The bacterial origins of plastid division and protein import by plastids are beginning to emerge — thanks largely to the availability of a total genome sequence for a cyanobacterium. Despite existing for hundreds of millions of years within the plant cell host, the chloroplast endosymbiont retains clear hallmarks of its bacterial ancestry. Plastid division relies on proteins that are also responsible for bacterial division, although may of the genes for these proteins have been confiscated by the host. Plastid protein import on the other hand relies on proteins that seem to have functioned originally as exporters but that have now been persuaded to operate in the reverse direction to traffic proteins from the host cell into the endosymbiont.
Addresses
Plant Cell Biology Research Centre, School of Botany, University of Melbourne, Parkville VIC 3010, Australia;
e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:513–519
1369-5266/99/$ — see front matter © 1999 Elsevier Science Ltd. All rights reserved.
Abbreviations
Fts filament temperature sensitive
SynToc75 Synechocystis homologue of Toc75
Toc75 channel protein in the outer chloroplast membrane
Introduction
‘Picture a palm tree growing peacefully on the shore of a spring, and a lion, lying hidden beside the palm, all its muscles tense, blood lust in its eyes, ready to pounce on an antelope and slaughter it. In order to understand fully the inner secret of this picture with its two such drastically different manifestations of life, a palm tree and a lion, it is essential to appreciate the theory of endosymbiosis. The life of the palm tree is so calm and peaceful because it is a symbiosis, it contains a legion of workers, green slaves (plastids) that work for it and nourish it. The lion has to feed itself.
Imagine that every cell of the lion’s body was filled with plastids, and I have no doubt that it would immediately lay itself peacefully by the palm, feeling replete with nothing more than some water and a few nutrient salts.’ Mereschkowsky, 1905 [1]
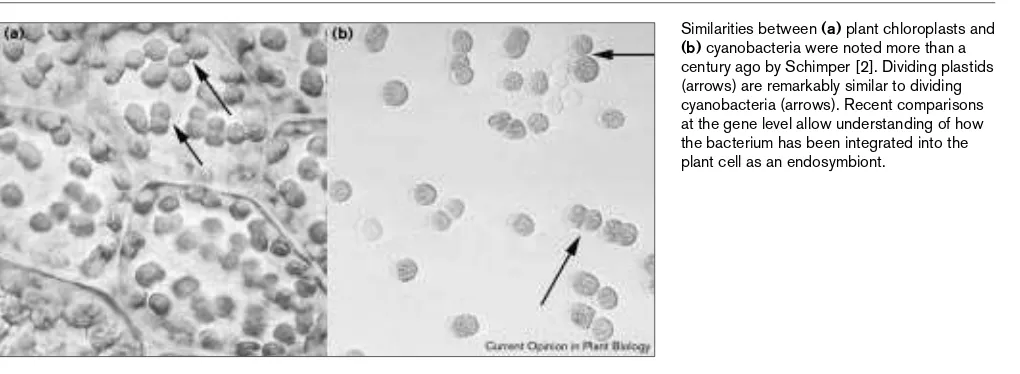
The acquisition of plastids by eukaryotic cells must rank as one of the most momentous events in the planet’s history; for plant cells it was their defining moment. Schimper [2] seems to have been the first biologist to realise that plas-tids derive from endosymbiotic photosynthetic bacteria
(Figure 1), but it was Mereschkowsky [1] who elaborated the concept in eloquent detail. Modern research is paint-ing in more and more detail Mereschkowsky’s idyllic picture of a bacterial cell living within a nucleated host cell. The availability of whole cyanobacterial genome sequences, combined with the advantage of being able to extrapolate from bacterial systems, which can be used to model events in chloroplasts, has allowed us to probe many of the questions of chloroplast biology with unprecedent-ed finesse. My review focuses on the recent rapid progress in our understanding of how the endosymbiont divides and how its need for host-encoded proteins is satisfied.
