the two groups as noted above for the whole series. A difference in exclusion criteria would
potentially
have had aprofound
effect on the relative remission rates of the twogroups.
DISCUSSION
We believe this
patient
series to berepresentative
of the full clinical spectrumof AML,
and have shown that if allpatients
are taken into account, the clinical course may be lesssuccessful than
previously
thought. 2-4
A
large
number ofpatients
(43/272)
were notgiven
chemotherapy.
In most cases, this was because theattending
physician
judged
thepatient
to beunlikely
to withstandchemotherapy.
Thesepatients
are never included intreatment series and yet a discussion of treatment results and
prognosis
with newpatients
should include this information.31
patients
were started onchemotherapy
but did notcomplete
asingle
course of treatment,usually
because of infectiouscomplications leading
toearly
death. Treatment series will often exclude suchpatients
as"inevaluable",
butthey
are betterregarded
as treatment failures.Many
treatment series report an averagepatient
age of 45 to50
years,’ 2-14
whereas the mean age of all AMLpatients
and the mean age in ourseries,
is60.15
Protocols may excludepatients
over50, 60,
or 70years,12,16,17
or thesepatients
maynot be referred to treatment centres. Patients with
preceding
myelodysplastic
syndromes,
andpatients
in whom AMLdevelops secondary
tocytotoxic chemotherapy
have been shown torespond
poorly
tochemotherapy
forleukaemia.6
Patients with poor
prognostic
variables have beensegregated
in separateseries.7,8,18
It islikely,
therefore,
that thesepatients’
are alsogenerally
excluded from recentchemotherapy
series. If we excludepatients
aged
70 and over,patients
withpreceding myelodysplastic syndromes,
andpatients
withprevious chemotherapy, analysis
shows ahigh
complete
remission rate(85-307o)
asreported
in otherseries.2-4
Reports
of recentimprovements
inchemotherapy
for AML may reflect inadvertent exclusions and refinements inleukaemia classification rather than true
improvement
intreatment
results.190ur
new treatmentregimen
led to animprovement
incomplete
remission rate but nosignificant
difference in overall mediansurvival,
when allpatients
are taken into account. Ifonly
the second series weresubject
tothe exclusions we have
discussed,
the apparentimprovement
could have been considerable. The remission rate for allpatients
in the first series(35%) might
then becompared
with the remission rate forpatients
with various exclusions in the second series(60 - 90%).
Results of treatment trials in AML that show an overall
improvement
in results should include ananalysis
ofpatients
who are untreated orpartially
treated,
and theproportion
ofpatients
with adverseprognostic
indicators.This work was supported by the National Cancer Institute of Canada and Grant CA31761-04A2 from the National Cancer Institute, USA, and the William J. Matheson Foundation.
Correspondence should be addressed to Dr Michael A. Baker, Oncology
Clinic, Toronto General Hospital, 657 University Avenue, Toronto, Ontario M5G 1L7, Canada.
REFERENCES
1. Bloomfield CD. Treatment of adult acute nonlymphocytic leukemia-1980 Ann Int Med 1980; 93: 133-34.
consolidation therapy in adult acute nonlymphocytic leukemia. Blood 1984, 63: 843-47.
6. Estey EH, Keating MJ, McCredie KB, Bodey GP, Freireich EJ. Causes of initial
remission induction failure in acute myelogenous leukemia. Blood 1982; 60: 309
7. Preisler HD, Early AP, Raza A, et al. Therapy of secondary acute nonlymphocytic
leukemia with cytarabine. N Engl J Med 1983; 308: 21.
8. Capizzi RL, Poole M, Cooper MR, et al. Treatment of poor risk acute leukemia with
sequential high dose ARA-C and asparaginase. Blood 1984; 63: 694-700. 9. Harousseau JL, Castaigne S, Milpied N, Marty M, Degos L Treatment of acute
non-lymphoblastic leukaemia in elderly patients. Lancet 1984; ii: 288
10. Yates J, Wallace J, Ellison RR, Holland JF Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chem Rep 1973; 57: 485-88.
