on observations of the distribution and activity of GS dur-ing N2fixation, it has been suggested that GS plays an important regulatory role in N2 fixation, either directly or indirectly, by preventing feedback inhibition from accumulated metabolites46
. Glutamine and glutamate are primary products of N metabolism via the GS/GOGAT metabolic pathway. Intracolonial Glu and Gln concentrations parallel the daily cycle of N2fixation
35,36and might
be factors in subcellular regulation of nitrogenase. However, in cultures of Trichodesmium NIBB1067 growing with and without combined N, the Gln:Glu ratio reflects the pattern of total N uptake (positive relationship)13. In natural populations, the magnitude and timing of the daily peak in Gln:Glu ratios varies among sites and populations. However, the highest Gln:Glu ratios are observed during the period when rates of N2fixation are highest
14,36 . Light might be an important determinant for N2fixation and the N status of Trichodesmium spp. cells. Higher Gln:Glu ratios (0.7) were observed in Trichodesmium spp. collected in the surface waters compared with colonies collected from deep waters14
. The decrease in the intracellular Gln:Glu ratios in colonies collected from deeper in the euphotic zone might be due to light or energy limitation of N2fixation rates. Higher rates of photosynthesis by populations growing in surface waters might result in increases in the supply of energy to support higher rates of N2 fixation. In cultures of Trichodesmium IMS101 and NIBB1067, grown on medium without added N substrates, the intracellular ratios of Gln:Glu are lower (a maximum of ~0.3) and this might reflect the low light levels at which these isolates are maintained13,14.
Nitrogen regulation
Investigators have suggested that the nitrogenase of Trichodesmium NIBB1067 is regulated at different levels. An endogenous rhythm
has been identified for nitrogenase transcription, translation and activity27. Alternatively, it has been suggested that the modification and expression of nitrogenase is also regulated by the availability of combined N (Ref. 39). Both regulatory mechanisms appear to be important. Recently, a global N-regulating gene, ntcA, has been identified in a Trichodesmium spp. isolate from the Red Sea (D. Lindell pers. commun.), but the conditions under which it is expressed have not been ascertained.
Community nitrogen dynamics and future directions
Although we are beginning to understand the physiology of N2 fixation by Trichodesmium spp., there remain many uncertainties regarding the mechanisms that support the growth of Tri-chodesmium spp. populations in nature and the growth of organisms living in association with Trichodesmium spp. colonies. For exam-ple, Trichodesmium spp. might have a larger role in the marine N cycle than previously suspected. Although net growth of popu-lations might depend on inputs of new N from N2 fixation, Trichodesmium spp. also contribute to overall N turnover in oceanic systems. They contribute both directly through the release of amino acids, DON and NH4
1
(Refs 13,36,37), and indirectly through the regeneration of dissolved inorganic and organic N by bacteria and grazers living in association with Trichodesmium spp. colonies47–49
. Pathways of N cycling among Trichodesmium spp. filaments and colonies and associated organisms remain to be directly quantified. Although Trichodesmium spp. alleviate N limitation of growth by fixing N2, the effects of deficits of other nutrients on Trichodesmium spp. growth and N2fixation are poorly understood. In particular, Fe, an essential component of the nitrogenase enzyme complex, might limit N2fixation in oceanic regions with low aeolian dust inputs. Phosphorous is also in short supply in oceanic regions where Trichodesmium spp. grow and form blooms. It is unclear what the nutrient demands are for Trichodesmium spp. growth and how they acquire sufficient P to support observed growth rates. High N:P ratios have been observed in populations from the Pacific Ocean10
. Although we are gaining in our understanding of how these species affect the marine N cycle where they occur, much needs to be done to integrate N2fixation into global C, N and P budgets.
Acknowledgements
We would like to thank three anonymous reviewers for their help-ful comments and suggestions. This work was supported by grants from the National Science Foundation Division of Ocean Sciences.