Chloroplast division
To establish a permanent endosymbiosis (i.e. one not requiring ongoing recruitment of fresh endosymbionts) the endosymbiont must divide within the host cells. Daughter endosymbionts must then be segregated into each daugh-ter host. Little is known about these processes at the molecular level [3•]. Most plant cells contain numerous chloroplasts (or plastids) that divide throughout the plant cell cycle (Figure 1), which perhaps suggests that the seg-regation process is only loosely regulated. However, many unicellular plants (algae) have a single plastid; its division precedes cytokinesis and the daughter plastids are subse-quently apportioned to daughter cells. How this is achieved is only understood at the structural level [4]. The division of plastids takes place by binary fission (Figure 1), and rings of material — both within the plastid and around its outer membrane — are visible at the division constric-tion [4]. Using bacterial division as a model, Osteryoung and Vierling [5] identified a key bacterial division protein (FtsZ) in Arabidopsis. Genes that encode similar proteins also occur in rice [6••,7], moss [8••] and cryptomonad algae [9•]. FtsZ is a tubulin-like protein [10•] that forms a ring around the isthmus of dividing bacterial cells. It is thought that the FtsZ ring interacts with several other division pro-teins and that it constricts during division [11]. FtsZ mutants (filament temperature sensitive) in bacteria fail to divide producing a long filament; a knockout of the gene in moss produces a similar-looking filamentous chloroplast [8••]. Most bacteria have a single ftsZ gene (but see the interesting discussion concerning the presence of multiple paralogues in Archaea and the origins of tubulin [10•]) but
Arabidopsishas multiple, nuclear ftsZgenes that comprise at least two families [6••,7]. The genes are clearly derived from the endosymbiont, and, like so many other endosym-biont genes (see below), have undergone transfer to the host nucleus. This transfer seems likely to allow the host to regulate endosymbiont division, particularly as levels of FtsZ are tightly linked with fission in bacteria [11]. One
ArabidopsisFtsZ family encodes proteins that are targeted to the stroma of the chloroplast, whereas the second family appears to encode cytosolic versions of the division protein
Endosymbiosis and evolution of the plant cell
[6••,7]. This finding is unexpected and suggests that a pro-tein that originally evolved to function within the bacterial cytoplasm can also function ‘outside’ the bacterium when resident within a host cell.
It will now be particularly interesting to follow the bacterial model further and explore whether accessory division pro-teins such as ZipA, FtsA and MinD function within, and even perhaps outside, the chloroplast. The antisense sup-pression of ftsZgenes in Arabidopsisresults in a reduction of the number of chloroplasts present in each cell [6••]. Intriguingly, the putative cytosolic FtsZ was also found to be essential for regulating chloroplast number [6••] and local-izations of the plastidic and cytosolic proteins during division are eagerly awaited. Several other proteins also have apparent roles in plastid division including the products of the ARC (accumulation and replication of chloroplasts) genes [3•], the minicell family [12], ftsH [13] and the dynamins — a strictly eukaryotic protein family that appear to have been recruited for mitochondrial [14,15] and chloro-plast division (Kim SH, Lim JH, Kim SJ, Kang SG, Hwang I, Abstract 5–25, 10th International Conference on Arabidopsis Research, 4–8 July 1999, Melbourne).
Intracellular gene transfer and transit peptides
A free-living cyanobacterium possesses about 3,000 genes [16] but a chloroplast has only 100–200 genes [17••]. Clearly, numerous genes such as those coding for peptido-glycan wall synthesis, became dispensable when the bacterium took up residence within the confines of a host, and these were probably lost; it is also clear that many indispensable genes have relocated to the plant cell nucle-us [17••]. Estimates of 1000–5000 nuclear genes encoding chloroplast proteins have been postulated [17••,18••]. Exactly what drives this transfer is not certain but the var-ious mutational pressures on the endosymbiont genome due to oxidative DNA damage, lack of recombination and genetic bottle necking (chloroplasts are clonal and asexual) probably all contribute to a Müller’s Ratchet situation(where deleterious mutations accumulate) that favours relocation of essential genes into the nucleus [18••,19]. Sex among the hosts has probably allowed them to share trans-ferred genes throughout their population. Once transferred, the genes must be expressed and, in most cases [18••], the gene product returned to the chloroplast across the two surrounding membranes. Martin and Herrmann [18••] argue that the expression of the newly relocated gene was a critical step, which, given that the gene was in a radically different environment (a nucleus as opposed to a prokaryotic compartment), was not automat-ic. Another critical step was for the transferred gene to acquire an amino-terminal (N-terminal) extension that could mediate chloroplast targeting. One proposed mecha-nism for such an acquisition is by exon shuffling; this has indeed been demonstrated for both chloroplast and mito-chondrial transit peptides in plants [20–24], as has alternate splicing to provide two proteins with the same transit peptides [25]. In plants, alternative promoters can provide versions of the gene product either with or without a transit peptide, resulting in a cytosolic and a chloroplast-targeted version of the same enzyme [26]. A recent paper shows that the chloroplast transit peptide for ribosomal proteins L12 and S9 can be duplicated [27] but the mech-anism for this replication remains to be established. An intriguing new hypothesis (Figure 2) proposes that the ultimate origin of the transit peptide is intimately linked to the origin of the import channel [28••] (see below).