11. Baker MA, Taub RN, Carter WH, and the Toronto Leukemia Study Group Immunotherapy for remission maintenance in acute myeloblastic leukemia Cancer Immunol Immunother 1982; 13: 85-88
12. Sauter C, Fopp M, Imbach P, et al Acute myelogenous leukaemia: maintenance
chemotherapy after early consolidation treatment does not prolong survival Lancet
1984; i: 379-82.
13. Buchner T, Urbanitz D, Fischer J, et al. Long-term remission in acute myelogenous
leukaemia Lancet 1984; i: 571.
14. Champlin R, Gale RP, Elashoff R, et al. Prolonged survival in acute myelogenous
leukaemia without maintenance chemotherapy. Lancet 1984, i: 894-96 15 Wintrobe MM, Lee GR, Boggs DR, et al, eds. Classification, pathogenesis and etiology
of neoplastic disease of the hematopoietic system. In Clinical hematology
Philadelphia Lea and Febiger, 1981: 1455-58.
16. Weinstein HJ, Mayer RJ, Rosenthal DS, Camitta BM, Coral FS, Nathan DG, Frei E Treatment of acute myelogenous leukemia in children and adults N Engl J Med
1980, 303: 473-78.
17. Cassileth PA, Begg CB, Bennett JM, et al A randomized study of the efficacy of
consolidation therapy in adult acute nonlymphocytic leukemia. Blood 1984; 63:
843-47.
18. Keating MJ, McCredie KB, Benjamin RS, et al. Treatment of patients over 50 years of
age with acute myelogenous leukemia with a combination of rubidazone and cytosine arabinoside, vincristine and prednisone (ROAP) Blood 1981, 58: 584
19. Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in
cancer. N Engl J Med 1985; 312: 1604-08
20 The Toronto Leukemia Study Group. Survival in acute myeloblastic leukemia is not
prolonged by remission maintenance or early reinduction chemotherapy Blood
1985; 66 (suppl): 210a.
Trial
Design
IS A CONTROLLED TRIAL OF LONG-TERM
ORAL ANTICOAGULANTS IN PATIENTS WITH
STROKE AND NON-RHEUMATIC ATRIAL
Walton
Hospital,
Liverpool;Radcliffe Infirmary, Oxford;
and Northern GeneralHospital,
Sheffield
Summary
A controlled randomised triallarge
enough
to assess the value of
anticoagulating
strokepatients
in atrial fibrillation would be difficult to conduct inthe UK and the results would be
applicable
toonly
a smallproportion
of strokepatients.
It would be more worthwhile toorganise
a trial that also assessed the value of other treatments that aresimpler
andapplicable
to all strokepatients.
A trialthat assessed the value of
aspirin
and beta-blockersagainst
control in all strokepatients
would not cost much more thanone restricted to
comparing
anticoagulants against
control inpatients
with stroke and atrial fibrillation but wouldprovide
information of more relevance to the managementof patients
with stroke in the UK.
INTRODUCTION
MANY stroke
patients
in atrial fibrillation(AF)
aregiven
TABLE I-POSSIBLE RANDOMISED TRIAL DESIGN TO INCLUDE ALL
STROKE PATIENTS (AF AND SINUS RHYTHM)
BB=betablocker. AC= oral anticoagulant.
To assess various anti-haemostatic therapies: X versus Y versus Z. To assess beta-blockade: 1 versus 2.
anticoagulant
versus controlought
to be conducted.However,
there are reasonswhy
a very different trialmight
bebetter. The alternative trial would include not
just
those in AF but all strokepatients;
it would be acomparison
ofanticoagulants
versus control versusaspirin;
andfinally
half of each of the three groups would begiven beta-blockers,
todiscover whether these agents were of any additional value
(table I).
Thedesign
of this trial was based on thefollowing
general
considerations;
that the type and number of strokepatients
in AFlikely
to enter the first type ofstudy
may belimited;
that the outcome forpatients
with AF is not much different from that for those in sinusrhythm
and that moststroke
patients
die not of stroke but of heartdisease,
sotreatments that
might
reduce heart disease need to bestudied;
thatanticoagulants
may reduce the incidence of heart disease and occlusive stroke but increase the incidence ofhaemorrhagic
stroke;
thataspirin
and beta-blockers may be as effective asanticoagulants
but are easier togive
and lesstoxic;
and
finally
that there are many more strokepatients
in sinusrhythm
than inAF,
which makes the former much easier tostudy
and of much greaterpublic
healthimportance.
