www.elsevier.comrlocateranireprosci
The bovine placenta before and after birth: placental
development and function in health and disease
D.H. Schlafer
), P.J. Fisher, C.J. Davies
Department of Biomedical Sciences, College of Veterinary Medicine, Cornell UniÕersity, T6-020, VRT,
Ithaca, NY 14853 USA
Abstract
Ž .
This paper reviews bovine placental development, anatomy microscopic and gross , nomencla-ture and classification. The paper focuses on the biology of those specialized cells that arise from the outermost layer of very early embryos, the trophoblast cells, and on placental macrophages, cells that play a key role in fetalrplacental defense. Data is presented from an immunohistochemi-cal quantitative study that characterizes the ontogeny of placental macrophages using placental
Ž .
tissues from 21 cows sampled from 4 months of pregnancy through the post partum period . Understanding of bovine placental development is essential for veterinarians, pathologists, diag-nosticians and researchers. Lesions of diagnostic significance can be recognized for many economically important infectious abortifacient diseases, and there is growing evidence that pregnancy failure of cloned calves is due in part to unexplained placental failure. Placentology and placental pathology are becoming of increasing importance.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Bovine; Placenta; Development; Anatomy; Histology; Macrophage; Disease; Review
1. Introduction
The cow is one of the ‘‘eutherian’’ species, i.e. those that have placentas. The word ‘‘placenta’’ is taken from latin meaning ‘‘flat cake’’ from the apparent gross similarity of the human discoid placenta to round flat loaves of unleavened bread commonly made in ancient times. The gross appearance of placentas varies greatly between species.
)Corresponding author. Tel.:q1-607-253-3352; fax:q1-607-253-3317.
Ž .
E-mail address: [email protected] D.H. Schlafer .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
146
There are very few similarities between the appearance of a placenta from a woman and one from a cow; but during very early embryonic development, before they enter the uterus, they appear nearly identical. Once placental development begins, dramatic differences become apparent.
When this great variation in anatomy of the definitive placenta from animal species to animal species is combined with the seemingly complex medical terms applied to developmental and histologic features, placentology can become quickly frustrating. The purpose of this paper is to present bovine placental development from a simplified perspective, to discuss and illustrate gross and microscopic features and to present preliminary quantitative data on the ontogeny of bovine placental macrophages, cells that are important in utero-fetal defense systems.
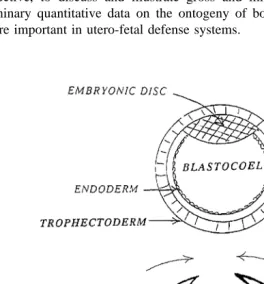
Fig. 1. Essential steps in early placental development.
The outer layer of the expanded blastocyst, the trophectoderm, is composed of trophoblast cells. This layer is
Ž
also referred to as ‘‘the trophoblast’’. Cells on the inner aspect of the blastocoel cavity which becomes the
.
2. Placental development
Following fertilization, the embryo goes through stages of division, modeling and
Ž .
cellular compaction morula stage . Cells that make up the early embryo are called ‘‘blastomeres’’. The ‘‘blastocyst stage’’ occurs when the embryo develops a fluid-filled central cavity, the ‘‘blastocoel’’, that is surrounded by a single layer of cells, the
Ž .
‘‘trophectoderm’’ Fig. 1 . These cells, forming this outer wall, have assumed special-ized functions and are known as trophoblast cells. They will remain as the outermost
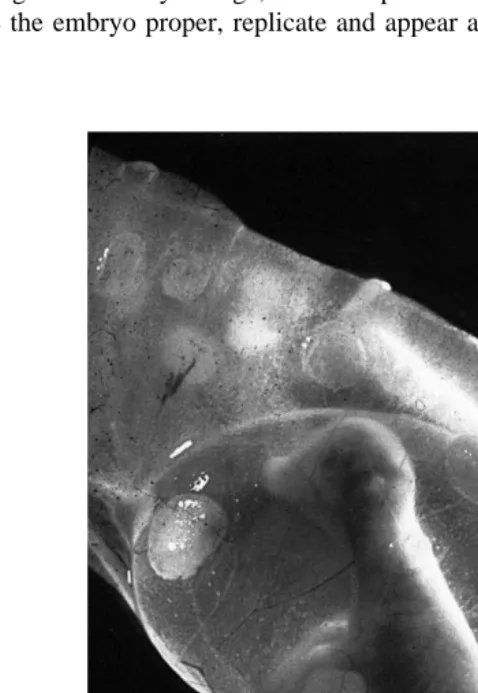
Fig. 2. Bovine Fetuses — Early Placentation.