References
1 Capone, D.G. et al. (1997) Trichodesmium, a globally significant marine
cyanobacterium. Science 276, 1221–1229
2 Carpenter, E.J. and Romans, K. (1991) Major role of the cyanobacteria
Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254,
1356–1358
3 Michaels, A. et al. (1996) Excess nitrate and the rate of nitrogen fixation in the
Sargasso Sea. Biogeochemistry 35, 181–226
4 Karl, D.M. et al. (1992) Trichodesmium blooms and new nitrogen in the North
Pacific gyre. In Marine Pelagic Cyanobacteria: Trichodesmium and Other
Diazotrophs (Carpenter, E.J. et al., eds), pp. 219–237, Kluwer
5 Gruber, N. and Sarmiento, J. (1997) Global patterns of marine
nitrogen fixation and denitrification. Global Biogeochem. Cycles 11, 235–266
6 Wada, E. and Hattori, A. (1991) Nitrogen in the Sea: Forms, Abundances and
Rate Processes, CRC Press
7 Carpenter, E.J. et al. (1997) Biogeochemical tracers of the marine
cyanobacterium Trichodesmium. Deep-Sea Res. 44, 27–38
8 Zehr, J.P. et al. (1999) New perspectives on nitrogen-fixing microorganisms in
9 Letelier, R.M. and Karl, D.M. (1996) Role of Trichodesmium spp. in the
productivity of the subtropical North Pacific Ocean. Mar. Ecol. Prog. Ser. 133, 263–273
10 Letelier, R.M. and Karl, D.M. (1998) Trichodesmium spp. physiology and
nutrient fluxes in the North Pacific subtropical gyre. Aquatic Microbial. Ecol. 15, 265–276
11 Karl, D.M. et al. (1997) The role of nitrogen fixation in biogeochemical
cycling in the subtropical North Pacific. Nature 388, 533–538
12 Carpenter, E.J. and Capone, D.G. (1992) Nitrogen fixation in Trichodesmium
blooms. In Marine Pelagic Cyanobacteria: Trichodesmium and Other
Diazotrophs (Carpenter, E.J. et al., eds), pp. 211–217, Kluwer
13 Mulholland, M.R. et al. (1999) N utilization and metabolism relative to patterns
of N2fixation in cultures of Trichodesmium NIBB1067. J. Phycol. 35, 977–988
14 Mulholland, M.R. and Capone, D.G. (1999) N2fixation, N uptake and N
metabolism in natural and cultured populations of Trichodesmium spp. Mar.
Ecol. Prog. Ser. 188, 33–49
15 Ohki, K. et al. (1986) Cultures of the pelagic cyanophytes Trichodesmium
erythraeum and T. thiebautii in synthetic medium. Mar. Biol. 91, 9–13
16 Prufert-Bebout, L. et al. (1993) Growth, nitrogen fixation, and spectral
attenuation in cultivated Trichodesmium species. Appl. Environ. Microbiol. 59, 1367–1375
17 Ben-Porath, J. et al. (1993) Genotype relationships in the genus
Trichodesmium based on nifH sequence comparisons. J. Phycol. 29, 806–809
18 Janson, S. et al. (1995) Cytomorphological characterization of the planktonic
diazotrophic cyanobacteria Trichodesmium spp. from the Indian Ocean and Caribbean and Sargasso Seas. J. Phycol. 31, 463–477
19 Janson, S. et al. (1999) Genetic analysis of natural populations of the marine
diazotrophic cyanobacterium Trichodesmium. FEMS Microbiol. Ecol. 30, 57–65
20 Carpenter, E.J. et al. (1993) The tropical diazotrophic phytoplankter
Trichodesmium: biological characteristics of two common species. Mar. Ecol. Prog. Ser. 95, 295–304
21 Bergman, B. et al. (1997) N2fixation by non-heterocystous cyanobacteria.
FEMS Microbiol. Rev. 19, 139–185
22 Chen, Y-B. et al. (1996) Growth and nitrogen fixation of the diazotrophic
filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS101 in defined media: evidence for a circadian rhythm. J. Phycol. 32, 916–923
23 Capone, D.G. et al. (1990) Basis for diel variation in nitrogenase activity in
the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl.
Environ. Microbiol. 56, 3532–3536
24 Zehr, J.P. et al. (1993) Modification of the Fe protein of nitrogenase in natural
populations of Trichodesmium thiebautii. Appl. Environ. Microbiol. 59, 669–676
25 Ohki, K. and Fujita, Y. (1988) Aerobic nitrogenase activity measured as
acetylene reduction in the marine non-heterocystous cyanobacterium
Trichodesmium spp. grown under artificial conditions. Mar. Biol. 98, 111–114
26 Wyman, M. et al. (1996) Temporal variability in nitrogenase gene expression
in a natural population of the marine cyanobacterium Trichodesmium
thiebautii. Appl. Environ. Microbiol. 62, 1073–1075
27 Chen, Y-B. et al. (1998) Circadian rhythm of nitrogenase gene expression in
the diazotrophic filamentous nonheterocystous cyanobacterium
Trichodesmium sp. strain IMS101. J. Bacteriol. 180, 3598–3605
28 Janson, S. et al. (1994) Compartmentalization of nitrogenase in a
non-heterocystous cyanobacterium: Trichodesmium contortum. FEMS Microbiol.
Lett. 118, 9–14
29 Fredriksson, C. and Bergman, B. (1995) Nitrogenase quantity varies diurnally
in a subset of cells within colonies of the non-heterocystous cyanobacteria
Trichodesmium spp. Microbiology 141, 2471–2478
30 Fredriksson, C. and Bergman, B. (1997) Ultrastructural characterization of
cells specialised for nitrogen fixation in a non-heterocystous cyanobacterium,
Trichodesmium spp. Protoplasma 197, 76–86
31 Lin, S. et al. (1998) Whole-cell immunolocalization of nitrogenase in marine
diazotrophic cyanobacteria, Trichodesmium spp. Appl. Environ. Microbiol. 64, 3052–3058
32 Bronk, D.A. et al. (1994) Nitrogen uptake, dissolved organic nitrogen release,
and new production. Science 265, 1843–1846
33 Glibert, P.M. (1993) The interdependence of uptake and release of NH41
and organic nitrogen. Mar. Microbial Food Webs 7, 53–67
34 Devassy, V.P. (1987) Trichodesmium red tides in the Arabian Sea. In
Contributions in Marine Sciences (Sastyabdapurti felicitation Vol.)