We concludeby asking
whether asimpler
trial(paradoxically,
without the twoanticoagulant subgroups) might
be evenbetter in a country, such as Great
Britain,
where computertomography (CT)
is often notreadily
available for the detectionof haemorrhagic
stroke(in
whichanticoagulants
are
contraindicated).
The evidence reviewed derives from manysources,
including
the OxfordshireCommunity
StrokeProject
(OCSP),’
in which 512 consecutive cases of first-ever stroke wereinvestigated .
WHAT IS A TYPICAL STROKE PATIENT IN AF LIKE?
The sort of stroke
patient
in whom thequestion
ofanticoagulation typically
arisesmight
be anelderly
womanwith a mild
right hemiparesis,
hypertension,
a left carotidbruit,
and atrial fibrillation but no evidence of rheumaticvalvular disease. The decision to
anticoagulate
shouldideally
be based on: whether the stroke was due to cerebral infarction orprimary
intracerebralhaemorrhage (this
requires
a CT scan of thebrain);
if the stroke was due to infarction whether it was a result ofhypertensive
small vessel disease within thebrain,
embolism from an atheromatousplaque
in the internal carotid artery, or embolism from thefibrillating
leftatrium;
and whether it is wise to
subject
anelderly hypertensive
patient
to the risks and inconvenience ofanticoagulation.
WHAT IS THE EFFECT OF AF ON RISK OF DEATH AND RECURRENT STROKE IN STROKE PATIENTS?
Although
AF not associated with rheumatic heart disease(NRAF)
may increase the risk of death in the first 30days
after astroke,
it has a less marked effect onmortality
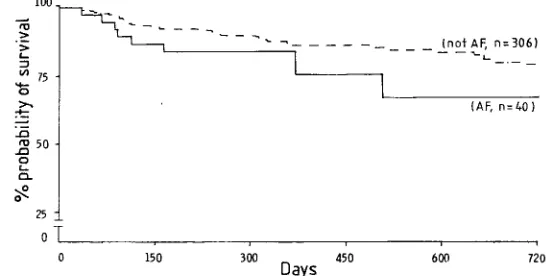
Fig 1-Probability of survival (Kaplan-Meier survival curve) among 346 patients who survived at least 30 days after a first cerebral
infarction.
Data from the Oxfordshire Community Stroke Project.
TABLE II-RISK OF DEATH OR RECURRENT STROKE IN PATIENTS WITH STROKE AND ATRIAL FIBRILLATION
RHD = cases ofAF associated with RHD; NRHD = cases ofAF not associated with RHD; NK = not known.
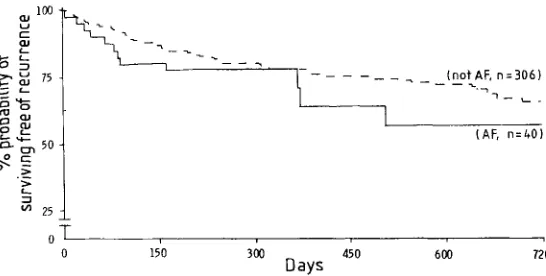
Fig 2-Probability of remaining free of recurrent stroke among 346
patients who survived at least 30 days after a first cerebral
infarction.
Data from the Oxfordshire Community Stroke Project.
thereafter.
However,
it is the30-day
survivors who would bemost
likely
to be entered into along-term
trial ofanticoagulants (table
II andfig 1).
In thelong
term NRAFseems to have little effect on the risk of recurrent stroke
(table
II andfig
2).
Thepossibility
thatpatients
with stroke and NRAF may be at greater risk ofearly
recurrence(within
the firstmonth)
thanpatients
in sinusrhythm3-’
has not beenconfirmed;6,7
the OCSP data so far show that the 6 recurrentstrokes in OCSP
patients
with AF have all occurred more than 1 month after the first stroke.WHAT IS THE EFFECT OF RHEUMATIC HEART DISEASE
(RHD)
ON RISK OF RECURRENT STROKE?In AF
patients
who have not had astroke,
the presence of RHD increases the relative risk of first-ever strokeapproximately
threefold,
from 5 - 6 to17. 6.8 However,
thereare no reliable data on how much RHD influences the
frequency
of recurrent stroke inpatients
withAF,
though
therisk is often assumed to be much
higher
with than without RHD. In the OCSPonly
5/68(7%)
of the cases of first-everstroke in AF had RHD.
