www.elsevier.com / locate / bres
Research report
Effects of substance P and calcitonin gene-related peptide on axonal
transport in isolated and cultured adult mouse dorsal root ganglion
neurons
a ,
*
a b c dHiromi Hiruma
, Ayako Saito , Takafumi Ichikawa , Yoriko Kiriyama , Sumio Hoka ,
e c a
Tatsumi Kusakabe , Hirosuke Kobayashi , Tadashi Kawakami
a
Department of Physiology, Kitasato University School of Medicine, 1-15-1 Kitasato, Sagamihara 228-8555, Japan
b
Department of Biochemistry, Kitasato University School of Medicine, 1-15-1 Kitasato, Sagamihara 228-8555, Japan
c
Department of Medicine, Kitasato University School of Medicine, 1-15-1 Kitasato, Sagamihara 228-8555, Japan
d
Department of Anesthesiology, Kitasato University School of Medicine, 1-15-1 Kitasato, Sagamihara 228-8555, Japan
e
Department of Sport and Medical Science, Kokushikan University, 7-3-1 Nagayama, Tokyo 206-8515, Japan
Accepted 22 August 2000
Abstract
Substance P and calcitonin gene-related peptide (CGRP) released from primary sensory neurons are known to play important roles in nociception and nociceptive transmission. In the present study, we attempted to clarify the roles of these neuropeptides in the regulation of axonal transport in sensory neurons. Cells were isolated from adult mouse dorsal root ganglia and cultured in F-12 medium containing fetal bovine serum for 48 h until their neurites were grown. These isolated and cultured DRG cells were mostly (.98%) small (diameter
,25mm) and medium (diameter, 25–40mm) in size, and were immunoreactive for substance P and CGRP (85.9 and 66.0% of total cells, respectively). Video-enhanced microscopy was applied to observe particles transported within neurites. Application of substance P (100 nM) decreased the number of particles transported in both anterograde and retrograde directions in each of DRG neurons tested (n55). The instantaneous velocities of individual particles transported in anterograde and retrograde directions were also reduced by substance P. In contrast,a-CGRP (100 nM) increased the number of particles transported in both directions in each of DRG neurons tested (n55), and also increased the instantaneous velocities of particles transported bidirectionally. Application ofb-CGRP (100–1000 nM) did not elicit any effect on axonal transport. Therefore, axonal transport in sensory neurons seems to be modulated by substance P anda-CGRP, both of which can be derived from its own and adjacent sensory neurons. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Axon guidance mechanisms and pathways
Keywords: Axonal transport; Dorsal root ganglion neuron; Substance P; Calcitonin gene-related peptide; Video-enhanced microscopy; Immuno-cytochemistry
1. Introduction dorsal root ganglion (DRG) [24–26,31,36,42] and axonally transported to the central and peripheral endings of sensory Neuropeptides such as substance P and calcitonin gene- nerve fibers [5,12,23,34,35]. They are released from cen-related peptide (CGRP) play a critical role in regulating tral terminals into the dorsal horn of the spinal cord to the inflammatory response in peripheral sensory neurons. convey noxious information as synaptic transmitters [53]. These neuropeptides are synthesized in cell bodies of the These neuropeptides are also released from peripheral terminals to induce extravasation, vasodilatation, inflam-mation, and nociception [29,40,45].
*Corresponding author. Tel.: 181-42-778-9159; fax: 1
81-42-778-Axonal transport is recognized as a cellular function 9841.