Prior to the era of genomics the identification of trans-ferred genes was a labour intensive task. Now, with the advent of sequencing projects, we can anticipate the avail-ability of a complete catalogue of these genes in the near future. Many transferred genes retain hallmarks of their prokaryotic ancestry and are identifiable by phylogenetics; others are known to have adopted functions in the cytosol [18••] and so phylogeny is an imperfect tool for predicting their subcellular location. The identification of those plant genes (potentially thousands) whose products are destined Figure 1
Similarities between (a)plant chloroplasts and
for the chloroplast will be a major challenge. The great majority of chloroplast-targeted proteins bear an N-terminal extension known as a transit peptide, which is both sufficient and necessary for targeting. Transit pep-tides can be identified experimentally either in vitro, by import assays with isolated, import-competent chloroplas-ts, or in vivo, by transforming plants with constructs that express a fusion of the putative transit peptide and a reporter protein such as green fluorescent protein [29•]. All of these approaches, including a recent protocol for identi-fying transit peptide-bearing genes from unidentified cDNA clones [30], are probably too labour intensive for use on a whole genome scale, and a bioinformatics approach has long been desired. However, no consensus of sequence or secondary structure in plant chloroplast transit peptides has, as yet, been observed. Two new develop-ments proffer a solution to this problem.
Wienk et al. [31•] have modelled the secondary structure of the preferredoxin transit peptide in trifluoroethanol, which mimics the properties of the galactolipids that are predom-inant in the outer membrane of chloroplasts [32••]. This transit peptide is found to form two amphipathic helices that are postulated to lie in the galactolipid layer with the hydrophobic face interacting with the acyl chains, while the hydrophilic face interacts with the sugar heads [31•]. The model is similar to that proposed for mitochondrial transit peptides, with the attractive distinction that the plastid membrane is the only surface within the plant cell presenting galactolipids and therefore the only surface on which the transit peptides of chloroplast-destined proteins would assume an active configuration [31•,32••]. This model (originally proposed by Keegstra [33]) provides a neat mechanism to distinguish between those proteins that are bound for the chloroplast and those bound for the other bacterial endosymbiont, the mitochondrion. The chloro-plast transit peptide–galactolipid interaction is probably reciprocal and modification of the lipid layer is potentially important in regulating import [32••]. Galactolipid-defi-cient chloroplasts have recently been shown to be defective in protein import [34].
Although this model is both attractive and consistent with the endosymbiosis model — cyanobacteria have galac-tolipids on their surface [35] — it has a nagging incongruity. Genes encoding galactolipid synthesis have recently been cloned [36,37] but they do not appear to derive from the galactolipid synthesis genes of cyanobac-teria. Indeed, the monogalactosyldiacylglyceride synthase of plants apparently derives from a peptidoglycan synthe-sis enzyme (MurG), which, though still of endosymbiotic origin, seems to have been recruited to a new function, rather than from the direct adoption of the cyanobacterial synthase [36]. The evolutionary origin of the digalactosyl-diacylglyceride synthase of plants is obscure [37].
von Heijne’s group have persued an informatics approach to identifying chloroplast transit peptides [38•]. They
trained a neural network to recognise chloroplast transit peptides and have made this network available as a Web service [38•]. Such tools will be important in making sub-cellular-targeting predictions from genomic data and should allow the identification of nuclear-encoded chloro-plast proteins. With a complete catalogue of transit peptides at hand, it will be fascinating to unravel their ori-gins in the genome (see above). The inventory will also give us a comprehensive picture of the various plastid metabolic pathways.