These bovine fetuses are contained in a clear membrane the ‘‘amnion’’ forming a round fluid-filled structure
Ž .
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
148
layer of cells essentially forming a single cell layer covering the outside of the placenta of all domestic animal species. This outer layer of trophoblast cells, together with the
somaticrparietal mesoderm, form the ‘‘chorion’’.
With time, more fluid is produced within the blastocoel resulting in an expansion of the size of the embryo, which is then called the ‘‘expanded blastocyst’’. The fluid-filled
Ž . blastocoel will become the cavity of the yolk sac Fig. 1 .
During the blastocyst stage, another specialized population of cells, those that will become the embryo proper, replicate and appear as a flat cluster of cells at one pole of
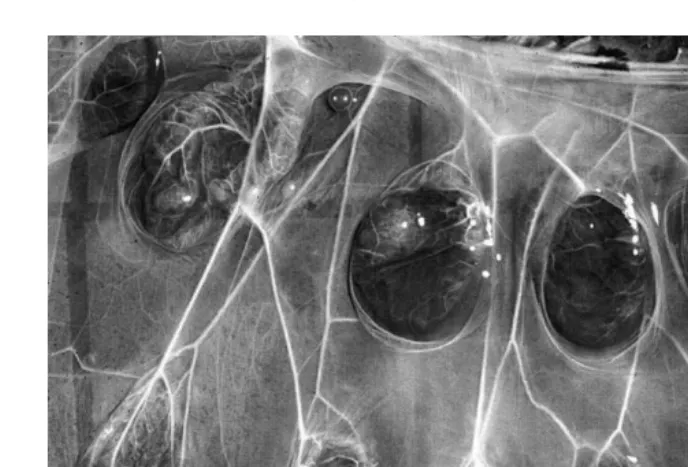
Fig. 3. Bovine fetus and placenta with cotyledons evident.
Ž . the hollow blastocyst. This cluster of cells is the embryonic disc inner cell mass that
Ž .
will become the embryo. Another cell layer endoderm grows from the inner cell mass Ž
to line the cavity of the yolk sac, and a third layer of cells, the mesoderm mesos
.
middle , will then extend from the embyonic disc and form a layer between the trophectoderm and the endoderm.
The expanded blastocyst maintains its round structure, with essentially three
concen-Ž .
tric layers of cells trophectoderm, mesoderm and endoderm surrounding the early yolk sac cavity with the mass of cells that will differentiate into the embryo located along one pole. Following this, the embryo will elongate. The amnion will form as folds of the trophectoblast cells, together with somatic mesoderm, rise up from around the embryo
Ž .
proper to ultimately join and create a fluid-filled space around the embryo see Fig. 2 . As the embryo begins to form into a fetus, but before the ventral abdominal wall is closed, an outpocketing of the hindgut extends from the fetus into the loose tissues of the splanchnic mesoderm. This sac-like stucture is the ‘‘allantois’’ and with the splanchnic mesoderm, it carries with it the vessels that will ultimately vascularize the
Ž . chorion and amnion Fig. 1 .
Only one additional major remodeling event remains. The expanding allantois becomes directly apposed to the chorion . The allantois and the chorion fuse to form the chorioallantois, which, with the amnion, are the ‘‘extra-embryonic fetal membranes’’. Depending on the species, the chorioallantois will assume different shapes and micro-scopic forms as it apposes and, in some cases, invades the endometrium of the gravid uterus.
()
D.H.
Schlafer
et
al.
r
Animal
Reproduction
Science
60
–
61
2000
145
–
160
150
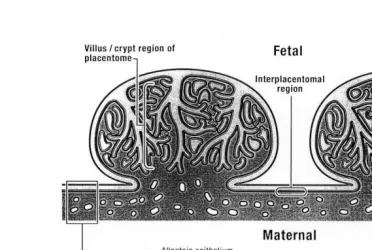
Fig. 5. Drawing of two bovine placentome and fetal membranes attached to the endometrium.