E-mail address: [email protected] (H. Hiruma). fundamental to synaptic transmission [11,18,50],
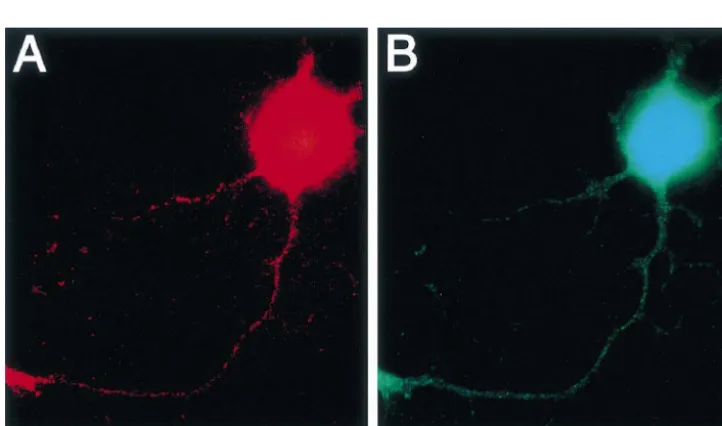
genesis [19], and synaptic formation [7]. Thus, axonal labelling immunofluorescent image, the immunostained transport is considered to be closely related to the develop- cells were examined with an inverted Zeiss Axiomat ment and function of the sensory nervous system as well as microscope (Carl Zeiss, Oberkochen, Germany) equipped other neuronal systems. In the present study, we attempted with exciting filters (546 nm for rhodamine, 450–490 nm to clarify the roles of major sensory neuropeptides, sub- for FITC) and emission filters (590 nm for rhodamine, stance P and CGRP, in the regulation of axonal transport in 515–565 nm for FITC). The number of immunoreactive isolated and cultured adult mouse DRG neurons. neurons was determined under microscopic observation and also by examining microphotographs. DRG neurons used for the experiments on axonal transport were also 2. Materials and methods double immunostained for substance P and CGRP.
2.1. Cell isolation and culture 2.3. Video-enhanced microscopic recordings
Adult male c57BL / 6 mice purchased from Japan SLC After a 48-h period of cell culture, the coverslip on (Hamamatsu, Japan) were killed with ether, and dorsal root which cells were plated was attached with waterproof tape ganglia were removed. The ganglia were incubated for 90 at the bottom of a 0.5-mm thick stainless steel chamber min at 378C in Hams’ F-12 medium (Gibco-BRL, Grand (50380 mm) whose center was hollowed out in the shape Island, NY, USA) containing 2 mg / ml collagenase (Worth- of a lozenge (25335 mm). The top of the chamber was ington Biochemical, Freehold, NJ, USA). Subsequently, covered with another coverslip, leaving small openings on
21
the ganglia were incubated for 15 min at 378C in Ca - both sides to perfuse the solution. The culture medium was 21
and Mg -free Hanks’ balanced salt solution (g / l: KCl, then replaced with Hepes-buffered saline (378C, pH 7.3) 0.4; KH PO , 0.06; NaCl, 8; Na HPO / 7H O, 0.09;2 4 2 4 2 D- containing 135 mM NaCl, 5 mM KCl, 1 mM CaCl , 1 mM2 glucose, 1; phenol red, 0.01; Hepes 3.6; NaOH, 0.3) MgCl , 10 mM Hepes, and 5.5 mM2 D-glucose. The containing 2.5 mg / ml trypsin (Sigma, St. Louis, MO, chamber was mounted onto the temperature-controlled USA). Trypsin activity was then inhibited by addition of stage (378C) of an inverted Zeiss Axiomat microscope, trypsin inhibitor (0.125 mg / ml, Sigma). Following a three- with an oil-immersed planapochromat 643 NA 1.40 times rinse with enzyme-free Hams’ F-12 medium, single objective (Carl Zeiss). The drug-containing solution (3 ml) cells were obtained by triturating the ganglia through was injected into one opening using a Pasteur pipette and fire-polished pipettes (0.2–0.5 mm inner diameter). The the solution spilled from another opening was removed cells were plated onto polylysine (Sigma)-coated glass using a peristaltic pump.
coverslips (30340 mm, 50 mm thickness), and were Nomarski images of axon-like neurites (length, >200 cultured in Ham’s F-12 medium containing 10% fetal mm; width,>1.0mm) obtained by the inverted microscope bovine serum and penicillin (100 units / ml)–streptomycin were transformed into video images by an analogue video (100 mg / ml) at 378C under humidified conditions in 95% camera (Harpicon, Hamamatsu Photonics, Hamamatsu,
air and 5% CO .2 Japan). The analogue signal was processed by a real-time
digital video image enhancement system (DVS-20, 2.2. Immunocytochemistry Hamamatsu Photonics). Video images were displayed on a video monitor (C1846, Hamamatsu Photonics) and were DRG neurons cultured for 48 h on a coverslip were stored on a video recorder (PVW-2800, Sony, Tokyo, fixed with 4% paraformaldehyde for 5 min at room Japan). Such processing provided a final magnification of temperature. After fixation, they were washed with 0.025 approximately310 000 on a video monitor.