Chloroplast protein import machinery
Intra-chloroplast protein targeting has long been known to derive from the endosymbiont secretion machinery [39] and several recent papers confirm this origin [40–44]. For many years the ∆pH-dependent pathway involved in thylakoid protein targeting was thought to be an excep-tion to this rule and was regarded as a specific invenexcep-tion of chloroplasts. It is now becoming clear, however, that chloroplast hcf106 (high chlorophyll fluorescence) — a protein central to ∆pH-dependent targeting — probably derives from the Tat (twin-arginine signal Figure 2
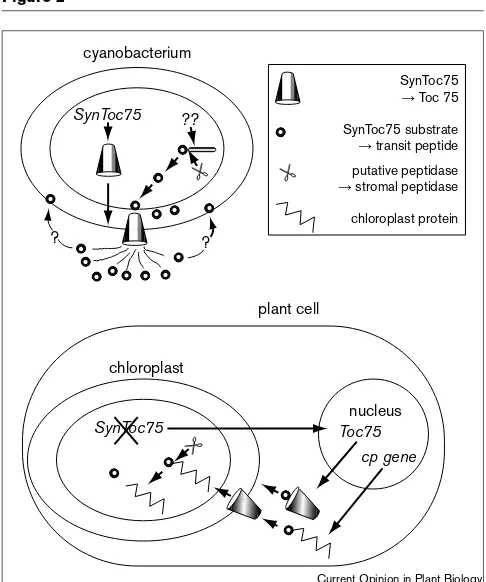
Scenario for the origin of part of the chloroplast import machinery (Toc75) and transit peptides from peptide secretion machinery of cyanobacteria like Synechocystisaccording to [28]. Transfer of the gene for the voltage-gated channel SynToc75 to the host nucleus results in an inside-out insertion of the channel into the outer endosymbiont membrane, effectively reversing the direction of transport. Derivatives of the cyanobacterial gene encoding the putative secretion substrate of SynToc75 are appended to chloroplast protein-encoding nuclear genes to mediate import by Toc75.
plant cell
chloroplast
nucleus Toc75
cp gene SynToc75
SynToc75 cyanobacterium
??
? ?
SynToc75 → Toc 75
SynToc75 substrate → transit peptide putative peptidase → stromal peptidase
chloroplast protein
sequence-dependent metalloenzyme translocation) pro-teins of bacteria [45]. The presence of twin arginines in the chloroplast leaders of ∆pH-dependent targeted pro-teins supports this concept.
The evolutionary origins of the protein import machinery of chloroplasts have only just begun to emerge — largely due to the availability of the Synechocystis genome sequence [16]. Details of recent advances have been exhaustively reviewed elsewhere [46–48,49•,50,51•], and here I only outline how these finding relate to endosym-biosis. The overall picture suggests that channels that originated in the cyanobacterial plasma membrane and the outer lipopolysaccharide layer have been recruited to create a system that translocates proteins into the endosymbiont. The first component to be identified in
Synechocystiswas Toc75, the channel in the outer chloro-plast membrane [28••,52••]. In chloroplasts, Toc75 is a voltage-gated ion channel formed by 16 amphipathic beta sheets that are arranged in the membrane so as to create a barrel-shaped channel with an internal diameter of
~2 nm [53]. A homologue (SynToc75 or SLR 1227) with
similar structure and gating properties occurs in the outer membrane of Synechocystis [28••,52••]. The role of this channel in Synechocystis is unknown but based on the known functions of related voltage-gated ion channels with analogous structures [28••,54•] it seems likely that it is a peptide exporter. If this is true, the endosymbiosis must have reversed the direction of this machinery, recruiting these channels to direct proteins into the organelle (Figure 2).
One can imagine that these ‘openings’ were initially exploited as a breach in the boundary that separated the endosymbiont from the host. Later, this breach would have been elaborated and regulated, perhaps by the addi-tion of host-derived regulatory components [49•,55]. In this respect, it is worth noting that most of the genes for translocon components that have obvious cyanobacterial homologues have been transferred to the nucleus allow-ing the host to control the supply lines. Reumann et al.