The relationship between the chorioallantois forming cotyledonary villi and the endometrium is demonstrated in this drawing. Branching cotyledonary villi penetrate
Ž Ž .
the crypts of the endometrium at specialized sites called caruncles. Together, they form the placentome. Reprinted from Davies et al. 2000 by permission of the
.
3. Gross anatomy
In the cow, the chorioallantois attaches at about 4 weeks of gestation to the lining of the uterus. This becomes more intimate over specialized areas of the endometrium called
Ž
caruncles approximately 100 are present, evenly distributed throughout the
en-.
dometrium . These sites can be seen grossly even in uteri of fetal calves. As the extra embryonic fetal membranes grow and expand within the lumen of the uterus, the flat surface of the chorioallantois will begin to become irregular over these caruncles. The remodeling of these areas, now recognized grossly as cotyledons, progresses to form
Ž villous projections that interdigitate with recesses in the surface of the caruncle Figs. 3,
.
5 and 6 . The surface of the caruncles develops crypts and the apposing chorioallantois is modeled to form finger-like villous projections that enter into them, thus, enhancing
Ž .
the contact surface area Figs. 4–6 . The combined cotyledonary and caruncular tissues together are called ‘‘placentomes’’. As gestation progresses, these become domed ovoid
Ž .
shaped structures ranging from 10 to 12 cm long and 2–3 cm thick Figs. 4 and 5 . Significant variation in size normally occurs. Placentomes are arranged in a very orderly
Ž .
manner in four rows that run lengthwise along both uterine horns Fig. 7 . They are largest in the horn in which the fetus develops and tend to decrease in size towards the apex of both horns. The surface area of contact between the cow and fetal calf is greatly
Fig. 6. Bovine placentome with the cotyledonary villi partially separated from caruncular crypts.
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
152
Ž .
Fig. 7. ‘‘Fetal membranes’’ chorioallantois with cotyledons expelled after delivery.
This placenta delivered at term demonstrates the regularity of cotyledons and their blood supply from branches of the umbilical arteries and veins. The chorioallantois is viewed from the allantoic surface. In utero, cotyledonary villi would extend from the opposite side into the endometrial crypts of the caruncle. Four rows of placentomes two demonstrated line each uterine horn.
Ž .
enhanced by the extensive interdigitation of fetal and maternal tissues Fig. 6 . This is
2Ž . Ž
estimated to be 130 m Russe and Sinowatz, 1991 equivalent to the entire surface area
. of a large room .
The chorioallantois that exists between placentomes opposes the endometrium in flat to gentle folds and is referred to as either the ‘‘interplacentomal’’ or the ‘‘smooth
Ž .
chorioallantois’’ Figs. 5 and 7 . Both the cotyledonary and the intercotyledonary chorioallantois function to meet the ever-increasing fetal metabolic demands, including the support of rapid tissue growth. Additionally, there is an extensive de novo protein synthesis within placental tissues and production of an ever-expanding list of hormones and growth factors.
Amniotic fluid is produced by several means which include fluxes of fluid across the amnionic epithelium and unkeratinized fetal skin, production by the lungs, and, in later pregnancy, by urine. During most of gestation, urine produced by the fetus is expelled into the bladder and through the urachus within the umbilical cord into the allantoic cavity. Near term, however, it has been shown that the composition of amniotic fluid changes to become more like urine. Fetuses older than 240 days gestation urinate
Ž .
through the urethra into the amniotic cavity Reeves et al., 1972 . The amount of amniotic and allantoic fluid is approximately equal in the 3-month gestation bovine
Ž .
fetus, but by term, the volume of allantoic fluid 10–15 l is greater than the volume of
Ž . Ž .
amniotic fluid 2.5–7.5 l Russe and Sinowatz, 1991 .
4. Histology
As noted, trophoblast cells form a continuous epithelial layer of the chorion, over the entire surface of the chorioallantois. Subpopulations of the trophoblast cells, however, are present, and have different functions.