Therefore,
even if it wereaccepted
that those with RHD should beanticoagulated,
the treatmentpolicy
for stroke and AF in the 90% or so without RHDmight
still be open to debate(but
this dilemma may not arise in theparts of world where the
prevalence
of RHD ishigh).
WHAT IS THE EFFECT OF ANTICOAGULANTS ON RISK OF RECURRENT STROKE?
The
only
randomised controlled trial ofanticoagulants
inpatients
with stroke attributed to embolism from the heartwas small
(28
patients)
and did not state the criteria fordiagnosis
of embolicstroke,
the number ofpatients
withAF,
or the number with
RHD.9
There have been extensive reviews of non-randomised studies ofanticoagulants
inpatients
with stroke that waspresumed
to be due to embolism from theheart,5,10-12
and some of the reviews have come outvery
strongly
in favour of the use ofanticoagulants. But,
non-randomised studies do not in
general
yield
reliable data and when historical controls have beenused,
claims such as "...in studies of
anticoagulation
... inpatients
with cerebralembolism ... there has been a 65 to 90% reduction in the number
ofembolic accidents"’2
should beinterpreted
withcaution. In
addition,
many of the studies were done before CTscanning
was available and most included ahigh
proportion
of cases withRHD,
so their relevance to thetreatment of strokes in the UK in the 1980s, when RHD is
relatively
uncommon, is debatable.A
rough
idea of thelikely
reduction in risk of stroke thatanticoagulation might
confer onelderly people
may beobtained from a
study
of the use ofanticoagulants
aftermyocardial
infarction inpatients aged
over60.’
Thisstudy
waslarge
and randomised andattempted
to determineby
CT scan and/or necropsy whether strokes that did occurwere ischaemic or
haemorrhagic.
There were 4 cases ofCT-proved
cerebral infarction among the 493patients
allocatedplacebo
andonly
1 among the 493 allocatedanticoagulants (a
risk reductionof 70%).
However,
there were 7 cases(6
fatal)
of definite intracranial
haemorrhage (ICH)
in the treated group andonly
1 in the control group.Thus,
if cases ofdefinite cerebral infarction and definite ICH were
counted,
anticoagulants
caused a 60% net increase in the risk of stroke. The numbers of cases were very small and the confidenceintervals
wide,
so no firm conclusions can be drawn.Nevertheless,
thefindings
show theimportance
ofbalancing
any reduction in the risk of cerebral infarction
against
anyincreased risk of ICH in studies of stroke
prevention.
WHAT IS THE EFFECT OF ANTICOAGULANTS ON RISK OF INTRACRANIAL HAEMORRHAGE?
Despite
thehigh
relative risk of ICH inanticoagulated.
patients,
the absolute number ofpatients
with ICH is still small.Unfortunately,
ICH may nevertheless be thecommonest fatal
complication
ofanticoagulant
therapy. IS
However,
itsfrequency
inpatients
with well-controlledprothrombin
times is still not known.Widespread,
inadequately
controlled use ofanticoagulants
in the 1960s may well have caused anappreciable
increase in thefrequency
of ICH inRochester,
Minnesota.16
InLeiden,17
where
anticoagulant
therapy
is centralised and veryclosely
supervised, anticoagulants
increased the risk of ICH ten-foldin
people
over 50. A similar increase in risk occurred inover-60s
given
oralanticoagulants
aftermyocardial
infarction.14
The risks of ICH in
patients
who havealready
had a strokeare rather different. For stroke due to
ICH,
anticoagulants
areobviously strongly
contraindicated,
soearly
CTscanning
torule out ICH before
starting
anticoagulant
treatment for anytype of stroke is
mandatory. 18-20
However,
a cerebral infarction may be followedby
spontaneoushaemorrhage
intothe infarcted area, and
anticoagulants
may exacerbate thistendency
(even
ifprothrombin
activity
remains within anacceptable
therapeutic
range). 111,11,21-2’
The risk of a"pale"
cerebral infarct
becoming
a"haemorrhagic"
infarct ishighest
in
patients
withlarge
infarcts orhypertension
and,
notsurprisingly,
inpatients given
excessive doses ofanticoagulants. 21-21
The difficulties ofcontrolling
anticoagulant
therapy
in strokepatients
with AF arecompounded by
the age of most of thepatients.