[28••] go one step further by suggesting that the reloca-tion of the Toc75 gene results in a different (i.e. inside-out) insertion of Toc75 into the outer plastid mem-branes causing it to function in reverse (Figure 2). It will be interesting to compare the relative orientations of the two channels in their respective membranes to test this hypothesis. Reumann et al. [28••] also posit that transit peptides may derive from the original substrate of SynToc75, and that the endosymbiont is, in effect, tricked into importing gene products along with the secretion substrate that is attached to their N-terminus (Figure 2). The irony of this is that SynToc75, which was originally a secreted protein, would now be led into the outer chloroplast membrane from the opposite side by its own substrate (Toc75 has a transit peptide [56]). Support for this hypothetical origin of transit peptides could come from the identification of the SynToc75 secretion
substrate. Since the stromal peptidase responsible for transit peptide cleavage in chloroplasts has a homologue in Synechocystis[49•], we can perhaps anticipate that the SynToc75 substrate undergoes processing prior to export (Figure 2). Moreover, since transit peptides interact with the outer, galactolipid-rich chloroplast membrane [32••], it may be that the hypothetical SynToc 75 secretion sub-strate is associated with the ancestral outer membrane of cyanobacteria (Figure 2).
An important piece of evidence is that the chloroplast import machinery has an independent origin to the similar-but-different mitochondrial import machinery. Intriguingly though, both systems seem to have recruited a similar mechanism, as the mitochondrial outer membrane channel (Tom40) also seems to have been a beta-barrel voltage-gated ion channel in the alpha proteobacteria that became mitochondria [57].
The inner chloroplast membrane channel Tic22 also has a homologue in cyanobacteria but its location and function in this bacterium are unknown [49•]. The mitochondrial inner membrane channel, a complex of Tim23 and Tim17, may have originated from a bacterial amino acid importer (LivH) [58].
Plastid targeting in unicellular ‘plants’
Conclusions
Chloroplasts arose from cyanobacterial-like endosym-bionts that were engulfed by a eukaryotic heterotroph about 1 billion years ago. The availability of the complete genome sequence for a cyanobacterium now allows us to frame hypotheses for the mechanisms of chloroplast divi-sion and the targeting of nuclear-encoded proteins into the organelle. Although much of the original endosym-biont machinery appears to be conserved, some components have undergone substantial modification to either change or remodel their function to suit their new environment within a host cell. Thus, even though Mereschkowsky’s ‘green slaves’ are severely subjugated by their host, becoming a chloroplast has been an immensely successful evolutionary stratagem — there are a lot of them out there.
Acknowledgements
I thank the Australian Research Council for support and Sue Barnes for Figure 1b. Errors in the translation of Mereschkowsky [1] are mine.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Mereschkowsky C: Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biol Zentralbl 1905, 25:593-604. [Title translation: On the origin of plastids in plants.]
2. Schimper AFW: Uber die entwicklung der chlorophyllkörner und farbkörper, Bot Zeitung 1883, 41:105-114. [Title translation: On the development of chloroplasts and chromoplasts.]
3. Pyke KA: Plastid division and development.Plant Cell 1999, • 11:549-556.
A review of plastid division in plants.
4. Pyke KA: Plastid division: the origin of replication.Plant Cell1998, 10:1971-1972.
5. Osteryoung KW, Vierling E: Conserved cell and organelle division. Nature1995, 376:473-474.
6. Osteryoung KW, Stokes KD, Rutherford SM, Percival AL,
•• Lee WY: Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ.Plant Cell1998, 10:1991-2004.
Analysis of ftsZ function using transgenics in Arabidopsis. The first identifica-tion of ftsZ genes whose products may funcidentifica-tion in the eukaryotic cytoplasm.
7. Osteryoung KW, Pyke KA: Plastid division: evidence for a prokaryotically derived mechanism. Curr Opin Plant Biol1998, 1:475-479.
8. Strepp R, Scholz S, Kruse S, Speth V, Reski R: Plant nuclear gene ·· knockout reveals a role in plastid division for the homolog of the
bacterial cell division protein Ftsz, an ancestral tubulin.Proc Natl Acad Sci USA 1998, 95:4368-4373.
A direct demonstration, by gene knockout, that FtsZ is essential for chloro-plast division.
9. Fraunholz MJ, Moerschel E, Maier UG: The chloroplast division · protein FtsZ is encoded by a nucleomorph gene in cryptomonads.
Mol Gen Genetics1998, 260:207-211.