Two populations of mononuclear trophoblast cells have phagocytic phenotypes. Ž
These are the trophoblast cells lining the arcade area located towards the fetal side of . Ž .
the placentome between the bases of cotyledonary villi Fig. 5 . These cells line the
‘‘hematophagus organs’’ where maternal erythrocytes accumulate between the surfaces of endometrial and chorioallantois tissues and are phagocytized by the trophoblast cells. A second area of specialized phagocytic trophoblast cells is found in the interpla-centomal chorioallantois overlying the openings of endometrial glands. Microscopic accumulations of uterine milk secreted by endometrial glands raise the overlying
Ž .
chorioallantois to form small microscopic dome-shaped spaces referred to as ‘‘areolae’’. Trophoblast cells over these chambers are tall and contain phagocytized uterine milk. While areolae are not visible grossly, careful examination of the surface of fresh placentomes reveals small uniformly distributed black areas that are the hematophagus organs.
Approximately one fifth of bovine trophoblast cells are binucleated. The cellular biology of these cells is amazing. Although they may have a similar microscopic appearance, lectin cytochemistry clearly demonstrates the existence of many
subpopula-Ž .
tions Munson et al., 1989; Jones et al., 1994 .
Trophoblast cells produce a number of hormones and growth factors including
Ž . Ž . Ž
progesterone Reimers et al., 1985 , bovine placental lactogen bPL Duello et al.,
. Ž . Ž
1986 , pregnancy associated glycoproteins bPAG-1, bPAG-2, bPAG-3 Roberts et al.,
. Ž .
1995 , and transforming growth factor beta Munson et al., 1996 . These hormones are associated with fetal growth and development and the maintenance of pregnancy. Progesterone receptors are present within nuclei of many different tissues of the
Ž
placentome, including trophoblast cells, suggesting paracrine function Shuler et al., .
1999 .
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
154
cotyledonary villi across to penetrate the single epithelial cell layer lining the caruncular
Ž .
crypts see Wooding et al., 1980; Wooding, 1982, 1992 . Similar migration occurs in the interplacentomal areas. As the binucleate cells migrate, they routinely fuse with a single endometrial epithelial cell and discharge their cytoplasmic granules. The fusion of a fetal cell, the binucleate trophoblast with a maternal cell temporarily forms a hybrid cell with three nuclei. This hybrid represents the union of two separate individuals into a single cell!
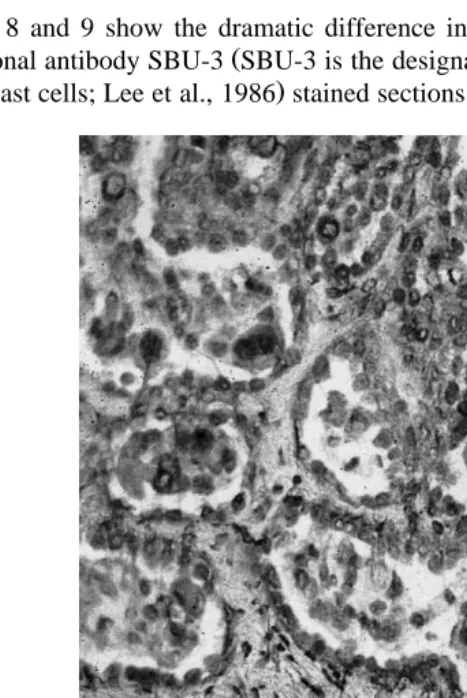
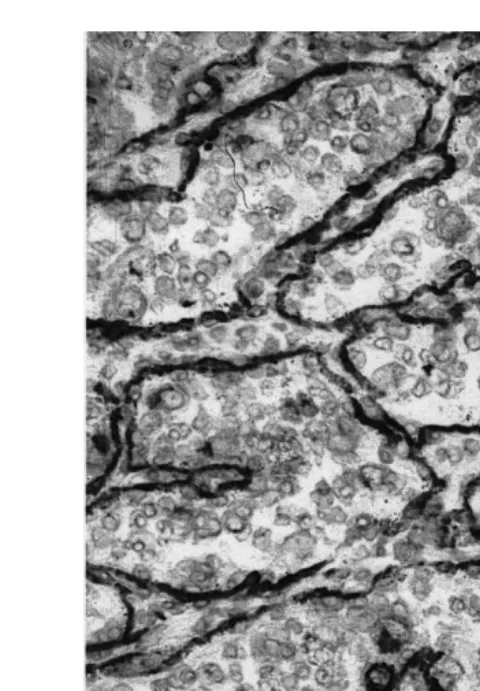
Figs. 8 and 9 show the dramatic difference in immunohistochemical staining using Ž
monoclonal antibody SBU-3 SBU-3 is the designation and antigen prepared from sheep .
trophoblast cells; Lee et al., 1986 stained sections of placentomal tissues comparing late
Fig. 8. Photomicrography of an SBU-3 immunohistochemically stained section of bovine placentome from the placenta of a cow in the 8th month of gestation.