In the OCSP57/68
(84%)
of thepatients
with first strokes in AF wereaged
70 or over and 24/68
(35%)
wereaged
80 or over.Although
age did not affect the relative risk of ICH due to
anticoagulants
in the Leidenstudy,l
the absolute risk mayrise very
steeply
withage.15,26-28
Moreover,
supervision
ofanticoagulants
inelderly
patients
may be difficult:they
maybe on several other
drugs,
so that interactions may occur,drug compliance
may be poor(especially
after astroke);
andthey
may havedifficulty
inattending
ananticoagulant
clinic.Hypertension,
whichgreatly
increased the risk of ICH forpatients
with stroke andAF;
forexample,
54% of the OCSP series weredefinitely hypertensive (at
least two bloodpressures greater than 160/90 mm
Hg
recorded before thestroke),
and inKelley’s
study29
78% ofpatients
with stroke and AF werealready
onantihypertensive
drugs
at the time ofadmission.
IS A RANDOMISED CONTROLLED TRIAL NEEDED?
As discussed
above,
after oralanticoagulation
the netreduction in risk of recurrent
stroke-and,
moreimportantly,
ofdisabling
and/or fatal stroke-could be modest inpatients
with NRAF. The risk of ICH may beparticularly
high
sincemany such
patients
areelderly
and/orhypertensive.
Arandomised trial is therefore necessary to assess the balance of risks and benefits
of anticoagulant
treatment inpatients
with stroke and non-RHD AF.7,3O A similarstudy might
also beappropriate
for the smallminority
offibrillating
strokepatients
who do haveRHD, though
few clinicians would consider such a trial ethical.WHAT PROPORTION OF STROKE PATIENTS ARE SUITABLE FOR ANTICOAGULATION?
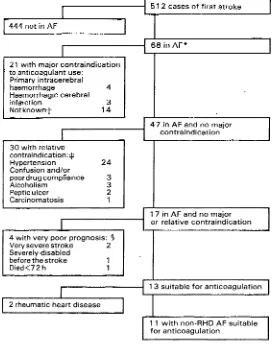
The OCSP data
(fig 3)
suggest thatonly
13/68(19%)
casesof first stroke with AF were suitable for
anticoagulant
Fig 3-Number of first strokes in AF suitable for anticoagulant therapy in the OCSP.
*Includes five cases ofAF associated with RHD.
tNo CT scan or necropsy performed.
$Some patients had more than one contraindication. Hypetension=two _ BP>160/90 mm Hg recorded before stroke.
§Severe stroke=hemiplegia, aphasia, and/or coma, survived >72 h; very disabled before stroke=needs assistance to walk and to attend to own body and/or bedbound.
TABLE
III-LIKELY
NUMBER OF PATIENTS WITH CT-PROVEN ISCHAEMIC STROKE AND NON-RHEUMATIC ATRIAL FIBRILLATIONAVAILABLE FOR CLINICAL TRIAL OF ANTICOAGULANTS Number available in England and Wales
First strokes in England and Wales each year* =96 000
ofwhoml3-3%inAF =12770
of whom 16-2% have non-RHD AF and "suitable for
anticoagulant therapy" = 2100
of whom 50% might enter a trialt = 1050
Number avazlable zn average datrict general hospital
(servmg about 250 OOO/yr)
Number of strokes per year =500
of whom 50% admitted to hospital =250
of whom 13.3% m AF = 33
of whom 16-2% have non-RHD AF and "suitable for
anticoagulant therapy" = 5
of whom 50% might enter a trial = 3
*From OCSP.1
t Previous multicentre trials suggest that about 50% of the patients eligible to enter the trial are not entered for a variety of reasons (patient refusal, follow-up
likely to be difficult, &c.).
therapy,
aproportion
similar to thatreported
fromScandinavia:31 however,
2 of these cases had RHD(and
1 of these wasalready
onanticoagulants).