M phosphate-buffered saline (PBS) containing 0.3% Triton
X-100 (PBST) for 3 min, and were treated for 10 min with 2.4. Analysis of axonal transport protein blocking agent (Immunon, Pittsburgh PA, USA) at
values obtained during application of neuropeptides were Immunocytochemical staining revealed that a large determined by Student’s paired t-test. The instantaneous portion of DRG neurons were immunoreactive for sub-velocity of the individual moving particles was also stance P and CGRP. Of total 674 neurons, 579 (85.9%) analysed. Serial video images were transferred at 5–20 ms were immunoreactive for substance P, and 420 (72.5%) of intervals to a Macintosh computer (Power Macintosh, substance P-immunoreactive neurons contained CGRP. 7600 / 200) equipped LG-3 video capture board (Scion, Immunoreactivity for CGRP was found in 445 (66.0%) of Frederick, MD) by using Scion image software (Scion). total 674 DRG neurons, and 420 (94.4%) of CGRP-Then, the movement distance / time ratio was analysed and immunoreactive neurons contained substance P. The re-defined as the instantaneous velocity. maining 69 (10.2%) of total neurons exhibited no reactivity for substance P or CGRP. The
immuno-2.5. Drugs reactivities for substance P and CGRP were found in both
the cell bodies and neurites (Fig. 1).
Substance P (Sigma), rat a-CGRP (Peptide Institude, Axonal transport of particles within the long axon-like Osaka, Japan), and rat b-CGRP (Peninsura Laboratories, neurite (length,$200mm; width,$1.0mm) was observed Belmont, CA, USA) were dissolved in Hepes-buffered under video-enhanced microscopy, and the particle move-saline at concentrations indicated in the text. ment measurements were performed at the axon hillock and proximal axon (distance from the cell body, ,100 mm). In control extracellular medium (Hepes-buffered 3. Results saline, pH 7.3, 378C), mean numbers of particles (per min) transported in anterograde and retrograde directions were 3.1. Morphological and immunocytochemical properties 63.3616.3 (mean6S.D., n519) and 63.1625.3 (n519), respectively. The instantaneous velocity of moving par-Most (.98%) of isolated and cultured adult mouse ticles varied from 0.03 to 2.48 mm / s in control medium. DRG neurons were small (diameter,,25mm) and medium
(diameter, 25–40mm) in size. This may be associated with 3.2. Effects of substance P on axonal transport loss of large cells during the process of cell isolation and
culture, because large cells are likely to be more abundant Application of 100 nM substance P decreased the in the intact mouse DRG [56]. In general, small- and number of particles transported in both the anterograde and medium-sized DRG neurons are chemosensitive whereas retrograde directions in each of five neurons tested (Fig. large neurons are not [9,17]. Therefore, small- and 2A). The maximum inhibition reached approximately 60% medium-sized DRG neurons were subjected to the follow- of control after 11 min of the application, and this value ing experiments where the effects of sensory neuropeptides was sustained throughout the application. Expressions of
were investigated. substance P and CGRP in the five neurons used for the
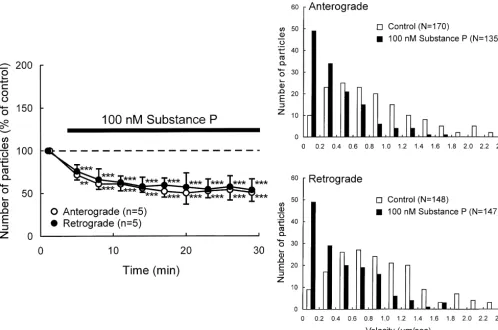
Fig. 2. Effects of substance P on axonal transport in DRG neurons. (A) Percent changes in the number of transported particles moving in anterograde and retrograde directions induced by application of 100 nM substance P. Each point indicates the mean (6S.D.). *P,0.05, **P,0.005, ***P,0.0005 compared to the value before the application. (B) Histograms of the instantaneous velocities of individual particles moving in anterograde and retrograde directions before (control) and during application of substance P.