Identifies an essential chloroplast protein in the reduced endosymbiont nucleus of a group of algae with second-hand plastids.
10. Faguy DM, Doolittle WF: Cytoskeletal proteins — the evolution of · cell division.Curr Biol 1998, 8:338-341.
No direct relevance to endosymbiosis but an intriguing discussion of homol-ogy between FtsZ and tubulin and the origin of the cytoskeleton.
11. Erickson HP: Ftsz, a tubulin homologue in prokaryote cell division. Trends Cell Biol 1997, 7:362-367.
12. Wakasugi T, Nagai T, Kapoor M, Sugita M, Ito M, Ito S, Tsudzuki J, Nakashima K, Tsudzuki T, Suzuki Y et al.: Complete nucleotide sequence of the chloroplast genome from the green alga Chlorella vulgaris— the existence of genes possibly involved in chloroplast division.Proc Natl Acad Sci USA 1997, 94:5967-5972.
13. Itoh R, Takano H, Ohta N, Miyagishima S-Y, Kuroiwa H, Kuroiwa T: Two ftsH-family genes encoded in the nuclear and chloroplast genomes of the primitive red alga Cyanidioschyzon merolae.Plant Mol Biol 1999:in press.
14. Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM: The dynamin-related GTPase, dnm1p, controls mitochondrial morphology in yeast.J Cell Biol 1998,143:333-349.
15. Smirnova E, Shurland DL, Ryazantsev SN, Vanderbliek AM: A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 1998, 143:351-358.
16. Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S et al.: Sequence analysis of the genome of the unicellular cyanobacterium Synechocystissp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions.DNA Research1996, 3:185-209.
17. Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, •• Kowallik K: Gene transfer to the nucleus and the evolution of
chloroplasts.Nature 1998, 393:162-165.
The first paper to make substantial use of the collected plastid genome sequences to investigate plastid evolution. Phylogenetic trees based on a massive dataset provide a strong picture of plastid history. Comparison of different gene cohorts in plastids suggests that relocation of plastid genes to the nucleus occurs frequently.
18. Martin W, Herrmann RG: Gene transfer from organelles to the ·· nucleus: how much, what happens, and why?Plant Physiol
1998,118:9-17.
An erudite analysis of possible drivers for intracellular gene transfer. Catalogues genes transferred from endosymbiont to the host whose products are used in the host rather than being targeted back to the endosymbiont.
19. Lynch M, Blanchard JL: Deleterious mutation accumulation in organelle genomes, Genetica 1998, 103:29-39.
20. Gantt J, Baldauf S, Callie P, Weeden N, Palmer J: Transfer of rpl22to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron.EMBO J 1991, 10:3073-3078.
21. Long M, de Souza S, Rosenberg C, Gilbert W: Exon shuffling and the origin of the mitochondrial targeting function in plant cytochrome c1 precursor.Proc Natl Acad Sci USA 1996,93:7727-7731.
22. Wischmann C, Schuster W: Transfer of rps10 from the
mitochondrion to the nucleus in Arabidopsis thaliana: evidence for RNA-mediated transfer and exon shuffling at the integration site.FEBS Lett 1995, 374:152-156.
23. Wegener S, Schmitz U: The presequence of cytochrome c1 from potato mitochondria is encoded on four exons.Curr Genet 1993,24:256-259.
24. Wolter F, Fritz C, Wilmitzer L, Schell J, Schreiber P: rbcSgenes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution.Proc Natl Acad Sci USA
1998,85:846-850.
25. Kubo N, Harada K, Hirai A, Kadowaki K-I: A single nuclear transcript encoding mitochondrial RPS14 and SDHB of rice is processed by alternative splicing: Common use of the same mitochondrial targeting signal for different proteins.Proc Natl Acad Sci USA 1999, 96:9207-9211.
26. Thorbjornsen T, Villand P, Kleczkowski L, Olsen O-A: A single gene encodes two different transcripts for the ADP-glucose
pyrophosphorylase small subunit from barley (Hordeum vulgare). Biochem J 1996, 313:149-154.
28. Reumann S, Davila-Aponte J, Keegstra K: The evolutionary origin of ·· the protein-translocating channel of chloroplastic envelope
membranes: identification of a cyanobacterial homolog.Proc Natl Acad Sci USA 1999, 96:784-789.