(Qasim, S.Z., ed.), pp. 61–66, India
35 Carpenter, E.J. et al. (1992) Glutamine synthetase and nitrogen cycling in
colonies of the marine diazotrophic cyanobacteria Trichodesmium spp. Appl.
Environ. Microbiol. 58, 3122–3129
36 Capone, D.G. et al. (1994) Amino acid cycling in colonies of the planktonic marine
cyanobacterium, Trichodesmium thiebautii. Appl. Environ. Microbiol. 60, 3989–3995
37 Glibert, P.M. and Bronk, D.A. (1994) Release of dissolved organic nitrogen
by marine diazotrophic cyanobacteria, Trichodesmium spp. Appl. Environ.
Microbiol. 60, 3996–4000
38 Ohki, K. et al. (1991) Regulation of nitrogen fixation by different nitrogen
sources in the marine non-heterocystous cyanobacterium Trichodesmium sp. NIBB1067. Arch. Microbiol. 156, 335–337
39 Ohki, K. et al. (1992) Regulation of nitrogenase activity in relation to the
light–dark regime in the filamentous non-heterocystous cyanobacterium
Trichodesmium sp. NIBB 1067. J. Gen. Microbiol. 138, 2679–2685
40 Tumer, N.E. et al. (1983) Different promoters for the Anabaena glutamine synthetase
gene during growth using molecular or fixed nitrogen. Nature 306, 337–342
41 Bergman, B. et al. (1985) Immuno-gold localization of glutamine synthetase
in a nitrogen-fixing cyanobacterium (Anabaena cylindrica). Planta 166, 329–334
42 Renstrom-Kellner, E. et al. (1990) Correlation between nitrogenase and
glutamine synthetase expression in the cyanobacterium Anabaena cylindrica.
Physiol. Plant. 80, 12–19
43 Rai, A.N. et al. (1992) Nitrogenase derepression, its regulation and metabolic
changes associated with diazotrophy in the non-heterocystous cyanobacterium
Plectonema boryanum PCC 73110. J. Gen. Microbiol. 138, 481–491
44 Smith, RJ. and Gallon, J. (1993) Nitrogen fixation. In Plant Biochemistry and
Molecular Biology (Lea, P.J. and Leegood, R.C., eds), pp. 129–153,
John Wiley & Sons
45 Kramer, J.G. et al. (1996) Diel variability in transcription of the structural gene
for glutamine synthetase (glnA) in natural populations of the marine diazotrophic cyanobacterium Trichodesmium thiebautii. FEMS Microbiol. Ecol. 21, 187–196
46 Flores, E. and Herrero, A. (1994) Assimilatory nitrogen metabolism and its
regulation. In The Molecular Biology of Cyanobacteria (Bryant, D.A., ed.), pp. 487–517, Kluwer
47 Mulholland, M.R. et al. (1998) Extracellular amino acid oxidation by
phytoplankton and cyanobacteria: a cross-ecosystem comparison. Aquat.
Microbial Ecol. 15, 141–152
48 O’Neil, J.M. et al. (1996) Ingestion of 15N
2-labelled Trichodesmium spp. and
ammonium regeneration by the harpacticoid copepod Macrosetella gracilis.
Mar. Biol. 125, 89–96
49 Paerl, H.W. et al. (1989) Bacterial associations with marine Oscillatoria sp.
(Trichodesmium sp.) populations: ecophysiological implications. J. Phycol. 25, 773–764
50 Mague, T.H. et al. (1977) Physiology and chemical composition of
nitrogen-fixing phytoplankton in the central North Pacific Ocean. Mar. Biol. 41, 213–227
51 Carpenter, E.J. and Price, C.C. (1977) Nitrogen fixation, distribution, and
production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnol. Oceanogr. 22, 60–72
52 McCarthy, J.J. and Carpenter, E.J. (1979) Oscillatoria (Trichodesmium) thiebautii
(Cyanophyta) in the central North Atlantic Ocean. J. Phycol. 15, 75–82
Margaret Mulholland*is at the Marine Sciences Research Center, SUNY Stony Brook, Stony Brook, NY 11794-5000, USA; Douglas Capone is at the Wrigley Institute for Environmental Studies and Dept of Biological Sciences, University of Southern California, Los Angeles, CA 90089, USA (e-mail [email protected]).
*Author for correspondence (tel 11 631 632 3163;