experiments were investigated by immunocytochemical CGRP but one was not immunoreactive for CGRP. One staining. Of the five neurons, four were immunoreactive neuron was not immunoreactive for either substance P or for substance P. Of these four substance P-immunoreactive CGRP. The instantaneous velocities of individual particles neurons, three were immunoreactive but one was not transported in anterograde and retrograde directions were immunoreactive for CGRP. One neuron was immuno- increased by a-CGRP (Fig. 3B).
reactive for neither substance P nor CGRP. When the
instantaneous velocity of particles was analyzed in one of 3.4. Effects ofb-CGRP on axonal transport the tested neurons, substance P was found to reduce the
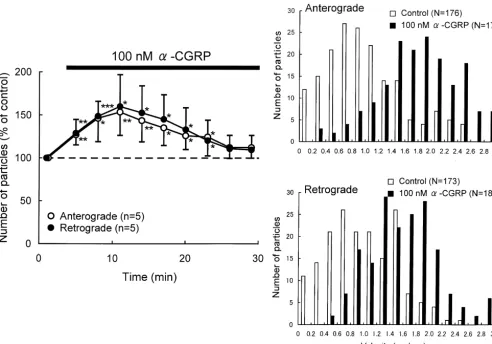
Fig. 3. Effects ofa-CGRP on axonal transport in DRG neurons. (A) Percent changes in the number of transported particles moving in anterograde and retrograde directions induced by application of 100 nMa-CGRP. Each point indicates the mean (6S.D.). *P,0.05, **P,0.005, ***P,0.0005 compared to the value before the application. (B) Histograms of the instantaneous velocities of individual particles moving in anterograde and retrograde directions before (control) and during application ofa-CGRP.
4. Discussion CGRP is modified by culture [8,54], influences of culture are also considerable factors.
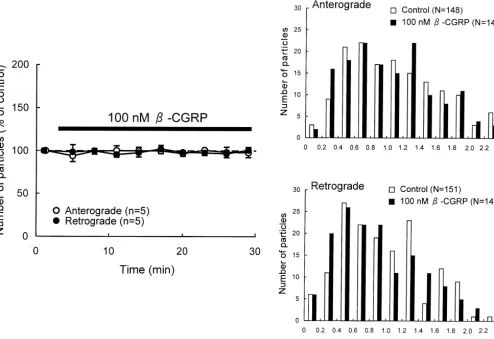
Fig. 4. Effects ofb-CGRP on axonal transport in DRG neurons. (A) Percent changes in the number of transported particles moving in anterograde and retrograde directions induced by application of 100 nMb-CGRP. Each point indicates the mean (6S.D.). (B) Histograms of the instantaneous velocities of individual particles moving in anterograde and retrograde directions before (control) and during application ofb-CGRP.
in DRG neurons. Substance P receptor (neurokinin-1 andb-CGRP can both couple all CGRP receptor subtypes receptor) immunoreactivities and binding sites have been [60]. Therefore, one possibility for explaining our results is found in isolated and cultured rat DRG neurons [55,59] as that mouse DRG neurons may express CGRP receptor well as in the intact rat DRG [58]. Neurokinin-1 receptor subtypes exclusively selective to a-CGRP.
mRNA has also been identified in the mouse DRG by It has been well documented that substance P and using reverse transcription-polymerase chain reaction (RT- mRNA encoding the precursor for substance P are present PCR) [1]. The neurokinin-1 receptor seems to be present in the rat DRG [3,25,46]. Botha-CGRP andb-CGRP and mostly in small- and medium-sized DRG cells [41,55]. their mRNAs are also expressed in rat DRG neurons Several electrophysiological studies have revealed that the [16,44,47,57], but a-CGRP is predominant [16,44,57]. majority (90%) of acutely isolated rat DRG neurons are Thus, axonal transport in sensory neurons may be con-sensitive to exogenously applied substance P [10,28], trolled by substance P anda-CGRP which can be released suggesting that substance P receptor is expressed on the from its own and adjacent sensory neurons, through cell membrane of most isolated DRG cells. Although autocrine and paracrine mechanisms. In addition, recent CGRP receptors have not yet been identified in DRG evidence has shown that substance P and CGRP are neurons, electrophysiological studies have shown that synthesized and released by lymphocytes [38,61]. Thus, CGRP elicits excitatory responses in rat DRG neurons in these neuropeptides derived from lymphocytes may also be situ [51,52], suggesting that DRG neurons functionally involved in the modification of axonal transport.