A key experimental analysis of Toc75 homologues in Synechocystis, a cyanobacterium related to the ancestor of chloroplasts. The authors develop fascinating hypotheses to explain how a channel that initially secreted pro-teins across the outer, Gram-negative, cyanobacterial membrane could be turned inside-out via gene transfer in endosymbiosis and caused to run back-wards by using the secretion substrate as leader peptides on cytoplasmi-cally synthesised gene products.
29. Kohler R: GFP for in vivo imaging of subcellular structures in plant · cells.Trends Plant Sci 1998, 3:317-320.
This article includes links to web movies of green fluorescent protein target-ed to plant organelles including the extraordinary tubules interconnecting chloroplasts.
30. Shimada Y, Wu G-J, Watanabe A: Screening of cDNA clones for plastid-targeted proteins.Plant Mol Biol Rep 1998, 16:199-200.
31. Wienk HLJ, Czisch M, de Kruijff B: The structural flexibility of the · preferredoxin transit peptide. FEBS Lett 1999, 453:318-326. The first paper to define a secondary structure for transit peptides in relation to the target membrane.
32. Bruce BD: The role of lipids in plastid protein transport. •• Plant Mol Biol 1998, 38:223-246.
A comprehensive review of models for galactolipid and transit peptide interactions.
33. Keegstra K: A new hypothesis for the mechanism of protein translocation into chloroplasts. In Photosynthesis. Edited by Briggs W. New York: Alan R Liss; 1989:347-357.
34. Chen LJ, Li HM: A mutant deficient in the plastid lipid dgd is defective in protein import into chloroplasts.Plant J 1998,16:33-39.
35. Slabas T: Galactolipid biosynthesis genes and endosymbiosis. Trends Plant Sci 1997, 2:161-162.
36. Shimojima M, Ohta H, Iwamatsu A, Masuda T, Shioi Y, Takamiya K: Cloning of the gene for monogalactosyldiacylglycerol synthase and its evolutionary origin.Proc Natl Acad Sci USA
1997,94:333-337.
37. Dormann P, Balbo I, Benning C: Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1.Science 1999, 284:2181-2184.
38. Emanuelsson O, Nielsen H, von Heijne G: ChloroP, a neural · network-based method for predicting chloroplast transit peptides
and their cleavage sites.Protein Sci 1999, 8:978-984.
From the people who brought you SignalP for identification of signal peptides. This paper describes an informatics tool for identification of chloroplast tran-sit peptides in plants. See URL http://www.cbs.dtu.dk/services/ChloroP/
39. Yuan J, Henry R, McCaffrey M, Cline KJ: SecA homolog in protein transport within chloroplasts: evidence for endosymbiont-derived sorting.Science 1994, 266:796-798.
40. Roy LM, Barkan A: A secYhomologue is required for the
elaboration of the chloroplast thylakoid membrane and for normal chloroplast gene expression.J Cell Biol 1998, 141:385-395.
41. Schuenemann D, Amin P, Hoffman NE: Functional divergence of the plastid and cytosolic forms of the 54-kDa subunit of signal recognition particle.Biochem Biophys Res Comm 1999,254:253-258.
42. Schuenemann D, Amin P, Hartmann E, Hoffman NE: Chloroplast SecY is complexed to SecE and involved in the translocation of the 33-kDa but not the 23-kDa subunit of the oxygen-evolving complex.J Biol Chem 1999, 274:12177-12182.
43. Schuenemann D, Gupta S, Persellocartieaux F, Klimyuk VI, Jones JDG, Nussaume L, Hoffman NE: A novel signal recognition particle targets light-harvesting proteins to the thylakoid membranes. Proc Natl Acad Sci USA 1998, 95:10312-10316.
44. Chaal BK, Mould RM, Barbrook AC, Gray JC, Howe CJ:
Characterization of a cDNA encoding the thylakoidal processing peptidase from Arabidopsis thaliana— implications for the origin and catalytic mechanism of the enzyme.J Biol Chem
1998,273:689-692.
45. Settles AM, Martienssen R: Old and new pathways of protein export in chloroplasts and bacteria.Trends Cell Biol 1998,8:494-501.
46. Soll J, Tien R: Protein translocation into and across the chloroplastic envelope membranes.Plant Mol Biol 1998,38:191-207.