Fig. 9. Photomicrography of an SBU-3 immunohistochemically stained section of bovine placentome from the placenta collected during cesarean section.
Note the deposition of stain along the area of the basement membrane of the caruncular crypts. Binucleate trophoblast cells migrate from the surface of the villi to embed within and fuse with an endometrial epithelial cell followed by dramatic degranulation. They are then lost. Several stained trophoblast cells remain in this photomicrograph. The exact role binucleate cell migration, fusion and degranualtion play in parturition andror placental release is not known.
Ž gestation with the degranulated binucleate cells found immediately before delivery Fig.
.
9 . The monoclonal antibody, originally ‘‘raised’’ against ovine placental tissues, specifically labels binucleate trophoblast cells in ruminant placentas. It is probable that the antigen labeled is a bPAG. The bPAGs have been localized to binucleate cells, and peripheral systemic levels have been shown to rise over the last 10 days of pregnancy
ŽPatel et al., 1997 . This is when marked binucleate cell degranulation occurs Figs. 8. Ž
.
and 9 . It has been suggested that bPAG-1 is a proteinase and may function as an
Ž .
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
156
Another important population of cells found in the bovine placenta are the fetal macrophages. These cells are referred to in human medicine as ‘‘Hofbauer Cells’’
ŽBenirschke and Kaufmann, 1995 . Fetal placental macrophages may originate either.
from chorionic mesenchyme early in gestation, or from fetal bone marrow-derived monocytes. These cells produce pro-inflammatory cytokines and function in antigen presentation suggesting that they can function as sentinel cells, are important in fetal defense and may contribute to transplacental transmission of microbial agents.
5. Disease states
In twinning during bovine pregnancy, placental vessels nearly always fuse. The establishment of shared vasculatures results in fetal chimarism. In male–female twin pairs, this causes characteristic, marked changes in development of the gonads, and internal tubular and external genitalia of the female fetus and is recognized as ‘‘free-martinism’’.
Placentation is important in understanding the pathogenesis of some congenital diseases, mechanisms of transplacental transmission of teratogenic microbial agents, and
Ž .
failure of pregnancy of cloned calves Hill et al., 1999 . It is beyond the scope of this review to address all aspects of in utero infection. Those infectious diseases that produce grossly evident lesions are limited to chronic fungal and bacterial infections. Mycotic infections, most commonly, Aspergillus sp., cause chronic placentitis with severe
Ž .
necrosis and destruction of cotyledonary villi. Brucellosis and yeast infection Candida also result in chronic infection with ‘‘functional amputation’’ of cotyledonary tissues leading to either in utero growth retardation, severe fetal distress, and commonly,
Ž .
abortion Anderson et al., 1986; Foley and Schlafer, 1987 . Transplacental transmission of most viral agents occurs with no grossly evident tissue destruction.
The most common disease of the bovine placenta is placental retention. The precise mechanisms that are triggered, and lead to placental release are not understood. Failure
Ž .
of placental release is common values cited range from 3% to 12% and is higher in individual animals that abort or calve prematurely, in cases of twinning, following
Ž .
induced deliveries, etc. see review by Laven and Peters, 1996 . During normal
maturation prior to delivery, binucleate trophoblast numbers decrease dramatically but Ž
this change does not occur in placentas that are retained Gross et al., 1991; Williams et .
al., 1987 . The mechanisms for this are not known.
Field studies have demonstrated an association between the major histocompatibility Ž .
complex MHC Class I compatibility of a dam and her fetus with placental retention
ŽJoosten et al., 1991; Joosten and Hensen, 1992 . In cases where dam and fetus share.
MHC Class I antigens, placental retention is more likely to occur. This suggests a central role for histocompatibility in triggering an immune reaction that contributes to placental release. Class I antigens have been detected on trophoblast cells in the
Ž . Ž .