[10] A. Dray, R.D. Pinnock, Effects of substance P on adult rat sensory synergic action of these neuropeptides [33,49]. However,
ganglion neurones in vitro, Neurosci. Lett. 33 (1982) 61–66. the effects of substance P and a-CGRP observed in this
[11] H.A. Fernandez, J.A. Donoso, Axonal transport involvement in study were opposite, the former was inhibitory and the long-lasting synaptic modifications in Blatta orientalis, J. Neurobiol. latter was stimulatory. In addition, the duration of the 14 (1983) 61–75.
effect of CGRP was transient whereas the effect of [12] H.L. Fernandez, C.A. Hodges-Savola, Axoplasmic transport of calcitonin gene-related peptide in rat peripheral nerve as a function substance P was prolonged. Thus, sensory neuropeptides
of age, Neurochem. Res. 19 (1994) 1369–1377. may contribute to the fine and balanced regulation of
[13] M.T. Galeazza, M.G. Garry, H.J. Yost, K.A. Strait, K.M. Har-axonal transport in sensory neurons. greaves, V.S. Seybold, Plasticity in the synthesis and storage of In the present study, we demonstrated that substance P substance P and calcitonin gene-related peptide in primary afferent inhibited and a-CGRP stimulated axonal transport in neurons during peripheral inflammation, Neuroscience 66 (1995)
443–458. sensory neurons. Axonal transport is fundamental to
[14] R. Gamse, A. Saria, Potentiation of tachykinin-induced plasma neuronal formation and synaptic transmission, therefore,
protein extravasation by calcitonin gene-related peptide, Eur. J. these neuropeptides can modify axonal transport to affect Pharmacol. 114 (1985) 61–66.
development and function of the sensory nervous system. [15] S.J. Gibson, J.M. Polak, S.R. Bloom, I.M. Sabate, P.M. Mulderry, M.A. Ghatei, G.P. McGregor, J.F. Morrison, J.S. Kelly, R.M. Evans, M.G. Rosenfeld, Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species, J. Neurosci. 4 Acknowledgements
(1984) 3101–3111.
[16] S.J. Gibson, J.M. Polak, A. Giaid, Q.A. Hamid, S. Kar, P.M. Jones, This work was partly supported by the Academic P. Denny, S. Legon, S.G. Amara, R.K. Craig, S.R. Bloom, R.J.S. Frontier Project from the Ministry of Education, Science, Penketh, C. Rodek, N.B.N. Ibrahim, A. Dawson, Calcitonin gene-related peptide messenger RNA is expressed in sensory neurones of Sports and Culture, Japan; a Grand-in-Aid for Scientific
the dorsal root ganglia and also in spinal motoneurones in man and Research (No. 11670638) from the Ministry of Education,
rat, Neurosci. Lett. 91 (1988) 283–288. Science, Sports and Culture, Japan; and Comprehensive
[17] M.S. Gold, S. Dastmalchi, J.D. Levine, Co-expression of nociceptor Research on Aging and Health (H10-Aging and Health- properties in dorsal root ganglion neurons from the adult rat in vitro, 008), Health Science Research Grants, from the Ministry Neuroscience 71 (1996) 265–275.
of Health and Welfare, Japan. [18] A. Gorio, The role of axoplasmic transport in the restoration of synaptic transmission and in the process of sprouting during nerve regeneration, Adv. Exp. Med. Biol. 209 (1987) 41–49.
[19] B. Grafstein, D.W. Burmeister, C.M. McGuinness, G.W. Perry, J.R. References Sparrow, Role of fast axonal transport in regeneration of goldfish optic axons, in: Neural Regeneration, F.J. Seil, E. Herbert, B.M. [1] T. Andoh, T. Nagasawa, Y. Kuraishi, Expression of tachykinin NK1 Carlson (Eds.), Progress in Brain Research, Vol. 71, Elsevier,
receptor mRNA in dorsal root ganglia of the mouse, Brain Res. Mol. Amsterdam, 1987, pp. 113–120.