47. Heins L, Collinson I, Soll J: The protein translocation apparatus of chloroplast envelopes.Trends Plant Sci 1998, 3:56-61.
48. Fuks B, Schnell DJ: Mechanism of protein transport across the chloroplast envelope.Plant Physiol 1997, 114:405-410.
49. Reumann S, Keegstra K: The endosymbiotic origin of the protein · import machinery of chloroplastid envelope membranes.Trends
Plant Sci 1999, 4:302-307.
A detailed database analysis of possible homologues of chloroplast transport machinery in cyanobacteria the ancestors of the chloroplast endosymbiont.
50. May T, Soll J: Chloroplast precursor protein translocon.FEBS Letts 1999, 452:52-56.
51. Keegstra K, Cline K: Protein import and routing systems of · chloroplasts.Plant Cell 1999, 11:557-570.
A detailed review of the protein import system of chloroplasts with a strong focus on internal sorting.
52. Bölter B, Soll J, Schulz A, Hinnah S, Wagner R: Origin of a ·· chloroplast protein importer.Proc Natl Acad Sci USA
1998,95:15831-15836.
A key paper that identifies a homologue of a central chloroplast protein import component (Toc75) in the cyanobacterium Synechocystis. The Synechocystis version is shown to have an identical structure and to be located in the equivalent membrane providing a strong proof of endosym-biosis as well as confirmation that the outer membrane of chloroplasts derives from the outer membrane of Gram-negative cyanobacteria.
53. Hinnah SC, Hill K, Wagner R, Schlicher T, Soll J: Reconstitution of a chloroplast protein import channel.EMBO J 1997, 16:7351-7360.
54. Bainbridge G, Gokce I, Lakey JH: Voltage gating is a fundamental · feature of porin and toxin beta-barrel membrane channels.FEBS
Letts 1998, 431:305-308.
Hypothesis paper suggesting that beta-barrel membrane channels are a broad family of proteins with roles in membrane transport in bacteria.
55. Heins L, Soll J: Chloroplast biogenesis — mixing the prokaryotic and the eukaryotic.Curr Biol 1998, 8:215-217.
56. Tranel PJ, Keegstra K: A novel, bipartite transit peptide targets OEP75 to the outer membrane of the chloroplastic envelope. Plant Cell 1996, 8:2093-2104.
57. Kunkele KP, Juin P, Pompa C, Nargang FE, Henry JP, Neupert W, Lill R, Thieffry M: The isolated complex of the translocase of the outer membrane of mitochondria — characterization of the cation-selective and voltage-gated preprotein-conducting pore.J Biol Chem 1998, 273:31032-31039.
58. Rassow J, Dekker PJT, van Wilpe S, Meijer M, Soll J: The preprotein translocase of the mitochondrial inner membrane: function and evolution.J Mol Biol 1999, 286:105-120.
59. Schwartzbach SD, Osafune T, Loffelhardt W: Protein import into cyanelles and complex chloroplasts.Plant Mol Biol
1998,38:247-263.
60. McFadden GI: Plastids and protein targeting.J Euk Microbiol
· 1999,46:339-346.
Yet another sesquipedalian offering from the McFadden review factory. Explores intracellular gene transfer with emphasis on secondary endosym-biosis and nucleomorphs.
61. Waller RF, Keeling PJ, Donald RGK, Striepen B, Handman E,
· Lang Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI: Nuclear-encoded proteins target to the plastid in Toxoplasma gondiiand Plasmodium falciparum.Proc Natl Acad Sci USA 1998,95:12352-12357.
62. Lang M, Apt KE, Kroth PG: Protein transport into complex diatom · plastids utilizes two different targeting signals.J Biol Chem
1998,273:30973-30978.
Analyses of leader function for nuclear-encoded plastid proteins of diatoms. Demonstrates that diatom transit peptides mediate uptake by plant chloro-plasts thereby suggesting that the systems are partially conserved.
63. Sulli C, Fang ZW, Muchal U, Schwartzbach SD: Topology of Euglena chloroplast protein precursors within the endoplasmic reticulum
to Golgi to chloroplast transport vesicles.J Biol Chem 1999,274:457-463.
64. Schatz G, Dobberstein B: Common principles of protein translocation across membranes.Science 1996, 271:1519-1526.