6. Placental macrophages: ontogeny during bovine pregnancy
Placentomes were collected at Taylor Packing Plant, Wyalusing, PA. Control tissues Ž
included fetal gut, thymus, lymph node, and maternal mesenteric lymph node 6th month
. Ž .
gestation . Gestation age was estimated from fetal crownrrump length Harris, 1983 .
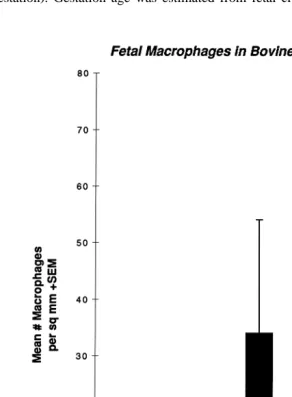
Fig. 10. Change in numbers of fetal macrophages throughout gestation.
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
158
Ž . Ž . Ž .
Placentas were sampled from cattle at approximately 4 ns3 , 6 ns2 , and 8 ns3
months gestation.
Periparturient placentomal samples were collected from seven first calf, holstein Ž
heifers at C-section performed following a drop in progesterone -2 ngrml
ProgestAs-.
say, Synbiotics, san Diego, CA . Placentomes were sampled from the mid-region of the uterine horn that contained the fetus. Post partum placentomes were collected from six cases, obtained per vagina within 30 min of normal vaginal delivery.
During sampling, slabs 5 mm in thickness were carefully sectioned from the central area of each placentome perpendicular to the allantoic surface. Samples were frozen in
chilled isopentane, cryosectioned at 8mm, and fixed in cold acetone for 15 min. After
blocking with normal serum, sections were incubated with primary monoclonal antibody
Ž . Ž
SBU-3 Ctr. An. Biotec., Melbourne, Australia or CD68 EBM 11 Dako, Carpenteria, .
CA , an antibody that labels macrophages, for 2 h at 378C in a humid chamber. Sections
Ž
were then incubated with a biotinylated anti-mouse secondary antibody Vector
Labora-. Ž
tories, Burlingame, CA followed by streptavidin peroxidase conjugate Zymed
Labora-. Ž .
tories, San Francisco, CA and visualized with AEC Zymed and hematoxylin. Non-im-Ž .
mune mouse ascites Zymed was used as a negative control.
Analysis of the number of CD68 EBM 11 positive cells per unit area of tissue was
Ž .
determined using Image-Pro Pluse Media Cybernetics image processing software. A
Ž .
total of 15 fields per placentome two per case were digitized. For each field, fetal villi Ž .
were traced and measured using a freeform ‘‘area of interest’’ AOI mechanism. For each AOI, macrophages were selected using the ‘‘color segmentation’’ function outlin-ing positively stained cells. Macrophage numbers were determined automatically, and calculated per unit area of AOI.
Results of this study are presented in Fig. 10. Relatively few fetal macrophages were present in cotyledonary villi from fetuses younger than 6 months, but by 8 months, macrophages had increased by over 10-fold. Samples collected at cesarean section and from placentas sampled after vaginal delivery contained similar numbers. In cases of mycotic, yeast, and bacterial placentitis, fetal macrophages become prominent within the allantoic mesenchyme in areas of inflammation. The role these cells play in fetal placental defense and their likely role in transporting microbial agents from sites of inflammation within the placenta to the fetus via umbilical veins needs further elucida-tion.
7. Conclusion
Acknowledgements
Funds in support of this work have been provided by the Harold Wetterberg Foundation and the USDA Animal Health and Diseases Research Program.
References
Anderson, T.D., Meador, V.P., Cheville, N.F., 1986. Pathogenesis of placentititis in the goat inoculated with Brucella abortus: Part I. Gross and histologic lesions. Vet. Pathol. 23, 219–226.
Benirschke, K., Kaufmann, P., 1995. In: Pathology of the Human Placenta. 3rd edn. Springer Verlag, New York, NY, pp. 84–92.
Davies, C.J., Fisher, P.J., Schlafer, D.H., 2000. Temporal and regional regulation of major histocompatability complex class I expression at the bovine uterinerplacental interface. Placenta 21, 194–202.