Brain Res. 35 (1996) 329–332. [20] P.G. Green, A.I. Basbaum, J.D. Levine, Sensory neuropeptide [2] G. Battaglia, A. Rustioni, Coexistence of glutamate and substance P interactions in the production of plasma extravasation in the rat,
in dorsal root ganglion neurons of the rat and monkey, J. Comp. Neuroscience 50 (1992) 745–749.
Neurol. 277 (1988) 302–312. [21] U. Hanesch, B. Heppelmann, R.F. Schmidt, Somatostatin-like [3] C.G. Boehmer, J. Norman, M. Catton, L.G. Fine, P.W. Mantyh, High immunoreactivity in primary afferents of the medial articular nerve levels of mRNA coding for substance P, somatostatin anda-tubulin and colocalization with substance P in the cat, J. Comp. Neurol. 354 are expressed by rat and rabbit dorsal root ganglia neurons, Peptides (1995) 345–352.
10 (1989) 1179–1194. [22] U. Hanesch, U. Pfrommer, B.D. Grubb, H.G. Schaible, Acute and [4] S.D. Brain, T.J. Williams, Inflammatory oedema induced by syner- chronic phases of unilateral inflammation in rat’s ankle are associ-gism between calcitonin gene-related peptide (CGRP) and mediators ated with an increase in the proportion of calcitonin gene-related of increased vascular permeability, Br. J. Pharmacol. 86 (1985) peptide-immunoreactive dorsal root ganglion cells, Eur. J. Neurosci.
855–860. 5 (1993) 154–161.
¨
[5] S. Brimijoin, J.M. Lundberg, E. Brodin, T. Hokfelt, G. Nilsson, [23] A. Harmar, P. Keen, Synthesis, and central and peripheral axonal Axonal transport of substance P in the vagus and sciatic nerves of transport of substance P in a dorsal root ganglion-nerve preparation the guinea pig, Brain Res. 191 (1980) 443–457. in vitro, Brain Res. 231 (1982) 379–385.
¨
[6] M. Burg, D.S. Zahm, M.M. Knuepfer, Immunocytochemical co- [24] T. Hokfelt, Neuropeptides in perspective: the last ten years, Neuron localization of substance P and calcitonin gene-related peptide in 7 (1991) 867–879.
¨
afferent renal nerve soma of the rat, Neurosci. Lett. 173 (1994) [25] T. Hokfelt, R. Elde, O. Johansson, R. Luft, G. Nilsson, A. Arimura, 87–93. Immunohistochemical evidence for separate populations of
somatos-ˆ
[7] R.E. Cull, Role of axonal transport in maintaining central synaptic tatin-containing and substance P-containing primary afferent neu-connections, Exp. Brain Res. 24 (1975) 97–101. rons in the rat, Neuroscience 1 (1976) 131–136.
¨
[8] P. Delree, D. Martin, C. Sadzot-Delvaux, B. Rogister, P. Leprince, P. [26] T. Hokfelt, J-O. Kellerth, G. Nilsson, B. Pernow, Experimental Robe, J.M. Rigo, P.P. Lefebvre, B. Malgrange, J. Schoenen, G. immunohistochemical studies on the localization and distribution of Moonen, In vitro and in vivo modulation of 5-hydroxytryptamine-, substance P in cat primary sensory neurons, Brain Res. 100 (1975) thyrotropin-releasing hormone- and calcitonin-gene related peptide- 235–252.
like immunoreactivities in adult rat sensory neurons, Neuroscience [27] L.C. Holford, P. Case, S.N. Lawson, Substance P, neurofilament, 51 (1992) 401–410. peripherin and SSEA4 immunocytochemistry of human dorsal root [9] A. Dray, Chemosensitivity of cultured dorsal root ganglia cells, ganglion neurons obtained from post-mortem tissue: a quantitative
[28] H.-Z. Hu, Z.-W. Li, J.-Q. Si, Evidence for the existence of substance [46] K. Noguchi, R. Dubner, M. De Leon, E. Senba, M.A. Ruda, P autoreceptor in the membrane of rat dorsal root ganglion neurons, Axotomy induces preprotachykinin gene expression in a subpopula-Neuroscience 77 (1997) 535–541. tion of dorsal root ganglion neurons, J. Neurosci. Res. 37 (1994) [29] J.L. Hylden, G.L. Wilcox, Intrathecal substance P elicits a caudally- 596–603.