Thusonly
11/512(2%,
95% confidence limits 0 - 8-3’
2%)
of all first strokes wouldhave been
eligible
for a trialof anticoagulation
in NRAF andstroke. Such a
study
would not be of direct relevance to 98%of strokes.
Nonetheless,
2% of all first strokes isequivalent
toabout 2000 cases in
England
and Wales a year. Inpractice,
only
a smallproportion
of thesemight
enter a randomisedtrial
(table III).
Would a trial infibrillating
strokepatients
thusbe feasible?
WHAT SIZE OF SAMPLE IS REQUIRED FOR A TRIAL OF ANTICOAGULANTS IN STROKE WITH NRAF?
If anticoagulants
can reduce thefrequency
of stroke and/or deathby
about30%,
then about1500
patients
would have tobe randomised and followed up for a few years if there is to be
a reasonable chance
(90%)
ofgetting
aconventionally
significant
result(p<O’ 05).32
It wouldprobably
bepracticable
to enteronly
inpatients,
and ourexperience
(table
III)
suggests that ahospital might
be able to enteronly
about 2 or 3patients
peryear,
so well over 100hospitals might
berequired.
Furthermore,
since allpatients
wouldrequire
a CT scan before randomisation to excludeICH,
only
the limitednumber of
hospitals
in the UK withrapid
access to a CT scanner couldparticipate,
so it wouldprobably
beimpossible
to recruit the numbers that
might
be needed for anadequate
study.
However,
if the true risk reduction is much better thanone-third,
then much smaller numbers ofpatients
wouldsuffice;
conversely
if attention was restricted to fatal ordisabling
strokes,
farlarger
numbersmight
berequired.
IS A TRIAL OF ANTICOAGULANTS IN NRAF WORTHWHILE?
A trial that had to recruit
subjects
from many centres would be difficult toorganise,
would beexpensive,
and wouldrequire
considerable effort to ensure strict adherence to theprotocol (especially
with respect toprothrombin
measure-ment for control oftherapy).
Furthermore,
if the resultswould be
applicable
toonly
about 2% of all firststrokes,
froma
public
healthviewpoint
at least, the value of a trial ofanticoagulants
restricted to strokepatients
with NRAF seemsless worthwhile than at first.
SHOULD THE TRIAL INCLUDE ALL PATIENTS WITH ISCHAEMIC STROKE?
Since the
long-term
outcome after ischaemic stroke seems to be similar whetherpatients
are in AF or sinusrhythm
(table
II)
and since the valueof anticoagulants
in either type ofpatient
has not beenreliably
established,33
there could be a case fortesting
anticoagulants
in allpatients
with ischaemic stroke.However,
we think that most clinicians would nothave as much enthusiasm for such a trial. Indeed many
might
be disturbed at the prospect, if the trial were to be
positive,
of thousandsof elderly
strokepatients being
given long-term
anticoagulants.
Furthermore,
the number of CT scans thatwould have to be done may be an
impossible
burden forBritain’s
already
stretched CTscanning
facilities.’9
ARE THERE OTHER TREATMENTS THAT ARE SIMPLER, MORE WIDELY APPLICABLE, AND LIKELY TO CONFER USEFUL
BENEFIT?
Death after transient ischaemic attack
(TIA)
and stroke ismore often due to ischaemic heart disease than to recurrent
stroke.34-36
Aspirin,
takendaily
for months or years aftermyocardial
infarction,
reduces the risk of deathby
about 15% and of furthermyocardial
infarctionby
about25%.
Beta-blockers reduce themortality
aftermyocardial
infarctionby
about25%.38
Furthermore,
there is somesuggestion
thataspirin given
after TIA and mild ischaemic stroke reduces the risk ofsubsequent
death and recurrentstroke;39
this effect isbeing
assessedby
the UK TIAaspirin
study
andby
a collaborativeanalysis
ofpublished
trials.Apart
fromsimplicity
and wideapplicability
these two agents have theadvantage
that a CT scan may not be necessary before start oftreatment to exclude ICH-a
simple
clinicalscoring
systemmight
beadequate. 21
WHAT IS THE DESIGN OF THE IDEAL TRIAL?