Duello, T.M., Byatt, J.C., Bremel, R.B., 1986. Immunohistochemical localization of placental lactogen in binucleate cells of bovine placentomes. Endocrinology 119, 1351–1355.
Foley, G.L., Schlafer, D.H., 1987. Candida abortion in cattle. Vet. Pathol. 24, 532–536.
Gross, T.S., Williams, W.F., Russek-Cohen, E., 1991. Cellular changes in the peripartum bovine fetal placenta related to placental separation. Placenta 12, 27–35.
Hill, J.R., Roussel, A.J., Cibelli, J.B., Edwards, J.F., Hooper, N.L., Miller, M.W., Thompson, J.A., Looney, C.R., Westhusin, M.E., Robl, J.M., Stice, S.L., 1999. Clinical and pathological features of cloned
Ž .
transgenic calves and fetusus 13 case studies . Theriogenology 51, 1451–1465.
Jones, C.J.P., Koob, B., Stoddart, R.W., Hoffmann, B., Leiser, R., 1994. Lectin-histochemical analysis of glycans in ovine and bovine near-term placental binucleate cells. Cell Tissue Res. 278, 601–610. Joosten, I., Hensen, E.J., 1992. Retained placenta: an immunological approach. Anim. Reprod. Sci. 28,
451–461.
Joosten, I., Sanders, M.F., Hensen, E.J., 1991. Involvement of major histocompatibility complex class I compatibility between dam and calf in the aetiology of bovine retained placenta. Anim. Genet. 22, 455–463.
Laven, R.A., Peters, A.R., 1996. Bovine retained placenta: aetioilogy, pathogenesis, and economic loss. Vet. Rec. 139, 465–471.
Lee, C.S., Gogolin-Ewens, K., Brandon, M.R., 1986. Comparative studies on the distribution of binucleate cells in the bovine placenta of the deer and cow using monoclonal SBU-3. J. Anat. 147, 163–179. Low, B.G., Hansen, P.J., Drost, M., Gogolin-Ewens, K.J., 1990. Expression of major histocompatibility
complex antigens on the bovine placenta. J. Reprod. Fertil. 90, 235–243.
Munson, L., Kao, J.J., Schlafer, D.H., 1989. Characterization of glycoconjugates in the bovine endometrium and chorion by lectin histochemistry. J. Reprod. Fertil. 87, 509–517.
Munson, L., Wilhite, A., Boltz, V.F., Wilkinson, J.E., 1996. Transforming growth factor beta in bovine placentas. Biol. Reprod. 55, 748–755.
Patel, O.V., Sulon, J., Beckers, J.F., Takahashi, T., Hirako, M., Sasaki, N., Domeki, I., 1997. Plasma bovine pregnancy-associated glycoprotein concentrations throughout gestation in relationship to fetal number in the cow. Eur. J. Endocr. 137, 428–432.
Reeves, J.T., Daoud, F.S., Gentry, M., Eastin, D., 1972. Changes in urinary flow in bovine fetuses during late gestation: composition of amniotic and fetal body fluids. Am. J. Vet. Res. 33, 2159–2167.
Reimers, T.J., Ullman, M.B., Hansel, W., 1985. Progesterone and prostanoid production by bovine binucleate trophoblast cells. Biol. Reprod. 33, 1227–1236.
Roberts, R.M., Xie, S., Nagel, R.J., Low, B., Green, J., Beckers, J.F., 1995. Glycoproteins of the aspartyl proteinase gene family secreted by the developing placenta. Adv. Exp. Med. Biol. 362, 231–240. Russe, I., Sinowatz, F., 1991. In: Embryologie der Haustiere., Imogen Russe. Paul Parey, Berlin, p. 164. Shuler, G., Wirth, C.K.K., Pfarrer, C., Leiser, R., Hoffmann, B., 1999. Immunolocalization of progesterone
( ) D.H. Schlafer et al.rAnimal Reproduction Science 60–61 2000 145–160
160
Williams, W.F., Margolis, M.J., Manspeaker, J., Douglass, L.W., Davidson, J.P., 1987. Peripartum changes in the bovine placenta related to fetal membrane retention. Theriogenology 28, 213–223.
Wooding, F.B.P., 1982. The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil., Suppl. 31, 30–31.
Wooding, F.B.P., 1992. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta 13, 101–113.