directed biting and scratching behavior in mice, Brain Res. 217 [47] K. Noguchi, E. Senba, Y. Morita, M. Sato, M. Tohyama,a-CGRP (1981) 212–215. and b-CGRP mRNAs are differentially regulated in the rat spinal [30] A. Ishida-Yamamoto, E. Senba, Cell types and axonal sizes of cord and dorsal root ganglion, Brain Res. Mol. Brain Res. 7 (1990)
calcitonin gene-related peptide-containing primary sensory neurons 299–304.
of the rat, Brain Res. Bull. 24 (1990) 759–764. [48] F. Nothias, A. Tessler, M. Murray, Restoration of substance P and [31] T. Jessell, A. Tsunoo, I. Kanazawa, M. Otsuka, Substance P: calcitonin gene-related peptide in dorsal root ganglia and dorsal horn depletion in the dorsal horn of rat spinal cord after section of the after neonatal sciatic nerve lesion, J. Comp. Neurol. 334 (1993) peripheral processes of primary sensory neurons, Brain Res. 168 370–384.
(1979) 247–259. [49] M. Otsuka, K. Yoshioka, Neurotransmitter functions of mammalian ¨
[32] G. Ju, T. Hokfelt, E. Brodin, J. Fahrenkrug, J.A. Fischer, P. Frey, R. tachykinins, Physiol. Rev. 73 (1993) 229–308.
P, J.C. Elde, Brown, Primary sensory neurons of the rat showing [50] G.A. Phares, P.E. Lloyd, Purification, primary structure, and neuro-calcitonin gene-related peptide immunoreactivity and their relation nal localization of cerebral peptide 1 from Aplysia, Peptides 17 to substance P-, somatostatin-, galanin-, vasoactive intestinal poly- (1996) 753–761.
peptide- and cholecystokinin-immunoreactive ganglion cells, Cell [51] P.D. Ryu, G. Gerber, K. Murase, M. Randic, Calcitonin gene-related Tissue Res. 247 (1987) 417–431. peptide enhances calcium current of rat dorsal root ganglion neurons [33] I. Kangrga, M. Randic, Tachykinins and calcitonin gene-related and spinal excitatory synaptic transmission, Neurosci. Lett. 89
peptide enhance release of endogenous glutamate and aspartate from (1988) 305–312.
the rat spinal dorsal horn slice, J. Neurosci. 10 (1990) 2026–2038. [52] P.D. Ryu, K. Murase, G. Gerber, M. Randic, Actions of calcitonin [34] Y. Kashihara, M. Sakaguchi, M. Kuno, Axonal transport and gene-related peptide on rat sensory ganglion neurones, Physiol.
distribution of endogenous calcitonin gene-related peptide in rat Bohemoslovaca 37 (1988) 259–265.
peripheral nerve, J. Neurosci. 9 (1989) 3796–3802. [53] H.-S. Schaible, On the role of tachykinins and calcitonin gene-[35] P. Keen, A.J. Harmar, F. Spears, E. Winter, Biosynthesis, axonal related peptide in the spinal mechanisms of nociception and in the transport and turnover of neuronal substance P, Ciba Found. Symp. induction and maintenance of inflammation-evoked hyperexcitability 91 (1982) 145–164. in spinal cord neurons (with special reference to nociception in [36] J.A. Kessler, I.B. Black, Similarities in development of substance P joints), in: T. Kumazawa, L. Kruger, K. Mizumura (Eds.), The and somatostatin in peripheral sensory neurons: effects of capsaicin Polymodal Receptor: a Gateway to Pathological Pain, Progress in and nerve growth factor, Proc. Natl. Acad. Sci. USA 78 (1981) Brain Research, Vol. 113, Elsevier, Amsterdam, 1996, pp. 423–441. 4644–4647. [54] J. Schoenen, P. Delree, P. Leprince, G. Moonen, Neurotransmitter [37] L. Kruger, C. Sternini, N.C. Brecha, P.W. Mantyh, Distribution of phenotype plasticity in cultured dissociated adult rat dorsal root calcitonin gene-related peptide immunoreactivity in relation to the ganglia: an immunocytochemical study, J Neurosci. Res. 22 (1989) rat central somatosensory projection, J. Comp. Neurol. 273 (1988) 473–487.