The
simplest
trial that would answer most of theimportant
questions
(ie,
areanticoagulants
andaspirin
eacheffective,
and, if so,
which is the moreeffective?)
would be to allocate all strokepatients (AF
and sinusrhythm
subgroups
can still beanalysed separately)
randomly
to one of three treatments(placebo, aspirin,
or oralanticoagulants).
This trial wouldrequire
an 11%larger sample
than a trial ofplacebo
vsanticoagulants.
A factorialdesign
would allow beta-blockersto be tested as well without further
increasing
thesample
size(table I).
Such a trial islikely
to be no moreexpensive
in termsof money or effort than one of
anticoagulants
versusplacebo
but would
provide
more answers.CONCLUSIONS
A trial of
long-term
oralanticoagulants
inpatients
with ischaemic stroke and NRAF or in all ischaemic stroke wouldperhaps
be done moreeasily
in countries(such
as the Netherlands or theUSA)
where ahigh
proportion
of strokesare admitted to
hospitals
with CT scanners. In theUK,
it would be more worthwhile to concentrate collaborative efforts(and
limitedresources)
on the reliable assessment ofsimpler,
morewidely practicable
treatments forsecondary
strokeprevention
whichmight
beapplicable
to allpatients
with ischaemicstroke;
aspirin
and beta-blockers are two suchagents which we believe could-and should be-tested.
Data from the Oxfordshire Community Stroke Project formed the basis for this paper and we would therefore like to thank all those who collaborated with
us in that study. The Oxfordshire Community Stroke Project is funded by the MRC with additional support from the Chest, Heart and Stroke Association.
Correspondence should be addressed to P. S., Department of Neurology,
Walton Hospital, Rice Lane, Liverpool L9 lAE.
REFERENCES
1 Oxfordshire Community Stroke Project Incidence of stroke in Oxfordshire: first year’s experience of a community stroke register Br Med J 1983, 287: 713-17 2 Lowe GDO, Forbes CD, Jaap AJ Relation of atrial fibrillation and high haematocrit to
mortality in acute stroke Lancet 1983, i: 784—86
3 Wolf PA, Kannel WB, McGee DL, Meeks SL, Bharucha NE, McNamara PM Duration of atrial fibrillation and imminence of stroke the Framingham study Stroke 1983, 14: 664-67
4. Easton JD, Sherman DG. Management of cerebral embolism of cardiac origin Stroke
1980, 11: 433-42
5. Sherman DG, Goldman L, Whiting RB, Jurgensen K, Kaste M, Easton JD,
Thromboembolism in patients with atrial fibrillation Arch Neurol 1984, 41: 708-10
6. Britton M, Gustafsson C Non-rheumatic atrial fibrillation as a risk factor for stroke
Stroke 1985, 16: 182-88
7 Sage JI, Van Uitert RL Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease Stroke 1983; 14: 537-40
8. Wolf PA, Dawber TR, Thomas HE, Kannel WB Epidemiologic assessment of chronic
atrial fibrillation and stroke the Framingham study Neurology 1978, 28: 973-77 9. Baker RN, Broward JA, Fang HC, et al Anticoagulant therapy in cerebral infarction
Report on cooperative study Neurology 1962; 12: 823-29
10 Genton E, Barnett HJM, Fields WS, Gent M, Hoak JC. XIV Cerebral ischaemia. the role of thrombosis and antithrombotic therapy Stroke 1977, 8: 150-75
11 Yatsu F, Mohr JP Anticoagulant therapy for cardiogenic emboli to brain Neurology,
1982, 32: 274-75
12 Mohr JP, Fisher CM, Adams RD Cerebrovascular Disease. In. Isselbacher KJ, Adams RD, Braunwald E, Petersdorf RG, Wilson JD, eds. Harrison’s principles of internal medicine (9th edn), New York McGraw-Hill, 1980 1932.
13 Sixty plus reinfarction study group A double-blind trial to assess long-term oral
anticoagulant therapy in elderly patients after myocardial infarction Lancet 1980, ii:
989-94.