149–162. [55] G. Segond von Banchet, M. Petersen, H.-G. Schaible, Expression of [38] J.-P. Lai, S.D. Douglas, W.-Z. Ho, Human lymphocytes express neurokinin-1 receptors on cultured dorsal root ganglion neurons
substance P and its receptor, J. Neuroimmunol. 86 (1998) 80–86. from the adult rat, Neuroscience 90 (1999) 677–684.
[39] Y. Lee, K. Takami, Y. Kawai, S. Girgis, C.J. Hillyard, I. MacIntyre, [56] E.W. Sommer, J. Kazimierczak, B. Droz, Neuronal subpopulations P.C. Emson, M. Tohyama, Distribution of calcitonin gene-related in the dorsal root ganglion of the mouse as characterized by peptide in the rat peripheral nervous system with reference to its combination of ultrastructural and cytochemical features, Brain Res. coexistence with substance P, Neuroscience 15 (1985) 1227–1237. 346 (1985) 310–326.
[40] F. Lembeck, P. Holzer, Substance P as neurogenic mediator of [57] C. Sternini, K. Anderson, Calcitonin gene-related peptide-containing antidromic vasodilation and neurogenic plasma extravasation, neurons supplying the rat digestive system: differential distribution Naunyn-Schmiedeberg’s Arch. Pharmacol. 310 (1979) 175–183. and expression pattern, Somatosensory Motor Res. 9 (1992) 45–59. [41] H.S. Li, Z.Q. Zhao, Small sensory neurons in the rat dorsal root [58] P. Szucs, E. Polgar, I. Spigelman, R. Porszasz, I. Nagy, Neurokinin-ganglia express functional NK-1 tachykinin receptor, Eur. J. Neuro- 1 receptor expression in dorsal root ganglion neurons of young rats, sci. 10 (1998) 1292–1299. J. Periph. Nerv. System. 4 (1999) 270–278.
[42] J.E. Maggio, J.C. Hunter, Regional distribution of kassinin-like [59] G.S. von Banchet, H-G. Schaible, Localization of the neurokinin 1 immunoreactivity in rat central andperipheral tissues and the effect receptor on a subset of substance P-positive and isolectin B4-of capsaicin, Brain Res. 307 (1984) 370–373. negative dorsal root ganglion neurons of the rat, Neurosci. Lett. 274 [43] P.W. McCarthy, S.N. Lawson, Cell type and conduction velocity of (1999) 175–178.
rat primary sensory neurons with calcitonin gene-related peptide-like [60] S.J. Wimalawansa, Calcitonin gene-related peptide and its receptors: immunoreactivity, Neuroscience 34 (1990) 623–632. molecular genetics, physiology, pathophysiology, and therapeutic [44] P.K. Mulderry, M.A. Ghatei, R.A. Spokes, P.M. Jones, A.M. potentials, Endocr. Rev. 17 (1996) 533–585.
Pierson, Q.A. Hamid, S. Kanse, S.G. Amara, J.M. Burrin, S. Legon, [61] L. Xing, J. Guo, J. Tang, Y. Tang, X. Wang, Morphological evidence J.M. Polak, S.R. Bloom, Differential expression of a-CGRP and for the location of calcitonin gene-related peptide (CGRP)
immuno-b-CGRP by primary sensory neurons and enteric autonomic neurons reactivity in rat lymphocytes, Cell Vision 5 (1998) 8–12. of the rat, Neuroscience 25 (1988) 195–205. [62] X-F. Zhou, W-P. Gai, R-A. Rush, CGRP immunoreactive neurons in [45] R.A. Nicoll, C. Schenker, S.E. Leeman, Substance P as a transmitter rat dorsal root ganglia do not all contain low-affinity NGF receptor