14. Sixty plus reinfarction group. Risks of long-term oral anticoagulant therapy in elderly
patients after myocardial infarction Lancet 1982, i: 64-68
15 Silverstein A Neurological complications of anticoagulant therapy. Arch Intern Med
1979, 139: 217-20
16 Furlan AJ, Whisnant JP, Elveback LR The decreasing incidence of primary intracranial haemorrhage a population study Ann Neurol 1979; 5: 367-73 17. Wintzen AR, de Jonge H, Loeliger EA, Bots GTAM Risk of intracerebral
haemorrhage during oral anticoagulant treatment, a population study Ann Neurol
1984, 16: 553-58
18 Ruff RL, Dougherty JH Evaluation of acute cerebral ischaemia for anticoagulant therapy computed tomography or lumbar puncture. Neurology 1981; 31: 736-40 19. Sandercock PAG, Molyneux A, Warlow CP The value of CT scanning in patients with
stroke The Oxfordshire Community Stroke Project Br Med J 1985, 290: 193-97
20. Sandercock PAG, Allen CMC, Corston RN, Harrison MJG, Warlow CP Clinical diagnosis of intracranial haemorrhage using the Guy’s Hospital Diagnostic Score Br Med J 1985; 291: 1675-77
21 Shields RW, Laureno R, Lachman T, Victor M. Anticoagulant-induced haemorrhage
in acute cerebral embolism Stroke 1984, 15: 426-37
22 Drake ME, Shin C. Conversion of ischaemic to haemorrhagic infarction by
anticoagulant administration Arch Neurol 1983, 40: 44-46.
23. Lodder J, Van der Lugdt PJM Evaluation of the risk of immediate anticoagulant
treatment in patients with embolic stroke of cardiac origin Stroke 1983; 14: 42-46
24 Cerebral embolism study group Immediate anticoagulation of embolic stroke Brain
haemorrhage and management options. Stroke 1984, 15: 779-89.
25 Ramirez-Lassepas M, Quinones MR. Heparin therapy for stroke haemorrhagic
complications and risk factors for intracerebral haemorrhage. Neurology 1984; 34: 114-17
26. Roos J, Joost HE The cause of bleeding during anticoagulant treatment Acta Med
Scand 1965, 178: 129-31.
27 Heyman A, Nefzger MD, Acheson RM. Epidemiotogic study of the relationship of
anticoagulant therapy to mortality from intracranial haemorrhage Trans Am Neurol Assoc 1912, 97: 152-55
28. Loeliger EA Drugs affecting blood clotting and fibrinolysis In: Dukes MNG, ed Side effects of drugs, 9th ed. Amsterdam- Excerpta Medica, 1980; 9: 587-613 29 Kelley RE, Berger JR, Alter M, Kovacs AG Cerebral ischaemia and atrial fibrillation
a prospective study Neurology 1984, 34: 1285-91
30. Starkey IR, Warlow CP The secondary prevention of stroke in patients with atrial fibrillation. Arch Neurol 1986; 43: 66-70
31. Norrving B, Nilsson B. The serious outcome in cerebral embolism of cardiac origin
J Neurol 1985, 232 (suppl) 115.
32. Taylor DW, Sackett DL, Haynes RB. Sample size for randomised trials in stroke prevention. How many patients do we need? Stroke 1984; 15: 968—71 33 Warlow CP Cerebrovascular disease Clins Haematology 1981; 10: 631-51 34. Marquardsen J The natural history of acute cerebrovascular disease: a retrospective
survey of 789 patients Copenhagen Munksgaard, 1969 147
35. Heyman A, Wilkinson WE, Hurwitz BJ, et al. Risk of ischaemic heart disease in
patients with TIA. Neurology 1984; 34: 626-30.
36. Matsumoto N, Wisnant JP, Kurland LT, Okazaki H. Natural history of stroke in
Rochester Minnesota 1955 through 1969: extension of a previous study 1945
through 1954 Stroke 1973, 4: 20-29
37. Anon (editorial) Aspirin after myocardial infarction. Lancet 1980; i: 1172-73
38 Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Betablockade during and after
myocardial infarction: an overview of the randomised trials. Progr Cardiovasc Dis
1985; 27: 335-71.
39 Canadian cooperative study group. A randomized trial of aspirin and sulphinpyrazone